SUMMARY
Case report data and a matched case-control study were used to investigate the epidemiological characteristics of hand, foot and mouth disease (HFMD) in children in Shenzhen, China between 2008 and 2011. Multivariate analyses were used to evaluate factors associated with severity of infection. Laboratory tests were performed to determine aetiological identification for samples from 163 severe and fatal cases as well as an outpatient-based HFMD sentinel surveillance system (n = 446). All identified EV71 belonged to sub-genotype C4a. No major changes in the CA16 and EV71 viruses were found until the end of 2011. Annual attack rates and the case-severity ratios (CSRs) rose from 0·82/1000 and 0·56/1000, respectively, in 2008 to 2·12/1000 and 6·13/1000 in 2011. The CSR was higher in migrants than in local residents. The adjusted odds ratio (OR) of having a severe attack for being a migrant was 2·45, having a fever >39°C (OR 5·77), visiting a private clinic (OR 2·65), longer time from symptom onset to diagnosis (OR 1·49), visiting a doctor (OR 1·51), early use of intramuscular pyrazolone (OR 3·36), early use of intravenous glucocorticoids (OR 2·28), or the combination of both (OR 3·75). The mortality and increasing case severity appears to be associated with socioeconomic factors including migration and is of worldwide concern.
Key words: China, epidemiological studies, hand foot and mouth disease, migrant children, surveillance
INTRODUCTION
Hand, foot and mouth disease (HFMD) is a common disease mainly occurring in children aged <5 years. It includes a group of diseases that are caused by numerous members of the Enterovirus genus of the family Picornaviridae, of which coxsackievirus type A (CA) and enterovirus 71 (EV71) are the most frequently seen [1]. The majority of cases only suffer mild symptoms but some individuals may become critically ill. Of all enteroviruses causing HFMD, EV71 has been recognized as the most important pathogen in the development of severe complications, hospitalizations, deaths and large-scale outbreaks [2–6]. In 1998, an unprecedented HFMD epidemic in Taiwan recorded 405 hospitalization, 78 deaths and a sentinel surveillance-based incidence of 129 106 cases [7]. However, in mainland China HFMD did not attract international attention until 2008 when several large outbreaks of HFMD occurred [8]. Deaths caused by this often benign disease were markedly high and directly led to a quick revision of China's Communicable Disease Prevention & Control Act in May 2008, when all health professionals were required to mandatorily report HFMD cases to the Ministry of Health (MOH) China through a real-time online system – the National Infectious Disease Surveillance and Reporting Information System (NIDSRIS), which is part of the China Information System for Disease Control and Prevention (CISDCP) [9]. At the local level before May 2008 in Shenzhen, clinically diagnosed cases of HFMD were allowed by Shenzhen Health Bureau to be reported to the local online real-time reporting system under category of ‘other infectious diseases’. Starting in May 2008, all doctors in Shenzhen were required by law to report HFMD cases. Those required to report included health professionals at community (community health centres and clinics), district (district general hospitals, health departments and women and children's hospitals), and city (city-level hospitals or medical centres) levels with a report timeline of within 24 h of diagnosis. Data auditing occurred at departments of disease prevention in each hospital, district CDC and municipal CDC to ensure adherence to reporting guidelines.
Genotype analyses of EV71 have not found persuasive evidence of change in virus lineages or genetic mutation with C4a being the most prevalent pathogen throughout the mainland since 2008 [10–12]. Very few studies in China have focused on discrepancies in both the disease rates as well as the severity of the disease between children with urban or rural social backgrounds. As China's rural–urban migration continues to affect the country's population distribution and health status of its people [13], concerns about the health of ‘left-behind’ children [14, 15] and rural–urban migrant children have been addressed, although mainly in the fields of mental and behavioural health. HFMD, as a paediatric infectious disease without a vaccine [16, 17], might yield important information when comparing migrant and non-migrant children and possible factors related to these differences in rates, ratios and outcomes of this and other infectious diseases.
METHODS
Data collection
Population: located in South China's Pearl River Delta, the metropolis of Shenzhen is a sub-provincial city with a high population density (population = 10·36 million, population density = 5201 persons/km2 in 2010, ranging from 1852 to 16 756 persons/km2 in eight districts) [18].
HFMD cases from 1 January 2008 to 1 May 2008 were extracted from the former Shenzhen local reporting system, and data from 2 May 2008 to 31 December 2011 were exported from the NIDSRIS based on the four initial digits of residence address codes for the case (4403 for Shenzhen). In addition, current hukou (household registration that reflects the child's rural or urban origin) status of the case was coded. Those without local hukou of Shenzhen were classified as migrant, and vice versa.
Currently, diagnosis of HFMD in China follows two guidelines: The Hand Foot Mouth Disease Prevention and Control Guidelines issued by the Chinese Centre for Disease Control and Prevention (China CDC) (updated in 2009), and the Hand Foot Mouth Disease Diagnosis and Treatment Guidelines (updated in 2010; http://www.moh.gov.cn/mohyzs/s3586/201004/46884.shtml) issued by the Ministry of Health China. Diagnostic standards and clinical classification given by the above guidelines are highly consistent, including a two-tier discriminative definition of ordinary and severe cases. In the guidelines, ordinary HFMD was defined as a case of vesicular-papular rash over the hands, soles and/or buttocks and/or mouth ulcers, with or without fever. Patients with any of the following clinical syndromes/manifestations (or plus the specific laboratory virology or serology results indicating enterovirus infection) are diagnosed as a clinically suspicious severe case (or a laboratory-confirmed suspicious severe case): neurological complications (including brainstem encephalitis, neurogenic pulmonary oedema, acute flaccid paralysis and aseptic meningitis, etc., or altered consciousness, e.g. lethargy, confusion or coma), and respiratory and circulatory dysfunction (e.g. acute myocarditis, shortness of breath, tachycardia). These manifestations may be supported by increased peripheral blood leukocytes, cerebrospinal fluid abnormalities, reduced arterial oxygen partial pressure, blood biochemical abnormalities including hyperglycaemia, abnormal outcomes with changes in EEG, brain/spinal cord MRI, chest X-ray, or echocardiography. The clinical, laboratory and epidemiological data of all suspicious fatal cases are submitted to a provincial diagnostic advisory board for final discussion and confirmation. The national guidelines also consider a differential diagnosis list of alternative diagnoses for HFMD, which contains five major categories of diseases sharing similar clinical symptoms with ordinary or severe enterovirus infection.
Severe and fatal cases were further investigated using a nationally adopted questionnaire that identifies symptoms, doctor visits, health insurance, and medication used. The face-to-face investigation was administered to parents/legal guardians of the sick children either in the hospital or at their residence soon after the case was reported online, by trained public health professionals. A nested case-control study was included with a randomly selected sample of non-severe cases identified using 1:1 matching with residential district and stratified by week of onset. The same questionnaire was administered to parents of these selected children using a telephone survey approach each month by the same group of trained public health workers. A chart review of all clinical information for cases in the control group was also performed by using available clinical records from the reporting health facilities. The study protocol received full approval from the Ethics Committee of Shenzhen Centre for Disease Control & Prevention (SZCDC) and informed consent agreements were obtained from the parents/legal guardian of all investigated cases.
Laboratory analysis
Of all confirmed severe HFMD cases, 163 faecal or rectal swab samples were collected, preserved at –80°C and transported to the designated key virus reference laboratory at the Department of Microbiology of SZCDC. In addition, 446 non-severe cases were also sampled in accordance with a HFMD outpatient sentinel surveillance sampling process and sent to the same laboratory for testing. Laboratory analyses were performed using reverse transcription–polymerase chain reaction (RT–PCR) and nucleotide sequencing. Specimens were mixed thoroughly with 500 μl of phosphate-buffered saline (pH 7·4) to yield homogeneous suspensions. Viral RNA was isolated from the supernatants of the suspensions using High Pure Viral RNA kits (Roche, Germany). Extracted RNA was processed with immediate RT–PCR tests or stored at –80°C for later use. All tests had the following cycling conditions: 30 min at 50°C, followed by 94°C for 3 min, 94°C for 30 s, 52°C for 30 s, and then 72°C for 1 min for 35 cycles, with another 10-min extension at 72°C. Primers used in EV71 and CA16 detection were: EV71-1 (5′-GTC CTT AAT TCG CAC AGC ACA GCT-3′); EV71-2 (5′-CGG TCC GCA CTGAGA ATG TAC CCA T-3′); CA16-1 (5′-CCT ATT GCA GAC ATG ATT GAC CAG-3′); CA16-2 (5′-TGT TGT TAA CTT GTC TCT ACT AGT G-3′). The PCR products (10 μl) were then examined by agarose gel electrophoresis. The potential target region for EV71 is 507 bp and for CA16 it is 880 bp. For EV71-positive samples, the complete VP1 coding gene was amplified with one step RT–PCR directly from the specimens, and outcomes were analysed using DNASTAR, BioEdit and Mega 4.0 software. Based on alignment of complete VP1 gene sequences of all the EV71 strains and supplemented GenBank EV71 reference strains (specifically C4 strains) collected in China, and using the neighbour-joining (NJ) tree approach, the genetic diversity and phylogenetic relationships were analysed. Virus test results for each sample were then combined into the online case profile using identifiers automatically generated when the case report in the real-time information system first occurred.
Data analysis
Frequency of cases reported by onset time (year, month), sex, age group, migratory status, educational status, and current residential district are reported. Attack rate (AR, the proportion of reported cases in the general population), case-severity ratio (CSR, the proportion of diagnosed severe cases in all reported HFMD cases), mortality rate (confirmed HFMD deaths/100 000 general population), case-fatality rate (CFR, the proportion of confirmed HFMD deaths of all reported cases), and severe CFR (the proportion of fatal cases in all diagnosed severe HFMD cases) were calculated and compared by both the year of onset and migratory status of the cases. Distributions of case severity were stratified by demographic factors including migratory status, year and month of onset, school attendance, and district of patients’ current address, etc. Time trends were plotted from 2008 to 2011. Odds ratios (ORs) and 95% confidence intervals (CIs) for case severity were calculated using univariate Generalized Linear Models (GLM) in the all-case-inclusive analyses as well as the case-control study. This was done because the response variable (case severity, yes or no) is not quantitative or normally distributed but belongs to an exponential binomial family. All-case-inclusive binary logistic regression models (dependent: 1 = severe cases, 0 = non-severe cases) were used to estimate ORs of potential risk factors (age, sex, incidence year, month of onset, school attendance information, migratory status, current residential districts, time between symptom onset and diagnosis) for case severity. Data from the case-control study (severe cases and the randomly sampled control cases) were used to calculate ORs of socioeconomic and clinically relevant factors based first on univariate and then multivariate analyses. The multivariate analysis for the nested case-control study used a Cox regression model to fit conditional logistic regression. This model sets the strata parameter as serial numbers of each pair (severe case and control non-severe case), and the time parameter as 1 (severe cases) or 2 (non-severe control cases) [19]. All analyses were completed using SPSS v. 16 (SPSS Inc., USA).
RESULTS
Time trend of HFMD in Shenzhen
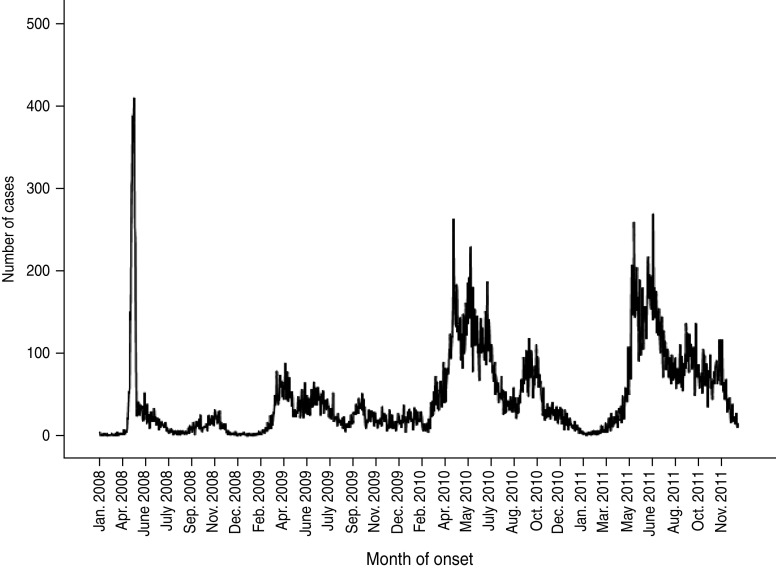
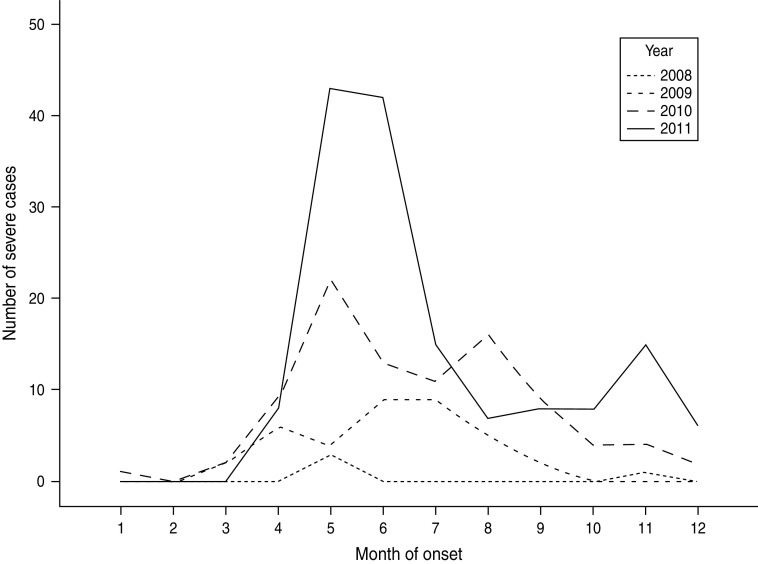
From January 2008 to December 2011, 64 428 cases of HFMD with a current Shenzhen residential address were reported to the local (on or before 1 May 2008) or NIDSRIS. Increases in the annual rate of cases were 28·12%, 154·39%, and 6·48% for periods 2008–2009, 2009–2010 and 2010–2011, respectively. During this period there were 286 severe cases and 18 fatal cases. The overall ratio of severe to non-severe cases (CSR) was 0·44% (95% CI 0·40–0·50), and the severe CFR was 6·29% (95% CI 3·50–9·00). In each year, the primary peak season of reports was between April and June, usually followed by a much smaller second peak emerging in October or November (Fig. 1). There was an increase in numbers of severe HFMD cases consecutively in 2010 and 2011, especially during the summer peak months of May and June and the second peak in autumn (Fig. 2).
Fig. 1.
Number of cases of hand, foot and mouth disease reported to the National Infectious Disease Surveillance and Reporting Information System by onset date (n = 64 428), Shenzhen, 2008–2011.
Fig. 2.
Number of severe cases of hand, foot and mouth disease reported to the National Infectious Disease Surveillance and Reporting Information System by onset month (n = 286), Shenzhen, 2008–2011.
Of all 64 428 cases, 89·7% were aged <5 years (mean = 2·42, s.d. = 3·040), and 1-year-olds accounted for 29·1% of all reports (Table 1). Infection was seen more frequently in males (63%) than females (37%) and the sex ratio of all cases was similar during the 4 years reported (P = 0·021). Only one quarter of the cases were schoolchildren. Migrant children accounted for two thirds of cases with the exception of 2008 (54%).
Table 1.
Characteristics of Reported HFMD Cases in Shenzhen, 2008–2011 (n = 64 428)
| Characteristics | 2008 | 2009 | 2010 | 2011 | Total (proportion%) |
|---|---|---|---|---|---|
| Population (million) | 8·77 | 8·91 | 10·36 | 11·69* | — |
| Annual attack rate (%) | 0·82 | 1·03 | 2·25 | 2·12 | — |
| Mortality rate (1/100 000) | 0·01 | 0·06 | 0·06 | 0·05 | — |
| Severe case | (P = 0·000) | ||||
| No | 7146 (99·9%)† | 9124 (99·6%) | 23 211 (99·6%) | 24 662 (99·4%) | 64 143 (99·6%) |
| Yes | 4 (0·1%) | 37 (0·4%) | 92 (0·4%) | 152 (0·6%) | 286 (0·4%) |
| Fatal case | (P = 0·393) | ||||
| Yes | 1 | 5 | 6 | 6 | 18 (0·02%) |
| No | 7149 | 9156 | 23 297 | 24 808 | 64 410 (99·98%) |
| Sex | (P = 0·021) | ||||
| Female | 2575 (36·0%) | 3330 (36·3%) | 8736 (37·5%) | 9011 (36·3%) | 23 652 (36·7%) |
| Age group (years) | (P = 0·000) | ||||
| <1 | 906 (12·7%) | 1323 (14·4%) | 3000 (12·9%) | 3617 (14·6%) | 8846 (13·7%) |
| 1–<2 | 1795 (25·1%) | 2623 (28·6%) | 6680 (28·7%) | 7633 (30·8%) | 18 731 (29·1%) |
| 2–<3 | 1484 (20·8%) | 2058 (22·5%) | 4653 (20·0%) | 5031 (20·3%) | 13 226 (20·5% |
| 3–<4 | 1291 (18·1%) | 1516 (16·5%) | 4145 (17·8) | 4106 (16·5%) | 11 058 (17·2%) |
| 4–<5 | 755 (10·6%) | 788 (8·6%) | 2199 (9·4%) | 2182 (8·8%) | 5924 (9·2%) |
| ⩾5 | 919 (12·9%) | 853 (9·3%) | 2626 (11·3%) | 2245 (9·0%) | 6643 (10·3%) |
| School | (P = 0·000) | ||||
| Yes | 2443 (34·2%) | 2110 (23·0%) | 5917 (25·4%) | 5629 (22·7%) | 16 099 (25·0%) |
| No | 4707 (65·8%) | 7051 (77·0%) | 17 386 (74·6%) | 19 185 (77·3%) | 48 329 (75·0%) |
| Migratory status | (P = 0·000) | ||||
| Local | 3273 (45·8%) | 3330 (36·3%) | 7926 (34·0%) | 8201 (33·3%) | 22 730 (35·3%) |
| Migrant | 3877 (54·2%) | 5831 (63·7%) | 15 377 (66·0%) | 16 613 (67·0)) | 41 698 (64·7%) |
| Residential district | (P = 0·000) | ||||
| Luohu | 1579 (22·1%) | 1604 (17·5%) | 2893 (12·4%) | 3041 (12·3%) | 9117 (14·2%) |
| Futian | 761 (10·6%) | 697 (7·6%) | 1753 (7·5%) | 1747 (7·0%) | 4958 (7·7%) |
| Nanshan | 794 (11·1%) | 752 (8·2%) | 2094 (9·0%) | 1627 (6·6%) | 5267 (8·2%) |
| Bao'an | 1791 (25·0%) | 1685 (18·4%) | 4040 (17·3%) | 4234 (17·1%) | 11 750 (18·2%) |
| Longgang | 2049 (28·7%) | 4188 (45·7%) | 11 896 (51·0%) | 13 479 (54·3%) | 31 612 (49·1%) |
| Yantian | 176 (2·5%) | 235 (2·6%) | 627 (2·7%) | 686 (2·8%) | 1724 (2·7%) |
| Laboratory results‡§ | (P = 0·000) | ||||
| EV71 | 121 (65·1%) | 66 (74·2%) | 112 (65·9%) | 118 (72·0%) | 417 (68·5%) |
| COX A16 | 62 (33·3%) | 21 (23·6%) | 57 (33·5%) | 25 (15·2%) | 165 (27·1%) |
| Other enterovirus | 3 (1·6%) | 2 (2·2%) | 1 (0·6%) | 21 (12·8%) | 27 (4·4%) |
| Overall reports | 7150 | 9161 | 23 303 | 24 814 | 64 428 |
Estimation based on population statistics in 2010.
Values in parentheses represent the proportion within each year.
Results based on cases that have been tested and confirmed in the Virology Laboratory of Shenzhen Centre for Disease Control & Prevention only.
n = 609 for laboratory viral tests.
There was an increasing trend of HFMD cases in children with migrant hukou status over time during 2008–2011 (P = 0·000). The proportion of HFMD patients with current residential addresses located in Bao'an (BA) or Longgang (LG) districts, two districts outside the Shenzhen Special Economic Zone (SEZ) with the highest and second highest migrant population proportions, rose from 53·7% in 2008, to 64·1% in 2009, 68·3% in 2010 and 71·4% in 2011.
Characteristics of severe cases and fatal cases
Of all 286 severe cases reported, no fatal case was seen in local populations (Table 2). There was an annual increasing pattern of severe cases seen in migrant populations, especially between 2011 and 2012. Overall the CSR in migrant populations was 2·34 times of that in local residents of Shenzhen. The rates varied significantly over the 4-year period. The overall severe CFR was 6·29%. In the 286 severe cases, 163 patients were sampled for viral tests, with 148 (90·8%) being confirmed as EV71 positive compared to a rate of 68·5% (417/609) in all samples with laboratory results during 2008–2011.
Table 2.
Case-severity ratios (per 1000), case-fatality rates (per 1000) and severe case-fatality rates (%) in Shenzhen for the years 2008–2011, by migratory status
| Year | Case-severity ratios (per 1000) | Case-fatality rates (per 1000) | Severe case-fatality rates (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Local | Migrant | All | Local | Migrant | All | Local | Migrant | |
| 2008 | 0·56 P = 0·630 | 0·31 | 0·77 | 0·14 | 0 | 0·26 | 25·00 | 0 | 33·33 |
| 2009 | 4·04 P = 0·001 | 1·20 | 5·66 | 0·55 | 0 | 0·86 | 13·51 | 0 | 15·15 |
| 2010 | 3·99 P = 0·000 | 1·77 | 5·14 | 0·26 | 0 | 0·39 | 5·43 | 0 | 7·60 |
| 2011 | 6·13 P = 0·008 | 4·27 | 7·04 | 0·24 | 0 | 0·36 | 3·95 | 0 | 5·13 |
| Overall | 4·44 | 2·38 | 5·56 | 0·28 | 0 | 0·43 | 6·29 | 0 | 7·76 |
| P = 0·000 | P = 0·000 | P = 0·000 | P = 0·393 | n.a. | P = 0·396 | P = 0·058 | n.a. | P = 0·094 | |
Of all 18 fatal cases reported, seven were female and 11 were male. With an average age of 1·39 years, all deaths occurred in children aged ⩽3 years with migrant hukou status. Sixteen fatal cases were children not attending school. Cases with symptom onset in April–August accounted for 77·8% (14/18) of all fatal cases. Twelve (66·7%) fatal cases lived in LG district. 78·0% of all severe cases and 88·9% (16/18) of all fatal reports were seen in BA and LG districts.
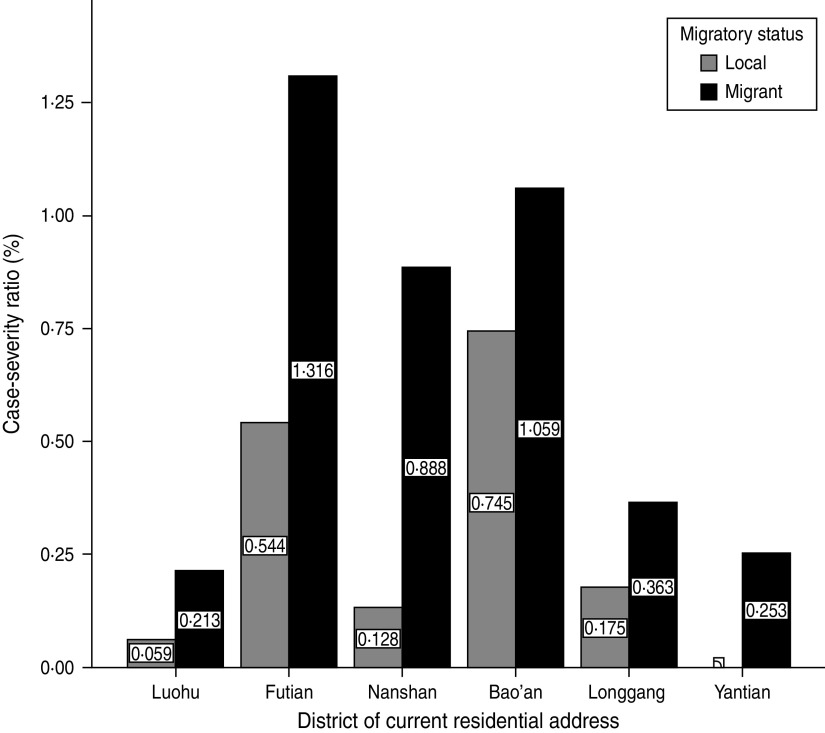
There was a variation in levels for CSR across all geographical districts in Shenzhen, with the migrant community having higher CSRs in all districts of Shenzhen during 2008–2011 compared to local populations (Fig. 3).
Fig. 3.
Case-severity ratios (%) for hand, foot and mouth disease by migratory status and geographical distribution in Shenzhen, 2008–2011 (n = 64 428).
Rates of severe HFMD varied over time in each year, with May and June having the highest risk (Table 3). Age and sex were not associated as risk factors for severity in the model. Children with migrant status were 2·3 times more likely to be a severe case than local children. Those attending school (including day care/kindergarten/elementary school/any other school) were less likely to be a severe case compared to non-schoolchildren after adjusting for migratory status, diagnosis delay, age and sex.
Table 3.
Effects of age, sex, year, month of onset, school, migratory status, residential district and time between symptom onset and diagnosis on likelihood of being a severe case, Shenzhen, China (n = 64 428)
| Variables | P* | OR | 95% CI |
|---|---|---|---|
| Year | |||
| 2008 | 0·000 | ||
| 2009 | 0·000 | 10·866 | 3·821–30·903 |
| 2010 | 0·000 | 9·717 | 3·536–26·699 |
| 2011 | 0·000 | 14·912 | 5·459–40·735 |
| Sex (female†) | 0·574 | 0·933 | 0·732–1·189 |
| School information | |||
| Stay at home† | 0·079 | ||
| Go to school‡ | 0·009 | 0·570 | 0·373–0·871 |
| Other | 0·786 | 0·847 | 0·254–2·821 |
| Migrant (local†) | 0·000 | 2·316 | 1·602–3·347 |
| Time between onset and diagnosis (days) | 0·000 | 1·493 | 1·415–1·576 |
| Month of onset | |||
| January† | 0·000 | ||
| February | 0·993 | 0·000 | 0·000–0·000 |
| March | 0·418 | 2·478 | 0·275–22·336 |
| April | 0·097 | 5·474 | 0·734–40·830 |
| May | 0·023 | 9·943 | 1·372–72·063 |
| June | 0·032 | 8·725 | 1·201–63·372 |
| July | 0·106 | 5·176 | 0·704–38·046 |
| August | 0·052 | 7·291 | 0·983–54·091 |
| September | 0·167 | 4·152 | 0·551–31·259 |
| October | 0·334 | 2·744 | 0·354–21·246 |
| November | 0·062 | 6·811 | 0·905–51·246 |
| December | 0·159 | 4·478 | 0·555–36·129 |
| Age group (years) | |||
| ⩾5† | 0·260 | ||
| <1 | 0·521 | 0·815 | 0·435–1·524 |
| 1–<2 | 0·824 | 1·068 | 0·600–1·901 |
| 2–<3 | 0·491 | 1·228 | 0·684–2·205 |
| 3–<4 | 0·309 | 1·350 | 0·757–2·408 |
| 4–<5 | 0·899 | 0·956 | 0·477–1·916 |
| District§ | |||
| Luohu† | 0·000 | ||
| Futian | 0·088 | 1·842 | 0·912–3·719 |
| Nanshan | 0·036 | 2·389 | 1·058–5·390 |
| Longgang | 0·000 | 6·611 | 3·152–13·866 |
| Bao'an | 0·000 | 4·262 | 2·108–8·618 |
| Yantian | 0·559 | 1·481 | 0·396–5·539 |
OR, Odds ratio; CI, confidence interval.
Adjusted R2 = 0·428.
Italic values indicate P < 0·05.
Reference category.
‘Go to school’ refers to any kind of school attendance 2 weeks before symptom onset (including day care, kindergarten, elementary school, secondary school).
Data from the cases living in the two newly established GM and PS new districts after 2010 were merged as part of data from their original BA and LG districts, respectively.
Findings from the nested case-control study confirmed some of these findings. The sex ratios of the case and control groups were not significantly different. Severe cases were statistically significantly younger than the control group. Being a migrant more than doubled the child's risk of developing severe complications. Children with fever >39°C were 2·8 times more likely to be a severe case, while patients with skin or mucous lesions 48 h after symptom onset were less likely to be severe (OR 0·010, 95% CI 0·006–0·019). Compared with visiting a city-level hospital or a medical centre as the first contact, visiting a private clinic significantly increased a child's likelihood of being a severe case. Data also showed that severe cases had significantly longer time intervals between onset and diagnosis and onset and first visit. Having health insurance decreased the likelihood of severity. Oral antipyretics, intramuscular pyrazolone and intravenous glucocorticoid use were all associated with higher risk of being a severe case. The most significant risk factor was the early combined use of intramuscular pyrazolone and intravenous glucocorticoids, with an OR of 14·576 (Table 4).
Table 4.
Analyses on potential risk factors in the nested case-control study, Shenzhen, China (n = 572)
| Characteristics | Severe cases (n = 286) | Non-severe cases (n = 286) | OR (95% CI) | P |
|---|---|---|---|---|
| Sex ratio (male:female) | 1·70:1 | 1·91:1 | 1·113 (0·802–1·591) | 0·485 |
| Age, mean (s.d.) | 2·01 (2·165) | 2·48 (3·112) | n.a. | 0·029 |
| Migrant children, n (%) | 232 (81·1) | 191 (66·8) | 2·137 (1·454–3·141) | 0·000 |
| Clinical symptoms, n (%) | ||||
| Fever >39°C | 186 (65·0) | 71 (24·8) | 2·832 (2·421–3·313) | 0·000 |
| Skin or mucous membrane lesions within 48 h of onset | 42 (14·7) | 270 (94·4) | 0·010 (0·006–0·019) | 0·000 |
| Aseptic meningitis | 54 (18·9) | — | — | — |
| Encephalitis | 40 (14·0) | — | — | — |
| Myocarditis | 34 (11·9) | — | — | — |
| Neurogenic pulmonary oedema | 82 (28·7) | — | — | — |
| Healthcare services, first visit, n (%) | ||||
| Private clinic | 161 (56·3) | 52 (18·2) | 5·796 (3·961–8·481) | 0·000 |
| Community health centre | 70 (24·5) | 57 (19·9) | 1·302 (0·876–1·935) | 0·191 |
| District hospital | 46 (16·1) | 102 (35·7) | 0·346 (0·232–0·515) | 0·000 |
| City-level hospital/medical centre | 9 (3·1) | 75 (26·2) | 0·091 (0·045–0·187) | 0·000 |
| Time between onset and first visit, mean (s.d.) | 2·13 (1·564) | 1·02 (1·677) | n.a. | 0·001 |
| Time between onset and diagnosis, mean (s.d.) | 3·27 (1·702) | 1·77 (1·592) | n.a. | 0·018 |
| Has health insurance, n (%) | 56 (19·6) | 80 (28·0) | 0·627 (0·425–0·926) | 0·018 |
| Medication within 72 h, n (%) | ||||
| (1) Self-medication | 152 (53·2) | 147 (51·4) | 1·073 (0·773–1·489) | 0·676 |
| (2) Any antibiotic use | 141 (49·3) | 132 (46·2) | 1·134 (0·817–1·575) | 0·451 |
| (3) Any antiviral medicine use | 192 (67·1) | 181 (63·3) | 1·185 (0·840–1·672) | 0·334 |
| (4) Any oral antipyretics use | 183 (64·0) | 122 (42·7) | 2·388 (1·706–3·344) | 0·000 |
| (5) Any intramuscular pyrazolone use | 122 (42·7) | 50 (17·5) | 3·511 (2·390–5·159) | 0·000 |
| (6) Any intravenous glucocorticoid use | 120 (42·0) | 35 (12·2) | 5·184 (3·391–7·926) | 0·000 |
| (2)+(3) | 104 (36·4) | 91 (31·8) | 1·224 (0·866–1·731) | 0·252 |
| (2)+(4) | 94 (32·9) | 57 (19·9) | 1·967 (1·344–2·879) | 0·000 |
| (3)+(4) | 126 (65·6) | 79 (27·6) | 2·063 (1·456–2·925) | 0·000 |
| (2)+(3)+(4) | 74 (25·9) | 40 (14·0) | 2·147 (1·402–3·288) | 0·000 |
| (5)+(6) | 49 (17·1) | 4 (1·4) | 14·576 (5·184–40·981) | 0·000 |
OR, Odds ratio; CI, confidence interval; n.a., not available; s.d., standard deviation.
In the adjusted multivariate model (Table 5), sex and age were not associated with risk of severity; however, being a migrant was still associated with an increased risk of severity (OR 2·452, 95% CI 1·722–4·327). Children with a fever >39°C were 5·8 times more likely to develop severe complications while the presence of a skin lesion within 48 h of disease onset was a protective factor. Those whose first visit was to private clinics had a 2·6 times greater likelihood of becoming a severe case compared to those whose first contact with the medical system was in a tertiary health facility. Medication within 72 h of the onset of symptoms was related to severe complications for cases. The use of antibiotics or antiviral drugs was not significantly associated with severity. In the adjusted model, any use of oral antipyretics, any use of intramuscular pyrazolone, any use of intravenous glucocorticoids and the combination of using the latter two types of medication in the initial 3 days were risk factors for the patient becoming a severe HFMD case.
Table 5.
Multivariate analyses for severity of case in patients with hand, foot and mouth disease adjusted for sex, age, migratory status, fever, skin lesion, health insurance, medication use and first visit to health facilities, Shenzhen, China (n = 572)
| Characteristics | OR (95% CI) | P |
|---|---|---|
| Sex | ||
| Male | 2·763 (0·029–5·275) | 0·663 |
| Female† | ||
| Age (years) | 0·976 (0·586–1·625) | 0·925 |
| Migratory status | 2·452 (1·722–4·327) | 0·015 |
| Migrant | ||
| Local† | ||
| Fever >39°C | 5·774 (2·344–14·225) | 0·000 |
| Yes | ||
| No† | ||
| Skin or mucous membrane lesions within 48 h | 0·045 (0·015–0·130) | 0·000 |
| Yes | ||
| No† | ||
| Type of health facility, first visit | ||
| Private clinic | 2·647 (1·245–5·632) | 0·011 |
| Community health centre | 1·921 (0·455–3·321) | 0·190 |
| District hospital | 1·837 (0·203–6·618) | 0·588 |
| City-level hospital/medical centre† | ||
| Time between onset and first visit (days) | 1·514 (1·194–1·919) | 0·001 |
| Time between onset and diagnosis (days) | 1·486 (1·090–2·025) | 0·012 |
| Insurance status | ||
| Has health insurance | 0·676 (0·434–1·055) | 0·085 |
| Has no insurance† | ||
| Medication within 72 h (answer of ‘no’†) | ||
| (1) Self-medication | 1·313 (0·496–3·475) | 0·584 |
| (2) Any antibiotics use | 0·649 (0·105–4·015) | 0·642 |
| (3) Any antiviral medicine use | 0·811 (0·185–3·568) | 0·782 |
| (4) Any oral antipyretics use | 1·832 (1·155–2·906) | 0·010 |
| (5) Any intramuscular pyrazolone use | 3·364 (2·181–5·187) | 0·000 |
| (6) Any intravenous glucocorticoid use | 2·284 (1·064–4·900) | 0·000 |
| (2)+(3) | 0·979 (0·069–13·979) | 0·988 |
| (2)+(4) | 1·046 (0·828–3·901) | 0·676 |
| (3)+(4) | 1·006 (0·724–2·348) | 0·218 |
| (2)+(3)+4) | 1·083 (0·262–4·299) | 0·208 |
| (5)+(6) | 3·752 (2·324–6·059) | 0·000 |
OR, Odds ratio; CI, confidence interval.
Reference.
From 2008 to 2011, 609 stool specimens or rectal swab samples from reported cases were collected for viral tests in the Key Virus Reference Laboratory (KVRL) of SZCDC. Of all samples, 417 (68·5%) were confirmed as EV71 positive, while CA16 accounted for 27·1% and the remaining 4·4% belonged to other types of enterovirus infection. By year, proportions of EV71 positive in tested samples ranged from the lowest (65·1%) in 2008 to the highest at (74·2%) in 2009, and that for CA16 ranged from the lowest (15·2%) in 2011 to the highest (33·5%) in 2010. If stratified by severity of clinical manifestation, this sample included 163 severe (inclusive of all 18 fatal) cases, of which 148 (90·8%) were EV71 positive, seven (4·3%) were CA16 positive and another eight (4·9%) were positive for other enteroviruses. All fatal cases were confirmed as EV71 positive and belonged to the C4 lineage and C4a subgenogroup. Of the 446 samples from non-severe (and non-fatal) cases, 269 (60·3%) were EV71 positive, 158 (35·4%) were CA16 positive and 19 (4·3%) belonged to other enteroviruses. Viruses that were neither CA16 nor EV71 increased from under 3% during 2008–2010 to 12·8% in 2011 (χ2 = 51·257, P = 0·000). The genetic compositions of CA16 and EV71 in both ‘sporadic’ and ‘outbreak’ cases have not substantially changed with all identified EV71 belonging to subgenotype C4a.
DISCUSSION
With a formal history of only 32 years, Shenzhen metropolis accommodates more than 10 million people with an average age of around 30. There are high proportions of rural–urban migrants and children aged 0–14 years. No major HFMD epidemic had been observed in Shenzhen until 2008 when case reports suddenly surged to an unprecedented high level, in parallel with the national epidemic situation background. Although a study in 2009 indicated that the composition of HFMD pathogens in different parts of mainland China were significantly different and could include EV71, COX A16, B5, A4, A6, A10, and A12 [1]; EV71 had been proven to be constantly related to severe HFMD cases reported in mainland China [8], and the EV71 C4a genotype had been the predominant circulating virus lineage responsible for all confirmed fatal and severe cases in Shenzhen since 2003 [20]. Although our study could not include all clinically diagnosed HFMD cases for laboratory viral tests, results from more than half (57%) of the severe (and all fatal) cases and 446 sentinel-based surveillance samples might point to a potential EV71 dominant epidemiological dynamic of the viral circulation in this part of China during 2008–2011, a phenomenon that had been continuously observed in community- and hospital-based HFMD sentinel surveillance systems at different levels in Shenzhen and Guangdong Province of South China. However, given that our laboratory sampling in this study used a mixed method and was not randomized, generalization of such a result needs to be treated with caution. The pathogenic background of isolated strains from severe and non-severe HFMD cases did not indicate massive changes in the EV71 and CA16 viruses at least not until the end of 2011. As it has been shown that EV71 viruses circulating in mainland China do not have as great a genetic diversity as those from Taiwan, Singapore, or Malaysia [21], our study confirms all EV71 strains found in Shenzhen belong to subgenotype C4a.
Annual incidence rates of HFMD in Shenzhen were higher than those from national reports of mainland China in the corresponding years (0·38 in 2008, 0·89 in 2009, 1·25 in 2010, 1·36 in 2011/1000) [22, 23]. The CSR (0·56–6·13/1000, average 4·44/1000) of HFMD observed in Shenzhen during 2008–2011 was much higher than that in Taiwan during the 1998 epidemic (0·08/1000) [24] and that in mainland China as a whole in 2009 (0·09/1000) [22]. The question that this research poses is, in the absence of change in virus, and a major change in diagnostic guidelines, why should the CSR of HFMD in Shenzhen in 2011 increase tenfold from that in 2008. Compared to its closest urban neighbour, Hong Kong, where only 39 severe cases and one fatal case of HFMD were reported to Health Protection (HP) Hong Kong during 2008–2011 [25], Shenzhen's epidemic situation of severe HFMD was far greater. The 4-year average severe CFR (6·29%), although lower than that of Taiwan (19%) more than 10 years ago, was still more than double that of mainland China in 2009 (2·55%) [22].
Our findings show that sex and age levels in severe case morbidity may not be as strong as they were in some other studies; however, they are similar to findings in Shanghai from 2009 to 2010 and 2007 to 2010. This study has confirmed very similar seasonality of incidence as found in Hong Kong, mainland China, and Singapore. The low rates of severe cases in schoolchildren might be related to a very strict general symptom-based screening system called ‘morning check’ in almost all schools in Shenzhen. This morning check is part of the National School Hygiene Regulation, implemented by school physicians in Shenzhen who are under technical support from district and municipal Centres for Disease Control & Prevention. The ‘red flag’ symptoms [26] provided in the training materials to school physicians includ fever and rashes or vesicular lesions on the hands, feet (or/and buttocks) and oral mucosa, which are typical symptoms for HFMD and predominantly exist in the majority of HFMD patients who are at the early disease progressive stage after symptom onset.
The high rate of serious disease in migrants, which is similar to findings from Shanghai [27], may reflect different disease behaviours/health service utilization of the parents, although many other potential baseline physical factors such as immunity levels [28] and/or nutritional status [29] may also play a role in the aetiological mechanisms. Three direct indices selected for disease behaviours (type of first contact, delay of contact, and delay of diagnosis) were quite sensitive to the final outcomes of disease severity.
Answering the question of why migrant children experienced severe complications more often than local children in this city remains a priority. The health outcome levels between local and migrant children that exist after controlling for age, sex, health insurance and month of onset, may not be a simple reflection of China's urban–rural HFMD levels in morbidity and mortality but reflect some other process. Since the clinical and public health definition of HFMD in China is actually a group of symptoms sharing human enterovirus infection of several lineages, our findings strongly support further aetiological studies of EV71 infection in migrant children.
For treatment of regular HFMD as a benign febrile exanthematous disease, no specific drug regimen other than symptom management is needed. However, severe symptomatic EV71 infection can develop from herpangina/HFMD to CNS involvement and cardiopulmonary failure, thus stage-based management is crucial to outcome [30]. Commonly used drugs include antiviral agents, antibiotics, and immunopotentiators, although there are few studies showing a clear improvement in severe HFMD/EV71 infection outcome. In this study, antipyretics use, intramuscular pyrazolone use, intravenous glucocorticoid use or the combination of the latter two within 3 days of symptom onset were very strong risk factors for severe HFMD.
In Shenzhen, unregulated private clinics which are predominantly located in migrant aggregating ‘villages-in-the-city’ (Chengzhongcun) provide more affordable and physically more accessible health services to residents living around them, compared to the more sophisticated community health centres or tertiary hospitals, both of which belong to the public sector and are thus under formal technical support from Centres for Disease Control & Prevention. The arrangements of payments in private and informal clinics are appealing to uninsured or under-insured families, but they may be of variable quality. In the case of fever, antipyretics and intravenous glucocorticoids are commonly used. In recent years, there has been increasing evidence showing that these two types of medication may be related to critical disease outcomes or death from infection [31, 32]. In China, it is common to see the use of over-the-counter antipyretics by parents for their febrile children at home. One study in Beijing found that migrants with low income tended to choose self-medication for themselves, while another survey in parents showed that the mother's educational level and socioeconomic status had the opposite effect on self-medication for their children, which is more affordable and cost-effective. It is highly likely that migrant children with fever, untypical rashes or lesions and possible EV71 infection in the early stage were given normal oral antipyretics at home and/or first brought to private clinics, where doctors gave them intravenous/intramuscular solutions that contained either non-steroidal anti-inflammatory drugs such as pyrazolone, or glucocorticoids, or a combination of both. This may have concealed or delayed the onset of red-flag symptoms and signs leading to delay in diagnosis. Given the high availability of private clinics providing care to Shenzhen's migrant communities, guidance delivered to these clinics on diagnosis and advice on optimal management for HFMD may be useful in reducing avoidable morbidity and mortality.
CONCLUSION
Severe EV71 infection with complications can be fatal and has caused 18 deaths in young children in the past 4 years in Shenzhen, making it an important public health issue. The early use of antipyretics and steroids is potentially a risk factor that may have a negative impact on the prognosis of HFMD in Chinese children. This finding in Shenzhen, South China may also apply to more than the Chinese population suffering from HFMD. The role played by socioeconomic inequalities between local and migrant children on HFMD rates should be further explored. Reinforcement of professional training of doctors in the private sector, health education in migrant communities and more sensitive screening in schools during peak seasons should be considered by the health authorities.
ACKNOWLEDGEMENTS
The study was supported by the Guangdong Provincial Medical Research Foundation (Grant number A2011554, Study on epidemiological characteristics of severe hand, foot and mouth disease in migrant children in Shenzhen).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Yang F, et al. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virology Journal 2011; 8: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsiung GD, Wang JR. Enterovirus infections with special reference to enterovirus 71. Journal of Microbiology, Immunology and Infection 2000; 33: 1–8. [PubMed] [Google Scholar]
- 3.Xu W, et al. Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. Virology Journal 2012; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landry ML, et al. Fatal enterovirus type 71 infection: rapid detection and diagnostic pitfalls. Pediatric Infectious Disease Journal 1995; 14: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 5.Yan XF, et al. Epidemic characteristics of hand, foot, and mouth disease in Shanghai from 2009 to 2010: enterovirus 71 subgenotype C4 as the primary causative agent and a high incidence of mixed infections with coxsackie virus A16. Scandinavian Journal of Infectious Diseases 2011; 44: 297–305. [DOI] [PubMed] [Google Scholar]
- 6.Modlin JF. Enterovirus déjà vu. New England Journal of Medicine 2007; 356: 1204–1205. [DOI] [PubMed] [Google Scholar]
- 7.Liu CC, et al. An outbreak of enterovirus 71 infection in Taiwan, 1998: epidemiologic and clinical manifestations. Journal of Clinical Virology 2000; 17: 23–30. [DOI] [PubMed] [Google Scholar]
- 8.Tan X, et al. The persistent circulation of enterovirus 71 in People's Republic of China: causing emerging nationwide epidemics since 2008. PLoS One 2011; 6: e25662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, et al. Emergence and control of infectious diseases in China. Lancet 2008; 372: 1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. Journal of Clinical Virology 2009; 44: 262–267. [DOI] [PubMed] [Google Scholar]
- 11.Mao LX, Wu B, Bao WX, et al. Epidemiology of hand, foot, and mouth disease and genotype characterization of enterovirus 71 in Jiangsu, China. Journal of Clinical Virology 2010; 49: 100–104. [DOI] [PubMed] [Google Scholar]
- 12.Li LJ. Review of hand, foot and mouth disease. Frontiers of Medicine in China 2010; 4: 139–146. [Google Scholar]
- 13.Gong P, Liang S, Carlton EJ, et al. Urbanisation and health in China. Lancet 2012; 379: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye J, Pan L. Differentiated childhoods: impacts of rural labor migration on left-behind children in China. Journal of Peasant Studies 2011; 38: 355–377. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, et al. The impact of parental migration on health status and health behaviours among left behind adolescent school children in China. BMC Public Health 2010; 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Lu J, Lu J. Enterovirus 71 vaccine: Close but still far. International Journal of Infectious Diseases 2010; 14: e739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, et al. EV71: an emerging infectious disease vaccine target in the Far East? Vaccine. 2010; 28: 3516–3521. [DOI] [PubMed] [Google Scholar]
- 18.List of administrative divisions of Shenzhen. (http://en.wikipedia.org/wiki/List_of_administrative_divisions_of_Shenzhen). Accessed 6 February 2013.
- 19.Conditional logistic regression using COXREG. (http://www-01.ibm.com/support/docview.wss?uid=swg21477360). Accessed 6 February 2013.
- 20.He YQ, et al. Genetic evolution of VP1 of enterovirus type 71 in Shenzhen. Chinese Journal of Experimental and Clinical Virology 2011; 25: 173–175. [PubMed] [Google Scholar]
- 21.Li L, et al. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. Journal of Clinical Microbiology 2005; 43: 3835–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, et al. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology 2011; 22: 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Statistical report on notifiable infectious diseases of the People's Republic of China: Year 2011. (http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohjbyfkzj/s3578/201202/54106.htm). Accessed 6 February 2013.
- 24.Ho M, et al. An epidemic of enterovirus 71 infection in Taiwan. New England Journal of Medicine 1999; 341: 929–935. [DOI] [PubMed] [Google Scholar]
- 25.EV SCAN. Enteric and Vector-Borne Disease Office of the Centre for Health Protection Hong Kong (http://www.chp.gov.hk/en/guideline1_year/29/134/441/502.html). Accessed 6 February 2013.
- 26.Haj-Hassan TA, et al. Which early ‘red flag’ symptoms identify children with meningococcal disease in primary care? British Journal of General Practice 2011; 61: e97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng M, et al. Epidemiology of hand, foot, and mouth disease in children in Shanghai 2007–2010. Epidemiology and Infection 2011; 140: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 28.Wong SSY, et al. Human enterovirus 71 and hand, foot and mouth disease. Epidemiology and Infection 2010; 138: 1071. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, et al. Influence of vitamin A status on the antiviral immunity of children with hand, foot and mouth disease. Clinical Nutrition 2012; 31: 543–548. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Zhu R, Qian Y, Deng J. The characteristics of blood glucose and WBC counts in peripheral blood of cases of hand foot and mouth disease in China: a systematic review. PLoS ONE 2012; 7: e29003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han K, et al. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clinical Infectious Diseases 2011; 53: 326–333. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Characterization of hand, foot, and mouth disease in China between 2008 and 2009. Biomedical and Environmental Sciences 2011; 24: 214–221. [DOI] [PubMed] [Google Scholar]