SUMMARY
A contact investigation following a case of infectious tuberculosis (TB) reported in a call centre in Milan (Italy) led to the identification of three additional cases that had occurred in employees of the same workplace during the previous 5 years, one of whom was the probable source case. Thirty-three latent infections were also identified. At the time of diagnosis, the source case, because of fear of stigma related to TB, claimed to be unemployed and a contact investigation was not performed in the workplace. Cases were linked through genotyping of Mycobacterium tuberculosis. TB stigma has been described frequently, mainly in high-incidence settings, and is known to influence health-seeking behaviours and treatment adherence. The findings in this report highlight that TB-associated stigma may also lead to incomplete contact investigations. Little is known about the causes and impact of TB-related stigma in low-incidence countries and this warrants further exploration. Research is also needed to evaluate the effectiveness of specific interviewing techniques and training interventions for staff in reducing feelings of stigma in TB patients. Finally, the outbreak emphasizes the importance of integrating routine contact investigations with genotyping.
Key words: Infectious disease control, outbreaks, tuberculosis (TB)
INTRODUCTION
Tuberculosis (TB) is still a major public health problem worldwide, with varying incidences between countries. In Italy, the incidence of notified TB cases in the last decade has constantly been below 10/100000 population, which is the threshold for definition of a low-incidence country. The nationwide incidence rate in 2011 was 5·8/100000 population [1]. Central and northern regions of Italy report higher incidences compared to southern areas of the country, probably due to undernotification of cases in southern regions and a greater proportion of foreign-born individuals in northern regions [2]. Lombardy, a large region in northern Italy, reported 11·7 cases/100000 population in 2009 [3] and 8·8 cases/100000 population in 2011 (Lombardy Regional Health Authority, personal communication). In the last decade, Milan, the largest urban area in the region (1·6 million inhabitants), has reported consistently higher incidences with respect to the rest of the region (16·6/100000 population in 2011, N = 266) [4]. This is consistent with a recent European study reporting higher TB notification and incidence rates in large metropolitan areas compared to non-urban areas [5].
TB control in Italy is based on timely diagnosis and adequate treatment of cases, screening of persons in high-risk groups and those in close contact with active TB cases (contact investigations), treatment of latent TB infection (LTBI) to prevent its progression to active disease, monitoring of treatment outcomes of active TB and LTBI cases, and vaccination of at-risk healthcare workers and children who live in close contact with a reported TB case [6].
Contact investigations are essential to identify secondary TB and LTBI cases at high risk of developing the disease [7, 8]. Various international, national and local guidelines for conducting contact investigations have been issued [6–7, 9, 10] which generally recommend the concentric circles approach for identifying contacts of infectious cases.
Incomplete investigations that do not allow for the identification and screening of all contacts at risk are one of the factors responsible for new outbreaks [11]. In a review of TB contact investigations in the USA from 2002 to 2008, an incomplete contact investigation was listed as the second most common contributing factor to TB outbreaks [11].
The aim of this study was to describe a workplace outbreak of TB in the city of Milan, caused by an incomplete contact investigation conducted 5 years previously.
METHODS
In December 2011, a hospitalized case of culture-positive pulmonary TB with cavitary involvement was reported to the local health authorities in Milan (Italy) and a contact investigation was initiated by the health department. The investigation was part of a routine public health response to a TB outbreak in a work environment and did not require ethics committee approval.
The traditional concentric circles approach was used, in accordance with national and regional protocols [6, 9]. This approach consists in systematically testing household, work/school, and leisure/recreation contacts according to the degree of exposure to the source case in the 3 months preceding the onset of symptoms. Close contacts (circle 1) are tested first and the contact investigation is expanded to regular (circle 2) and occasional (circle 3) contacts if there is evidence of recent transmission of infection (i.e. if the observed group prevalence of infection is higher than that in the local community). Contacts are screened by clinical examination and tuberculin skin testing (TST) using the Mantoux method. A positive TST result is defined as induration of ⩾5 mm in contacts of circles 1 and 2 or as induration of ⩾10 mm in contacts of circle 3. Contacts with a positive TST undergo further evaluation at the regional TB reference centre, by chest X-ray, pulmonary assessment and, in some cases (e.g. in the case of previous bacillus Calmette–Guérin vaccination), an interferon gamma release assay as a confirmatory test. If a diagnosis of active TB is ruled out, subjects are prescribed chemoprophylaxis with isoniazid for 6 months, as recommended by national and international guidelines [6, 12].
Since 2012, genotyping in Lombardy has been conducted routinely by a regional reference laboratory, on all isolates identified as Mycobacterium tuberculosis, using 24-loci mycobacterial interspersed repetitive units of variable numbers of tandem repeats (MIRU-VNTR) [13]. Results are collected in a regional databank and compared with other sequences present in the database.
RESULTS
The index case (case 1) was a 39-year-old Italian female with no known contact with a TB case. She was a 20 pack-year smoker and reported having a productive cough for the previous 4 months. Sputum smear, bronchial aspirate cultures and polymerase chain reaction were all positive for M. tuberculosis. Sixteen family contacts and 11 other contacts were tested, of whom two (12·5%) and zero, respectively, were TST positive (Table 1).
Table 1.
Demographic, clinical and laboratory characteristics of tuberculosis cases reported in a call centre in Milan (Italy) over a 5-year period, and results of contact investigations performed
| No. | Sex, age (years) | Date of symptom onset | Date of report | Laboratory and other investigations | Contacts tested | No. of latent infections | |
|---|---|---|---|---|---|---|---|
| Type of contact | No. | ||||||
| 1* | F, 39 | August 2011 | December 2011 | Clinical exam + | Close (family and other) | 2 | 0 |
| Chest X-ray + | Close (work) | 33 | 8 | ||||
| Smear + | Regular (family) | 16 | 2 | ||||
| Culture + | Regular (work) | 16 | 1 | ||||
| PCR + | Occasional (work) | 107 | 21 | ||||
| Occasional (other) | 9 | 0 | |||||
| Total | 183 | 32 | |||||
| 2 | F, 26 | October 2009 | April 2010 | Clinical exam + | Close (family) | 3 | 1 |
| Chest X-ray + | Regular (work) | 16 | 3 | ||||
| Smear − | Occasional (other) | 4 | 0 | ||||
| Culture + | |||||||
| PCR + | |||||||
| TST − | |||||||
| Total | 23 | 4 | |||||
| 3† | F, 40 | July 2007 | August 2007 | Clinical exam + | Close (family) | 4 | 3 |
| Chest X-ray + | |||||||
| Smear + | |||||||
| Culture + | |||||||
| Total | 4 | 3 | |||||
| 4 | M, 32 | April 2006 | May 2006 | Clinical exam + | Contact investigation not performed (non-contagious, case) |
0 | 0 |
| Chest X-ray − | |||||||
| Smear − | |||||||
| Culture − | |||||||
| TST + | |||||||
| Total | 0 | 0 | |||||
| Total | 210 | 39 (18·6%) | |||||
Polymerase chain reaction (PCR) test used: Gene Xpert.
Index case.
Source case.
+, Positive; –, negative.
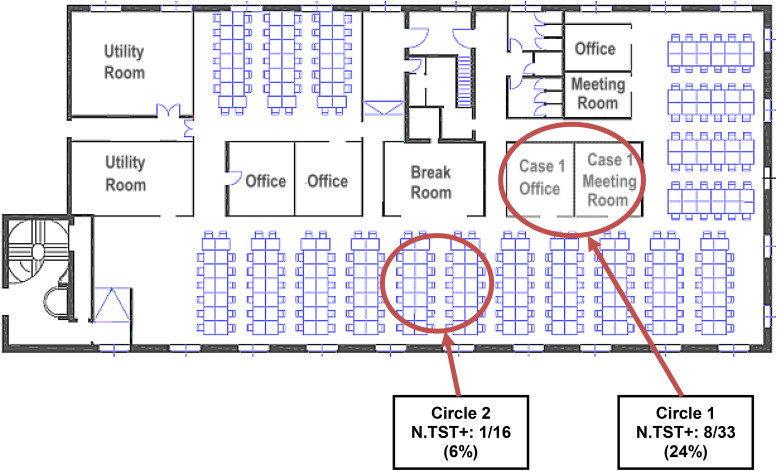
The patient had been employed for over 20 years as the manager of a call centre in Milan where about 156 operators worked in 4-, 6- or 8-h shifts. The work environment consisted of an open space of about 450 m2 located on a single floor, naturally illuminated by many windows and equipped with air conditioning (Fig. 1). Besides using her own office where she held daily meetings (Fig. 1, circle 1), the index case also spent time near one of the work stations (Fig. 1, circle 2). Initially, 49 work contacts [33 in circle 1 (close contacts) and 16 in circle 2 (regular contacts)], were tested, of whom eight (24·2%) and one (6·2%), respectively, had a positive TST (Table 1). No active TB cases were identified. However, during the investigation it was discovered that a case of pulmonary TB (case 2) had been reported in the call centre 20 months previously (in April 2010), in an Italian 26-year-old female employed by the call centre since 2005 (Table 1). Case 2, a smoker, had been diagnosed with TB after a 6-month period of weight loss and night sweats. Although a contact investigation had been conducted at the time of diagnosis (Table 1, Fig. 2), the investigation had not been extended beyond close work contacts because of the low contagiousness of the case (negative sputum smear) and because the patient had a limited number of contacts as she worked at a single work station.
Fig. 1.
Layout of the call centre in Milan (Italy) where four tuberculosis cases were reported over a 5-year period (2006–2011) showing areas used by close (circle 1) and regular (circle 2) workplace contacts of the index case (case 1). N.TST+, N (%) of co-workers tested with a positive TST.
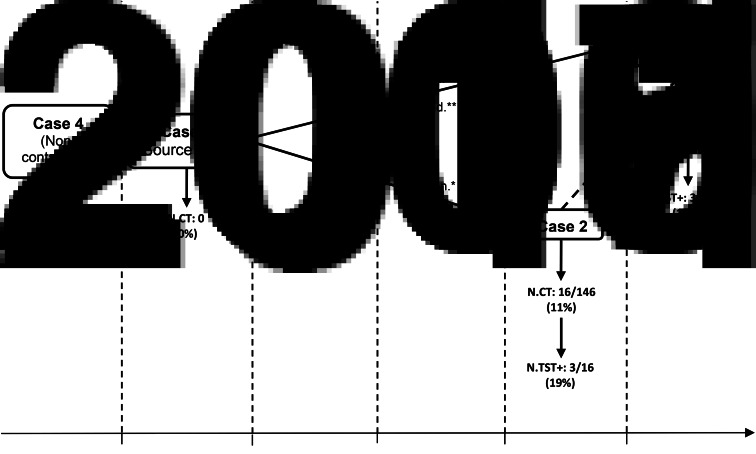
Fig. 2.
Chronological description of the hypothetical relationship between tuberculosis cases in a call centre in Milan (Italy) from 2006 to 2011; epidemiological and genotype links identified between cases, number and percentage of workplace contacts investigated for each case and number of latent tuberculosis infection cases diagnosed. N.CT, Number of co-workers tested; N.TST+, Number of positive TST results; * link established by genotyping; ** epidemiological link.
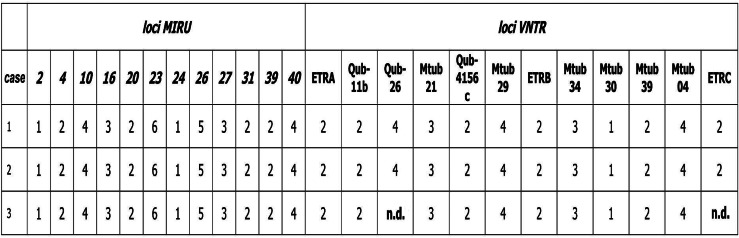
M. tuberculosis isolates from cases 1 and 2 belonged to the Latin American-Mediterranean (LAM) family and showed identical MIRU-VNTR patterns (Fig. 3). In view of the linkage between the two cases, the work setting (consisting of many work stations in a restricted space) and the high rate of transmission in contacts tested, the contact investigation was expanded by testing the 107 remaining workers. Overall, 30 (19·2%) of 156 workplace contacts had a positive TST (Fig. 2).
Fig. 3.
24-locus mycobacterial interspersed repetitive units of variable numbers of tandem repeats (MIRU-VNTR) results for M. tuberculosis isolates (Latin American-Mediterranean family) from cases of a tuberculosis outbreak in a call centre in Milan (Italy), 2006–2011. n.d., Not determined.
The expanded contact investigation revealed that two additional TB cases had occurred in employees in August 2007 (case 3) and May 2006 (case 4), respectively (Table 1). Case 3 was a case of pulmonary TB in a 40-year-old Italian female with a positive family history of disease, hospitalized for a worsening cough that had started in the previous month (Table 1). The patient's father had been diagnosed with TB in 1998 but she had not been screened because she was not considered to be at high risk. At the time of admission, the woman had been employed by the call centre since January 2005, but she had claimed to be unemployed so the contact investigation was restricted to family members (Table 1). Genotype testing was performed and the identified LAM strain displayed a MIRU-VNTR pattern, covering 22 of 24 loci, identical to that of isolates from the first two cases (Fig. 3). Due to technical problems, it was not possible to identify two MIRU-VNTR loci; nevertheless, given the strong epidemiological link between the three cases, the strain was considered to be genotypically identical to the strains identified in isolates from cases 1 and 2. Case 4 (Table 1) was a case of extrapulmonary TB affecting peripheral lymph nodes in a 32-year-old Italian male with no risk factors for TB, employed by the call centre since 2004. He had a negative sputum culture and was therefore considered to be non-contagious so a contact investigation was not indicated at that time.
Figure 2 shows the hypothetical relationship between TB cases in the call centre, epidemiological and genotype links identified between cases, number and percentage of workplace contacts investigated for each case and number of LTBI cases diagnosed.
Antibiotic susceptibility testing of all culture-positive TB cases revealed susceptibility to first-line TB drugs and all completed treatment. Follow-up of cases was completed in 2014. None of the LTBI cases developed active disease.
DISCUSSION
The described outbreak involved three cases of active pulmonary TB and 33 cases of latent infection that occurred in the same workplace over a 5-year period. Case 3, diagnosed in 2007, was the probable source of the outbreak. There was a high overall rate of transmission of infection in the workplace, with 19% of contacts tested found to have LTBI.
Few workplace TB outbreaks are reported in the international literature [14–16] and none from Italy, where reported outbreaks have involved mainly schools [17, 18]. Reported transmission rates are variable, ranging from 8·5% to 51% of workplace contacts being diagnosed with a latent infection [14–16]. However, it is difficult to compare attack rates between outbreaks that have occurred in settings with different environmental characteristics and a different likelihood of transmission of infection.
The most likely cause of the present outbreak was the failure to perform a complete contact investigation after the probable source case (case 3) was reported in 2007. It is very likely that the outbreak originated from this case who then transmitted the infection to two co-workers (cases 1 and 2) whose infection progressed to active disease in 2010 and 2011, respectively. MIRU-VNTR typing of the outbreak isolates yielded identical patterns, confirming the existence of a link between the three cases and transmission in the workplace. LAM lineage strains are relatively frequent in Lombardy and represent 12% of isolates from 2013, of which 50% are from foreign-born cases [19]. This is in accordance with data reported from another Italian region [20]. However, no other M. tuberculosis isolates displaying the specific MIRU-VNTR profile of the present cluster were identified in the regional database.
With regards to LTBI cases, no additional risk factors for TB, such as previous history of TB or significant contact outside the workplace were identified during contact investigation. Epidemiological data indicate that these cases are all likely to have been infected in the workplace.
Cases 1 and 2 had failed to be identified at the time that the source patient (case 3) was diagnosed because case 3 did not disclose information about her work contacts for fear of stigma related to a diagnosis of TB. In particular, she was concerned that her temporary contract with the call centre, due to end in the forthcoming months, might not be renewed if it became known that she had TB.
Failure to be identified as a contact was identified as the primary reason for disease development in 54% of case patients in one US study [21]. Other studies have shown similar results, with epidemiological links identified in only a small percentage of case patients with the same M. tuberculosis strain [21].
TB-related stigma has been frequently described, mainly in low-income countries with a high prevalence of disease, and is due to the association of TB with poverty, low social class, malnutrition, HIV, or alcohol and drug abuse [22–24]. Shame, isolation and fear are the main themes that emerge in studies assessing the stigma of TB. These can have a negative impact on patients and their families and may lead them to conceal their disease [24]. Cultural variations have been described with respect to knowledge, attitudes and health responses to TB and the potential for stigma [25]. However, little is known about the impact and causes of TB-related stigma in low-incidence countries and this needs to be explored further [26].
TB-related stigma has major implications on the efficacy of TB control programmes because it is a barrier to seeking treatment and a cause of social suffering, leading to diagnostic delays and non-adherence to treatment [22–30]. The present outbreak highlights that stigma may also lead to the incomplete identification of contacts of infectious TB cases if patients do not disclose information about their contacts. Precarity and job insecurity are factors which played an important role in this outbreak. It is known that socioeconomically disadvantaged populations are more prevalent in many big cities, inside and outside the European Union [5]. Although we found no supporting evidence in the literature, it is plausible to assume that, in low-incidence countries, these factors may contribute more frequently to fear of stigma and discrimination in metropolitan areas, compared with rural areas.
There is little published research on the effectiveness of stigma reduction strategies [26, 31]. A promising approach, in regions where TB stigma is common, seems to be helping TB patients resist stigma, especially through TB groups [26, 30]. Educational interventions regarding TB transmission routes, aimed at the community and patients, have also been proposed but additional research is needed [24, 26]. According to some authors, it would be useful to assess whether interventions known to reduce HIV/AIDS stigma have any effect on TB diagnosis and treatment [26].
Establishing trust and rapport between public health workers and patients is critical to gain full information from infectious cases during contact investigations. However, current national and international guidelines generally lack guidance for staff involved in TB control on how to establish rapport with cases during individual interviews. Some guidelines do highlight the importance of periodic on-the-job training for staff who conduct interviews [7]. However, no specific interviewing techniques have been described in the literature, and no training interventions for interviewers have been shown, in studies, to decrease feelings of stigma in TB patients interviewed. This is an area of research that should be addressed.
Considering the limits of traditional contact investigations, especially in high-risk groups such as the homeless or illegal immigrants, and also in other situations such as that described in the present outbreak, genotyping of all M. tuberculosis isolates and collection of results in a centralized database, as is being done in Lombardy, are fundamental for linking cases and confirming suspected chains of transmission [13, 32]. In addition, new approaches, such as the use of social network analysis and geographical information systems, are also being used increasingly [33].
The findings in this report are subject to the following limitations. First, because part of the investigation was conducted retrospectively and covered a long time period, it proved difficult to completely and accurately reconstruct outbreak transmission dynamics. For example, it was not possible to link case 4, who was non-contagious, to the other outbreak cases, as no M. tuberculosis isolates were available for this case, and we were unable to determine if case 4 had been infected in the workplace. Second, baseline TST information was not available for most contacts identified, thus limiting the ability to definitively attribute many LTBI cases to the workplace outbreak. However, DNA fingerprinting confirms that TB transmission occurred in the call centre and demographic and epidemiological data regarding LTBI cases indicate that they are all likely to have acquired the infection in the workplace. Third, genotyping in the Lombardy region has been performed on all culture-positive cases only since 2010, meaning that isolates from the source case (2007) were only examined years later, during the outbreak investigation of 2011. As a result, it was not possible to verify whether strains with the same genetic profile were circulating in the region at the time the source case was diagnosed.
In conclusion, the described outbreak highlights how TB-related stigma may lead to incomplete contact tracing and negatively influence the efficacy of TB control even in low-incidence countries. Research is needed on the causes and impact of TB-related stigma in such settings. The outbreak also highlights the need for training in effective interviewing skills for staff involved in TB control and emphasizes the importance of integrating routine contact investigations with genotyping and other methods.
ACKNOWLEDGEMENTS
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.European Centre for Disease Control and Prevention/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2014. Stockholm: European Centre for Disease Prevention and Control, 2014. (http://www.ecdc.europa.eu/en/publications/Publications/tuberculosis-surveillance-monitoring-Europe-2014.pdf). Accessed 6 October 2014
- 2.Italian Ministry of Health, Italian National Health Institute, Regional Health Authority of Emilia-Romagna. Tuberculosis in Italy. Report 2008 [in Italian] (http://www.salute.gov.it/imgs/c_17_pubblicazioni_1472_allegato.pdf). Accessed 6 October 2014
- 3.Regional Health Authority of Lombardy. Infectious diseases surveillance. Report 2010 [in Italian] (http://www.arca.regione.lombardia.it/shared/ccurl/280/67/report29.04.2011Aprile.pdf). Accessed 6 October 2014.
- 4.Local Healh Unit of Milan. Report on prevention activities, 2013 [in Italian] (http://www.asl.milano.it/ita/Default.aspx?SEZ=10&PAG=88&NOT=6343). Accessed 6 October 2014.
- 5.van Hest NA, et al. Tuberculosis control in big cities and urban risk groups in the European Union: a consensus statement. Eurosurveillance 2014; 19: pii=207286. [DOI] [PubMed] [Google Scholar]
- 6.Italian Ministry of Health. Updated guidelines on the management of contacts of tuberculosis and of nosocomial cases, 2010 [in Italian] (http://www.salute.gov.it/imgs/C_17_pubblicazioni_1661_allegato.pdf). Accessed 5 October 2014.
- 7.Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. Morbidity and Mortality Weekly Report 2005; 54: 1–47. [PubMed] [Google Scholar]
- 8.Sia IG, Wieland ML. Current concepts in the management of tuberculosis. Mayo Clinic Proceedings 2011; 86: 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regional Health Authority of Lombardy. Decree no. 19767 of 10 December 2004. Prevention, surveillance and control of tuberculosis [in Italian] (http://normativasan.servizirl.it/port/GetNormativaFile?fileName=536_DGR2004_19767.pdf). Accessed 6 October 2014.
- 10.European Centre for Disease Control and Prevention. Guidance: investigation and control of tuberculosis incidents affecting children in congregate settings. Report 2013. Stockholm: European Centre for Disease Prevention and Control, 2013 (http://www.ecdc.europa.eu/en/publications/Publications/guidance-investigation-control-tb-incidents-children-in-congregate-settings.pdf). Accessed 6 October 2014.
- 11.Mitruka K, et al. Tuberculosis outbreak investigations in the United States, 2002–2008. Emerging Infectious Diseases 2011; 17: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Latent tuberculosis infection: a guide for primary healthcare providers. Atlanta: Centers for Disease Control and Prevention, 2013. (http://www.cdc.gov/tb/publications/ltbi/default.htm). Accessed 6 October 2014.
- 13.Crawford JT. Genotyping in contact investigations: a CDC perspective. International Journal of Tuberculosis and Lung Disease 2003; 7: S453–457. [PubMed] [Google Scholar]
- 14.McElnay C, Thornley C, Armstrong R. A community and workplace outbreak of tuberculosis in Hawke's Bay in 2002. New Zealand Medical Journal 2004; 117: U1019. [PubMed] [Google Scholar]
- 15.Raffalli J, Sepkowitz KA, Armstrong D. Community-based outbreaks of tuberculosis. Archives of Internal Medicine 1996; 156: 1053–1060. [PubMed] [Google Scholar]
- 16.Davidow AL, et al. Workplace contact investigations in the Unites States. International Journal of Tuberculosis and Lung Disease 2003; 7: S446–452 [PubMed] [Google Scholar]
- 17.Faccini M, et al. Tuberculosis outbreak in a primary school, Milan, Italy. Emerging Infectious Diseases 2013; 19: 485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filia A, et al. Tuberculosis in kindergarten and primary school, Italy, 2008–2009 Emerging Infectious Diseases 2011; 17: 514–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regional Health Authority of Lombardy. Surveillance of infectious diseases in Lombardy. Report July 2014 [in Italian] (http://www.sanita.regione.lobardia.it/shared/ccurl/1018/855/REPORT_MALATTIE_INFETTIVE_2014_Dati2013.pdf). Accessed 6 October 2014.
- 20.Garzelli C, et al. Impact of immigration on tuberculosis in a low-incidence area of Italy: a molecular epidemiological approach. Clinical Microbiology and Infection 2010; 16: 1691–1697. [DOI] [PubMed] [Google Scholar]
- 21.Chin DP, et al. Spread of Mycobacterium tuberculosis in a community implementing recommended elements of tuberculosis control. Journal of the American Medical Association 2000; 283: 2968–2974. [DOI] [PubMed] [Google Scholar]
- 22.Gebremariam MK, Bjune GA, Frich JC. Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: a qualitative study. BMC Public Health 2010; 10: 1471–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kipp AM, et al. Socio-demographic and AIDS-related factors associated with tuberculosis stigma in southern Thailand: a quantitative, cross-sectional study of stigma among patients with TB and healthy community members. BMC Public Health 2011; 11: 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juniarti N, Evans D. A qualitative review: the stigma of tuberculosis. Journal of Clinical Nursing 2011; 20: 1961–1970. [DOI] [PubMed] [Google Scholar]
- 25.Chang SH, Cataldo JK. A systematic review of global cultural variations in knowledge, attitudes and health responses to tuberculosis stigma. International Journal of Tuberculosis and Lung Disease 2014; 18: 168–173. [DOI] [PubMed] [Google Scholar]
- 26.Courtwright A, Turner AN. Tuberculosis and stigmatization: pathways and interventions. Public Health Reports 2010; 125: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atre S, et al. Gender and community views of stigma and tuberculosis in rural Maharashtra, India. Global Public Health 2011; 6: 56–71. [DOI] [PubMed] [Google Scholar]
- 28.Zetola NM, et al. Measuring stigma: are we looking in the right places? International Journal of Tuberculosis and Lung Disease 2012; 16: 1130–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubel AJ, Garro LC. Social and cultural factors in the successful control of tuberculosis. Public Health Reports 1992; 107: 626–636. [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly P. Isolation and stigma: the experience of patients with active tuberculosis. Journal of Community Health Nursing 1999; 16: 233–241. [DOI] [PubMed] [Google Scholar]
- 31.Heijnders M, Van Der Meij S. The fight against stigma: an overview of stigma-reduction strategies and interventions. Psychology, Health and Medicine 2006; 11: 353–363. [DOI] [PubMed] [Google Scholar]
- 32.Kato-Maeda M, Metcalfe JZ, Flores L. Genotyping of Mycobacterium tuberculosis: application in epidemiologic studies. Future Microbiology 2011; 6: 203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook VJ, et al. Recommendations on modern contact investigation methods for enhancing tuberculosis control. International Journal of Tuberculosis and Lung Disease 2012; 16: 297–305. [DOI] [PubMed] [Google Scholar]