SUMMARY
Hepatitis B virus (HBV) can be eliminated by effective universal vaccination. In Belgium, a free-of-charge HBV vaccination programme in infants with catch-up in adolescents was introduced in 1999. To evaluate the effects in <20-year-olds, seroprotection (anti-HBs >11 mIU/ml, according to the assay) and markers of infection (anti-HBc, HBsAg) were assessed in 2443 residual sera collected 7–8 years after implementation of the programme. The maximal prevalence of a solely anti-HBs seroprotective (‘vaccinated’) serostatus was 82·9% at age 1 year and 60·5% at age 13 years. A clear increase was found in age cohorts targeted by the campaign after a similar serosurvey conducted 4 years earlier. The prevalence of HBV infection remained unchanged at a low level (1·8% in 2006) similar to pre-vaccination data (1993–1994). We conclude that universal HBV vaccination has achieved overall high levels of vaccine-induced immunity, despite regional variations, which may give rise to pockets of susceptible young adults in the future.
Key words: Children's vaccines, epidemiology, hepatitis B, serology
INTRODUCTION
Hepatitis B virus (HBV) is the causal agent of 80% of primary liver carcinoma, which is still in the top ten of the most common cancers worldwide [1]. Hepatitis B is an exclusively human viral infection, and could thus be eliminated by effective vaccination programmes [2, 3]. Therefore, in 1992 the World Health Organization (WHO) recommended the introduction of universal HBV vaccination in all countries by 1997. In the WHO European region, 47/53 countries had implemented this recommendation by February 2012 (WHO database [4]).
In Belgium, HBV vaccine had been recommended and the cost partially refunded for children aged <13 years since 1996, but universal free-of-charge vaccination only began in September 1999 [5]. Infants received the vaccine from age 3 months initially (and from age 8 weeks since 2004), whereas adolescents received catch-up vaccination at age 10–13 years. Since January 2004, a hexavalent combination vaccine containing a HBV component has been generally used in infants. The uptake of HBV vaccine, as measured through vaccination coverage surveys at different ages (Table 1a) was not always similar in the different regions (Flanders, Wallonia, Brussels-Capital Region).
Table 1.
HBV vaccination coverage (%) in Belgium, measured by EPI-based surveys, by age and by year of survey [7–13]
| (a) At age 18–24 months (3 or 4 doses) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | 1999 | 2000 | 2003 | 2005 | 2006 | 2008 | 2009 | |
| Wallonia | 50·2* | 64·8* | 92·7* | 90·4† | ||||
| Flanders | 68·4* | 92·2† | 95·1† | |||||
| Brussels | 42·1* | 88·4† | ||||||
| (b) In school-aged children (3 doses) | ||||||||
| Region | Age (yr) | Survey (cohort)‡ | 2000 (1988) | 2001 (1989) | 2004 (1992) | 2005 (1997 + 1991) | 2006 (1994) | 2008 (1994) |
| Wallonia | 11–12 | 41·4 | 37·1 | 64·2 | 68·6 | |||
| Flanders | 7–8 | 72·0 | ||||||
| Flanders | 14 | 75·7 | 89·2 | |||||
EPI, Extended programme of immunization.
Third dose coverage.
Fourth dose coverage.
Year of birth of the majority of the surveyed children. In the French community, school-based surveys included children in the 6th year of primary school regardless of their year of birth, whereas in the surveys in Flanders children were primarily selected based on their year of birth. Adolescents were invited for HBV vaccination in the last year of primary school in Wallonia, and in the first year of secondary school in Flanders.
In infants, HBV vaccination coverage (three doses) exceeded 90% from the 2005 and 2006 surveys onwards in Flanders and in Wallonia, respectively, whereas in Brussels it was still below 90% in 2006. In school-aged children, coverage was below 90% in Flanders up to 2008 and in Wallonia up to 2005 (Table 1b), [6–13].
The prevalence of hepatitis B infection in the pre-vaccination era was assessed in Belgium through seroprevalence studies as part of the decision-making process to introduce universal HBV vaccination. Serological markers of HBV infection (anti-HBc, HBsAg), were evaluated by two regional studies in 1993–1994 [14, 15]. In Flanders, the northern region (about 60% of the population), markers of previous (recovered) or active infection were positive in 6·4% (0·7% HBsAg positives) of the general population, but in only 1% of the 0–24 years age group. In Wallonia, markers of HBV infection were only evaluated in children aged 5–9 years and adults aged 18–29 years and found positive in 2·0% (0·8% HBsAg carriers) and 5·8% (1·1% HBsAg carriers), respectively. These findings confirmed the low-endemic HBV state of the country.
Since the start of the vaccination campaign, HBV seroprevalence in children up to the age of 18 years was evaluated for the first time as part of the European Sero-Epidemiology Network 2 project (ESEN2), on residual sera collected in diagnostic laboratories in 2002–2003 [16]. In this survey, prevalence of solely anti-HBs seropositivity was high in the age cohorts that had been targeted by the universal vaccination campaign, but was also significant in non-targeted age groups. HBsAg prevalence was low at 0·9% [16]. In 2003, a saliva-based survey performed in a sample covering the Belgian population similarly found 0·5–1% HBsAg prevalence in individuals aged 0–24 years [17].
To further evaluate the effects of the universal HBV campaign in the targeted age cohorts, in terms of seroprotection on the one hand and residual infections (programme failure) on the other, and to detect any regional differences, a similar analysis is presented on sera collected in 2006–2007.
METHODS
This study was conducted as part of a larger seroprevalence study on vaccine-preventable diseases in individuals aged 1–65 years [18]. Residual sera from children aged 1–19 years were prospectively collected from 15 diagnostic laboratories, with a representative geographical distribution over the country's 10 provinces. All participating laboratories collected samples in 2006, but three of them experienced logistical problems and were allowed to extend their collection period up to October 2007. Laboratories were allocated fixed numbers of samples per annual age group to enable collection proportionally to (1) the population of each region (Flanders, Wallonia, Brussels-Capital Region), and (2) the population of each province (within the regions). We also aimed for equal numbers of males and females in each age group. Only children living in Belgium were included, as derived from their residence postal code. To avoid selection of immunodeficient children, the collecting laboratories excluded samples from oncology or intensive care wards as well as from patients for whom there was any other indication of an immunosuppressed condition or multiple transfusions. For each sample, the birth date, sample date, gender and postal code of the place of residence were provided by the collecting laboratories. The region (Brussels-Capital Region, Flanders, Wallonia) and province of residence were derived from the postal code. The protocol was approved by the Ethics Committee of the University of Antwerp, conditional on the samples being delivered unlinked and anonymous to the investigators.
The samples consisted of residual serum, or heparin or EDTA plasma, and were stored frozen (−20°C) until testing. They were analysed with commercially available enzyme-linked immunosorbent assays (ELISAs) in the virology laboratory at the Scientific Institute of Public Health (IPH), Brussels, Belgium, with a semi-automatic pipetting system. The ETI-AB-COREK PLUS (N0137), ETI-AB-AUK-3 (P001603) and ETI-MAK-4 (N0019) kits from Diasorin (Italy) were used for anti-HBc, anti-HBs, and HBs-Ag, respectively. Quantitative results were obtained for anti-HBs only, from the optical density (OD) values as specified by the manufacturer. Samples were considered seronegative for anti-HBs if the titre was <9 mIU/ml and seroprotective if the titre was >11 mIU/ml. Equivocal samples (9–11 mIU/ml) were not retested. A stepwise algorithm was applied: in case of insufficient serum, anti-HBs was measured first.
Based upon combined results for the three measured HBV markers, the serostatus of each sample was categorized as ‘vaccinated’ if the sample was solely anti-HBs positive, ‘ever infected’ if HBsAg or anti-HBc were positive, and ‘no history’ if all markers were negative. The latter category represents not only children who were never infected nor vaccinated, but also non-responders or responding vaccinees who lost their antibodies at a later stage. In the case of conflicting (all markers positive or anti-HBs as well as HBsAg positive) or equivocal results, children were labelled as ‘undetermined’. Logistic regression analysis evaluated age, gender and region as predictors of HBV serostatus, after exclusion of samples with ‘undetermined’ status. Final models were selected using stepwise backward selection, omitting terms with a P value >0·1. Statistical significance was defined as a P value <0·05. SPSS version 15.0 (SPSS Inc., USA) was used for the regression analysis, exact binomial 95% confidence intervals (CIs) on prevalence estimates were calculated with Excel 2010 (Microsoft, USA).
The 2006 results were compared with HBV marker results from another panel of residual sera collected in 2002 as part of ESEN2 [16]. This serum panel also consisted of samples from children aged 1–19 years and was collected using a similar design in 2002–2003 by nine of the 15 laboratories who participated in 2006. Anti-HBs, anti-HBc and HBsAg were measured with the same ELISA kits as used in 2006, but the titre results were standardized to enable comparison with the other participating countries in ESEN2 [19, 20].
RESULTS
HBV serological data on samples collected in 2006–2007
In 2006–2007 a total of 2443 samples from children aged 1–19 years were collected, of which 2379 contained enough serum to enable testing for HBV markers. The gender distribution (50·1% males) and the geographical distribution (10·0% living in Brussels, 58·4% in Flanders and 31·6% in Wallonia) was an accurate representation of the Belgian population aged 1–19 years in 2006 (Belgian official statistics) [21].
Not all tested samples contained sufficient serum for determination of all three HBV markers. Anti-HBs was found positive (>11 mIU/ml) in 1457 (61·3%) and equivocal (9–11 mIU/ml) in 44 (1·8%) samples, and missing for one sample. Anti-HBc and HBsAg results were available for 2369 samples, 37 (1·6%) were positive and 13 (0·5%) equivocal for antiHBc, whereas for HBsAg 35 (1·5%) were positive and five (0·2%) were equivocal.
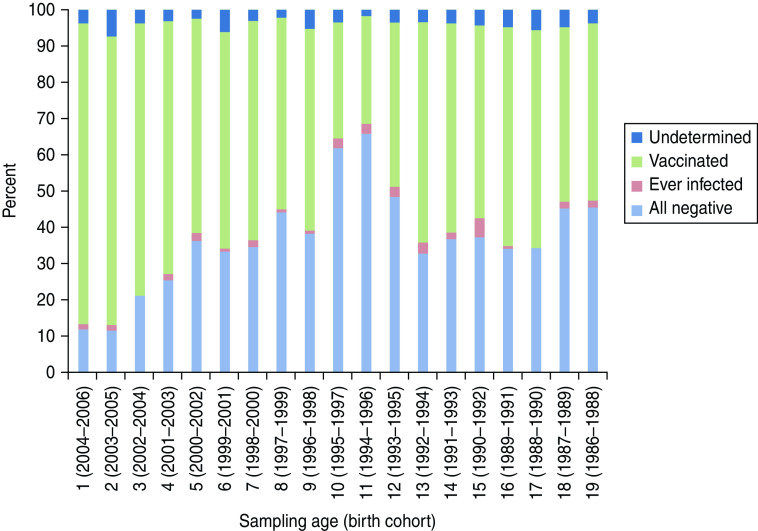
The HBV serostatus based on combining the results for the three markers (see Methods section) is summarized in Figure 1. Incomplete (0·5%, n = 14), equivocal (2·6%, n = 61) and conflicting (1·1%, n = 24, positive for anti-HBs as well as HBsAg) results left the HBV serostatus of 4·2% of the tested samples ‘undetermined’; 89/99 of these undetermined samples had anti-HBs >9 IU/ml.
Fig. 1.
Hepatitis B seroprofiles according to age in 1- to 19-year-olds, Belgium, 2006–2007 (N = 2379). ‘Ever infected’ = seronegative for anti-HBs and seropositive for anti-HBc and/or HBsAg or seropositive for anti-HBs as well as anti-HBc; ‘Undetermined’ = incomplete, equivocal or conflicting results (i.e. positive for anti-HBs as well as HBsAg); ‘Vaccinated’ = solely anti-HBs > 11 IU/ml. ‘All negative’ = anti-HBs <9 IU/ml and seronegative for antiHBc as well as HBsAg.
A small proportion of children had clear signs of ‘current or previous infection’ (overall 1·8%, 95% CI 1·3–2·4). They were either seronegative for anti-HBs and seropositive for anti-HBc and/or HBsAg (0·8%, of whom 11/18 were HBsAg seropositive) or seropositive for anti-HBs as well as anti-HBc (1·0%, n = 24). The ‘ever infected’ status is further specified in Table 2 [22]. Markers of HBV infection were more prevalent in Flanders (2·3%, 95% CI 1·6–3·2) than in Wallonia (0·5%, 95% CI 0·1–1·4).
Table 2.
Prevalence of different combinations of HBV markers consistent with recent or past HBV infection (‘ever infected’ serostatus), in 2002 and 2006, in Belgium, with their interpretation [22]
| ‘Ever infected’ serostatus | HBsAg | anti-HBc | anti-HBs | 2002 | 2006 |
|---|---|---|---|---|---|
| Chronic carrier | + | + | N | 0·7 (0·3–1·1) | 0·2 (0–0·4) |
| Recent infection (or false positive) | + | N | N | 0·2 (0–0·4) | 0·3 (0·1–0·5) |
| Past infection, recovered | N | + | + | 0·8 (0·3–1·2) | 1·0 (0·6–1·4) |
| Past infection, recovered or low-level chronic (or false positive) | N | + | N | 0·4 (0·1–0·7) | 0·3 (0·1–0·5) |
| Not further classified | IS | + | N | 0·1 | 0·0 |
Values given are % (95% confidence interval).
N, negative; +, positive; IS, insufficient serum.
Table 3 presents the prevalence of infected and vaccinated serostatus by birth cohort and age at sampling, and indicates which cohorts were not yet (fully) targeted by the programme (shaded cohorts) at the moment they were sampled in the 2002–2003 and/or in the 2006–2007 survey. In 2006–2007 (right-hand panel), the ‘vaccinated’ serostatus was more prevalent in birth cohorts targeted in infancy (1998–2006) than in birth cohorts targeted in adolescence (1988–1993). The ‘ever infected’ serostatus was not more prevalent in non-targeted than in targeted cohorts.
Table 3.
Prevalence of ‘vaccinated’ and ‘ever infected’ serostatus (combined results for anti-HBs, HBsAg and anti-HBc) per birth cohort, in two consecutive serosurveys in Belgium
| Birth cohorts | Serosurvey | ||||||
|---|---|---|---|---|---|---|---|
| Collected in 2002–2003 (N = 1528) | Collected in 2006–2007 (N = 2379) | P value | |||||
| Age (years) | Vaccinated% (95% CI) | Ever infected% (95% CI) | Age (years) | Vaccinated% (95% CI) | Ever infected% (95% CI) | ||
| 2004–2006 | n.a. | 1 | 83·5 (77·4–88·4) | 1·4 (0·3–4·5) | |||
| 2003–2005 | n.a. | 2 | 78·9 (73·0–84·0) | 1·2 (0·2–3·8) | |||
| 2002–2004 | n.a. | 3 | 75·2 (68·0–81·4) | 0 | |||
| 2001–2003 | n.a. | 4 | 68·6 (60·9–75·7) | 2·5 (0·7–6·4) | |||
| 2000–2002 | 1 | 60·8 (50·9–70·0) | 1·3 (0·1–5·9) | 5 | 59·5 (51·6–67·0) | 1·7 (0·3–5·1) | |
| 1999–2001 | 2 | 68·8 (59·0–77·5) | 1·3 (0·1–6·0) | 6 | 60·5 (52·6–68·0) | 0·8 (0·0–3·9) | |
| 1998–2000 | 3 | 65·4 (55·5–74·3) | 3·8 (1·1–9·6) | 7 | 60·1 (53·2–66·8) | 2·0 (0·5–5·0) | |
| 1997–1999 | 4 | 51·3 (41·5–60·9) | 1·3 (0·1–5·8) | 8 | 51·2 (43·4–58·9) | 0·8 (0·0–3·8) | |
| 1996–1998 | 5 | 64·6 (55·0–73·4) | 1·2 (0·1–5·6) | 9 | 56·5 (48·5–64·4) | 0·9 (0·0–4·1) | |
| 1995–1997 | 6 | 49·4 (39·8–59·0)* | 0 | 10 | 31·9 (24·6–39·8)* | 2·7 (0·7–6·7) | 0·017 |
| 1994–1996 | 7 | 42·5 (33·1–52·3) | 1·3 (0·1–5·8) | 11 | 31·0 (24·0–38·8) | 2·6 (0·7–6·5) | |
| 1993–1995 | 8 | 34·1 (25·5–43·7) | 2·4 (0·4–7·5) | 12 | 43·8 (35·5–52·3) | 2·9 (0·8–7·2) | |
| 1992–1994 | 9 | 29·6 (21·4–39·1)* | 1·2 (0·1–5·7) | 13 | 62·0 (54·1–69·3)* | 3·3 (1·1–7·4) | <0·001 |
| 1991–1993 | 10 | 22·8 (15·3–31·9)* | 2·5 (0·4–7·6) | 14 | 55·5 (48·6–62·2)* | 1·9 (0·5–4·9) | <0·001 |
| 1990–1992 | 11 | 28·4 (20·2–37·8)* | 0 | 15 | 55·4 (47·5–63·0)* | 5·0 (2·2–9·5) | <0·001 |
| 1989–1991 | 12 | 38·3 (29·2–47·9)* | 2·5 (0·4–7·6) | 16 | 60·0 (52·3–67·4)* | 0·8 (0·0–3·8) | 0·003 |
| 1988–1990 | 13 | 55·6 (45·8–65·0) | 3·7 (1·0–9·3) | 17 | 55·6 (47·9–63·2) | 0 | |
| 1987–1989 | 14 | 68·8 (59·2–77·3) | 5·0 (1·7–11·1) | 18 | 54·5 (46·7–62·2) | 1·7 (0·3–5·1) | |
| 1986–1988 | 15 | 57·3 (47·6–66·6) | 1·2 (0·1–5·6) | 19 | 46·5 (37·9–55·2) | 2·0 (0·4–6·2) | |
| 1985–1987 | 16 | 34·6 (25·8–44·2) | 1·2 (0·1–5·7) | n.a. | |||
| 1984–1986 | 17 | 34·6 (25·8–44·2) | 4·9 (1·7–10·9) | n.a. | |||
| 1983–1985 | 18 | 14·8 (8·8–22·9) | 1·2 (0·1–5·7) | n.a. | |||
| 1982–1984 | 19 | 29·6 (21·4–39·0) | 4·9 (1·7–10·9 | n.a. | |||
CI, Confidence interval; n.a., not available; ‘Vaccinated’ = solely anti-HBs positive; ‘Ever infected’ = seronegative for anti-HBs and seropositive for anti-HBc and/or HBsAg or seropositive for anti-HBs as well as anti-HBc; P value (Fisher's exact) for significant difference in prevalence of ‘vaccinated’ [indicated by an asterisk (*)]; grey shading = not (yet) fully targeted by the vaccination programme. Samples were collected based on the age at sampling, in 2006–2007 and in 2002–2003. As a consequence, each 1-year age band contains 3 birth years, in both surveys.
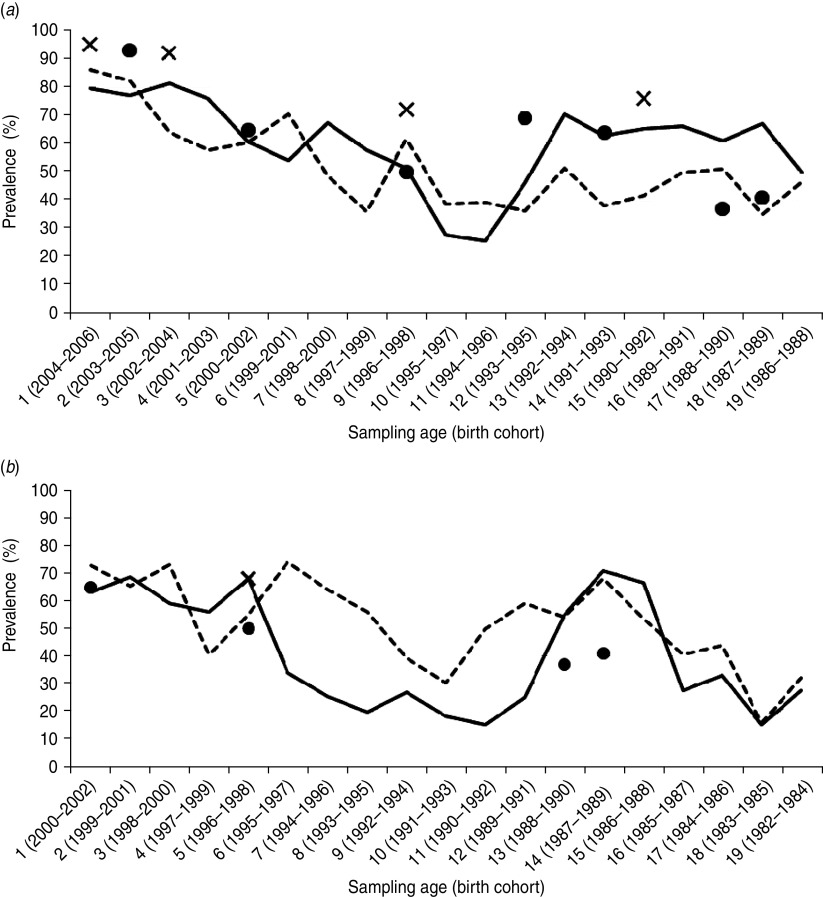
In Figure 2a, the regional prevalence of a ‘vaccinated’ HBV serostatus is plotted by age. For Flanders, extended programme of immunization (EPI) coverage estimates were consistently higher than the prevalence of a ‘vaccinated’ serostatus in the corresponding birth cohorts. Regional differences in ‘vaccinated’ serostatus were obvious in the 10–19 years age group.
Fig. 2.
Regional prevalence of ‘vaccinated’ HBV serostatus by age, together with available HBV vaccine coverage estimations from regional surveys, (a) 2006–2007 and (b) 2002–2003. X axis: sampling age and birth year of the corresponding age cohort. Y axis: prevalence of ‘vaccinated’ HBV serostatus (anti-HBs seropositive, anti-HBc and HBsAg seronegative) in Flanders (––) and Wallonia (- - -); coverage estimates from surveys performed up to (a) 2006 or (b) 2002, within the corresponding birth cohort (×, Flanders; ●, Wallonia).
Statistical analysis on HBV data collected in 2006–2007
Well fitting models (Hosmer & Lemeshow test for goodness of fit) could only be obtained using binary logistic regression, and after inclusion of a variable called ‘age cohort’ that indicated whether the child was aged >11 years. Separate models compared once ‘vaccinated’ and once ‘ever infected’ to all other serostatus outcomes. Region was a significant predictor for both being ‘vaccinated’ (solely anti-HBs positive) and ‘ever infected’ (HBsAg or anti-HBc positive). Children living in Flanders were more frequently ‘vaccinated’ than children living in Wallonia (OR 1·49, 95% CI 1·23–1·80), but also more frequently ‘ever infected’ (OR 4·45, 95% CI 1·57–12·64). Similarly, children living in Brussels were more frequently ‘ever infected’ than children in Wallonia (OR 4·95, 95% CI 1·38–17·68). Being ‘vaccinated’ was more prevalent in children aged >11 years than in younger ones (OR 0·24, 95% CI 0·17-0·34), but less prevalent with older age (OR 0·84, 95% CI 0·81-0·86) in each of the age groups. Gender was not statistically significant. Inclusion of interaction terms in the model predicting ‘vaccinated’ serostatus revealed that the difference between Flanders and Wallonia was significantly higher in the >11 years age cohort, and that the decrease with age was significantly higher in the younger age cohort.
Comparative analysis on HBV data collected in 2002 (ESEN2)
In 2002–2003, 1955 samples were collected from children aged 1–19 years. The samples from one laboratory could not be used for HBV analysis because this laboratory had erroneously excluded samples with positive HBV serology from their collection. This left 1528 samples in which HBV markers were evaluated, they were well distributed over the country (59·8% from Flanders, 8·1% from Brussels, 32·1% from Wallonia). Anti-HBs was measured on 1514 samples and was positive in 710 (46·9%); anti-HBc was measured in 1510 samples and positive in 33 (2·2%) and HBsAg was measured in 1390 samples and positive in 19 (1·4%). For the analysis that was published earlier in a European comparison, 32 samples with incomplete HBV results were excluded to conform to a common test algorithm [16]. For the current analysis we re-included those samples and re-categorized the HBV serostatus in a similar way as described above based on the combination of standardized HBV marker results. Serostatus could not be determined in 4·2% of the children due to conflicting or missing marker results. Based on their combined HBV serostatus, 2·2% (95% CI 1·5–3·0) had markers of previous HBV infection of whom 0·9% were HBsAg positive (Table 2). There were no significant regional differences, prevalence of markers of infection were 3·1% (95% CI 1·7-5·0), 1·8% (95% CI 1·0-2·8) and 1·6% (95% CI 0·2-5·7) in Wallonia, Flanders and Brussels, respectively.
The prevalence of ‘vaccinated’ serostatus in 2002–2003 was similar in birth cohorts targeted in infancy or adolescence, but increased significantly in cohorts that were targeted between the 2002–2003 survey and the 2006–2007 survey (1989–1994) (Table 3). Regional figures for ‘vaccinated’ serostatus are plotted in Figure 2b. A bimodal pattern with highest prevalence of ‘vaccinated’ serostatus in the birth cohorts targeted by the programme (1999–2002, 1987–1989) was obvious in each region, but the dip in between was less expressed in Wallonia.
DISCUSSION
Substantial progress has been made in the European region to introduce the hepatitis B vaccine into national programmes since the WHO recommended universal vaccination for all countries in 1997.
This study portrays the implementation of universal hepatitis B vaccination in Belgium, a low-endemic country, as well as its effect on the prevalence of HBV infection in the targeted population, using information on hepatitis B prevalence and vaccine-induced immunity from representative serological surveys in children aged 1–19 years.
First, we focused on the vaccine-induced immunity. The maximal prevalence of the ‘vaccinated’ serostatus (solely anti-HBs seropositive) was found at ages 1 and 13 years (83·5 and 62·0%, respectively) in 2006–2007, and at ages 2 and 14 years in 2002–2003 (68·8% each). These ages correspond fairly well to the ages at which universal vaccination was implemented in practice, i.e. in the first year of life and, as a catch-up, in the last year of primary school (in Wallonia) or the first year of secondary school (in Flanders); the delay in 2002–2003 corresponds to a shortage of vaccine in 2000 which delayed implementation in the first targeted cohorts.
When comparing the age-specific HBV seroprofiles of the 2006–2007 and the 2002–2003 study (Table 3), immunity to HBV clearly rose in birth cohorts that were targeted by the programme at ages 11 or 12 years in between both studies (1989–1995). Vaccine-induced immunity in the second year of life rose from 68·8% in 2002 to 83·5% in 2006, which corroborates the rising coverage reported in infancy (Table 1a). In age groups who had not been targeted with free-of-charge vaccine between 2002 and 2006 (1994–1998) a substantial part had nonetheless been vaccinated, either at infant age before the universal campaign had started or later in childhood than recommended, leaving only 40–70% susceptible (Fig. 1, Table 3). The gradual decrease of seronegativity from the 1994–1996 birth cohort to the 2004–2006 birth cohort probably reflects a gradual increase of HBV vaccine uptake due to the changing recommendations and reimbursement conditions during the long preamble to universal free-of-charge HBV vaccination in Belgium [5]. In contrast, the flat pattern in the birth cohorts aged >12 years who had all been targeted by 2006 (Fig. 1) suggests a fairly constant uptake of the vaccine by adolescents.
Regional figures (Fig. 2) from 2002 suggest more vaccination in non-targeted age groups in Wallonia, whereas 2006 figures suggest a lower coverage in the adolescent age group in this region compared to the other regions (Fig. 2). The latter finding was statistically significant in the regression analysis. As a consequence, the total proportion of children aged 1–19 years with a ‘vaccinated’ serostatus did not increase much in Wallonia from 2002 to 2006, in contrast to Flanders where it rose by 20%.
The serological data described above offer useful complementary information to the regional vaccination coverage surveys. It should be noted that these surveys are usually conducted at different times in the different regions (cf. Table 1). Nonetheless, the available survey-based HBV coverage estimates were usually higher than the prevalence of a ‘vaccinated’ serostatus in the same birth cohort (Fig. 2). Primary vaccination failure could explain part of this difference, but is known to be rare (<5%) in infants as well as in adolescents [22]. Waning of anti-HBs antibody titres, although limited, inevitably causes overestimation of susceptibility to HBV in serosurveys and may have overestimated the cohort effect found in this study. Loss of anti-HBs seropositivity after successful infant vaccination has been reported as 15–50% at 5–15 years [22] and 36% at 10 years after a 3–5–11 months schedule [23], and 12–14% at 5 or 10 years after (pre)adolescent vaccination [24]. But long-term protection against HBV was demonstrated after successful primary vaccination regardless of declining or disappearance of antibodies afterwards [25–27]. The 2002–2003 serosurvey results agreed better with coverage estimates from EPI surveys than the 2006–2007 results (Fig. 2). The standardization procedure within the ESEN2 project showed that the ELISA kit used for both the 2002–2003 and 2006–2007 serosurveys tended to underestimate anti-HBs seropositivity by 1–5% compared to the results of the reference laboratory, and only the 2002–2003 results have been adjusted for this assay effect [16]. Moreover, a fixed cut-off at 10 IU/ml was used in 2002–2003, although equivocal results had not been retested. Applying this cut-off on the 2006–2007 anti-HBs results would categorize 23 (23%) of the 99 samples currently classified as ‘undetermined’ as being ‘vaccinated’. Exclusion of ‘undetermined’ samples would also increase the prevalence of the ‘vaccinated’ status, but would introduce bias because ‘undetermined’ samples were not equally distributed over age groups.
Of the nine other European countries for which HBV seroprevalence was evaluated in the ESEN2 project, six had introduced a similar HBV vaccination strategy as Belgium. For only two of them, Italy and Luxembourg, the HBV seroprofile agreed with successful implementation in both infants and adolescents [16]. In Italy, HBV vaccine is mandatory and provided through vaccination clinics within the Italian National Health Service, whereas in Luxembourg it is not mandatory but offered free of charge by private physicians [28]. High HBV vaccine coverage in adolescents was reported by Hungary in a recent European survey [29]. In Belgium as well as in Hungary, a school-based approach is used, which has been demonstrated to be very successful in reaching adolescents [30–32]. In Belgium, school health centres invite adolescents for HBV vaccination at school (free of charge) or at a private practice; vaccination is not mandatory and there are no school entry requirements.
According to the serological profiles, in the coming decade a marked decrease of HBV incidence in young adults can be expected in Belgium, as has been demonstrated in Italy with a similar vaccination strategy [33, 34]. However, to fully prevent HBV carriage, the programme should also ensure vaccination of newborns of infected mothers at birth. A selective at-risk strategy including HBV screening of pregnant women was recommended in Belgium by the National Health Council in 2004, but its implementation has not yet been extensively evaluated. In 2009 the WHO recommended that HBV vaccination at birth should be considered for all children [2].
Second, the information on prevalence of infection merits discussion. The seroprevalence of an ‘ever infected’ HBV serostatus (antiHBs negative and anti-HBc and/or HBsAg positive) in 2006–2007 (1·8%) and 2002–2003 (2·2%) was similar to earlier reported findings in Belgium within the same age group (1–2% in 1993–1994; 0·5–1% in a postal saliva survey in 2003 [14, 15, 17]). The most plausible interpretation would be that the incidence of HBV infection in children has remained unchanged despite universal vaccination. This is not unexpected since childhood HBV infection and perinatal transmission were already rare in Belgium before vaccination was started. The samples with ‘ever infected’ serostatus were probably mainly imported cases from endemic countries, but we cannot exclude that perinatal transmission was not accurately prevented. Nevertheless, in a low-endemic country the effect of vaccination is to be expected mostly in adults. Adult samples were collected for the 2002–2003 and 2006–07 serosurveys to study other vaccine-preventable disease [18] but could not be used for estimation of HBV serology as they were collected in a blood donor population, which would introduce a clear bias. The saliva-based survey performed in a population-based sample in 2003 [17], only 3 years after the start of the universal HBV vaccination, was unable to demonstrate any effect on HBsAg seropositivity in adults aged >24 years, which were the most affected group in 1993–1994 (1·1% HBsAg seropositives).
HBsAg seropositivity in those aged 1–19 years was 0·5% in 2006–2007 and 0·9% in 2002–2003, but prevalence of both HBsAg- and anti-HBcpositive chronic carriers was 0·2% and 0·7%, respectively. Comparison within the ESEN2 project as well as with other recently published surveys demonstrated a similar prevalence of chronic carrier status in this age group in other low-endemic European countries irrespective of universal HBV vaccination [16, 35–38]. However, as samples from gastroenterological wards were not excluded from the serosurveys, overselection of children with a past or recent HBV infection cannot be excluded.
A limitation of the study was that no information was available on immigrant status of the children or their parents. In other low-endemic countries, immigrant children have been found to be an important risk group for HBV infection [36]. Another limitation which is inherent in cross-sectional serosurveys is that the timing of onset of infections is unknown, and thus incidence of HBV infection cannot be derived.
From the data presented in this paper we may conclude that the prevalence of HBV infection in those aged <20 years remains very low in Belgium and that universal HBV vaccination was well implemented in infants as well as in adolescents (catch-up), although regional differences exist and might result in unequal susceptibility in young adults in the near future.
ACKNOWLEDGEMENTS
This project was funded by the Institute of Public Health and the University of Antwerp. For the serum samples we are indebted to the collaborating laboratories, to doctors D. Baetens, M. Martin, J. Billiet, S. Vanderschueren, H. De Puydt, W. d'Hoore, A. Mewis, P. Couck, C. Neve, C. Fillee, T. Ledant, A. Dediste, C. Potvlieghe, P Goffinet and C. Pacco as well as their staff from the diagnostic laboratories; to doctors A. De Smet and M. C. Frère from the regional laboratories of the Red Cross, and to Dr M. Stalpaert of AML-Riatol who contributed logistical support. We also thank Professor Niel Hens (Universities of Antwerp, Leuven and Hasselt; I-Biostat) for his statistical assistance.
DECLARATION OF INTEREST
K. Hoppenbrouwers and P. Van Damme have been principal investigators of several vaccine trials for different vaccine manufacturers for which their universities (K.U. Leuven and University of Antwerp, respectively) obtained research grants.
REFERENCES
- 1.Franceschi S, Raza SA. Epidemiology and prevention of hepatocellular carcinoma. Cancer Letters 2009; 286: 5–8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis B vaccines. WHO Weekly Epidemiological Record 2009; 84: 405–419. [Google Scholar]
- 3.Romano L, et al. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Digestive and Liver Disease 2011; 43 (Suppl. 1): S2–7. [DOI] [PubMed] [Google Scholar]
- 4.Anon. WHO year of introduction of selected vaccines database (http://www.who.int/entity/immunization_monitoring/data/year_vaccine_introduction.xls). Accessed 13 July 2012.
- 5.Van Damme P, Keunen K, Beutels P. Universal hepatitis B vaccination in Flanders: a martyrdom or an example of good policy? Anatomy of policy making. Tijdschrift voor Geneeskunde 2002; 58: 231–239. [Google Scholar]
- 6.Vellinga A, Depoorter AM, Van Damme P. Vaccination coverage estimates by EPI cluster sampling survey of children (18–24 months) in Flanders, Belgium. Acta Paediatrica 2002; 91: 599–603. [DOI] [PubMed] [Google Scholar]
- 7.Swennen B, et al. Analysis of factors influencing vaccine uptake: perspectives from Belgium. Vaccine 2001; 20: S5–S7. [DOI] [PubMed] [Google Scholar]
- 8.Theeten H, et al. Infant vaccination coverage in 2005 and predictive factors for complete or valid vaccination in Flanders, Belgium: an EPI-survey. Vaccine 2007; 25: 4940–4948. [DOI] [PubMed] [Google Scholar]
- 9.Vandermeulen C, et al. Vaccination coverage in 14-year-old adolescents: Documentation, timeliness, and sociodemographic determinants. Pediatrics 2008; 121: E428–E434. [DOI] [PubMed] [Google Scholar]
- 10.Swennen B, et al. Vaccination coverage survey in infants at 18–24 months of age in Brussels-Capital Region. Report requested by the Common Community Commission of Brussels-Capital Region, 2000.
- 11.Hoppenbrouwers K, et al. Vaccination coverage survey in infants and adolescents in Flanders in 2008. Brussels, Belgium, 2009. [Google Scholar]
- 12.Swennen B, Robert E. Vaccination coverage survey in infants at 18–24 months of age in the French community (except Brussels). Brussels, Belgium: PROVAC-Université Libre de Bruxelles, 2003.
- 13.Swennen B, Robert E. Vaccination coverage survey in infants at 18-24 months of age in the French community (except Brussels). Brussels, Belgium: PROVAC-Université Libre de Bruxelles, 2009.
- 14.Beutels M, et al. Prevalence of hepatitis A, B and C in the Flemish population. European Journal of Epidemiology 1997; 13: 275–280. [DOI] [PubMed] [Google Scholar]
- 15.Van Loock F, Rubbens C. Survey of hepatitis B prevalence in the French Community, Belgium. Brussels, Belgium: Scientific Institute of Public Health, 1994. [Google Scholar]
- 16.Nardone A, et al. A comparison of hepatitis B seroepidemiology in ten European countries. Epidemiology and Infection 2009; 137: 961–969. [DOI] [PubMed] [Google Scholar]
- 17.Quoilin S, et al. A population-based prevalence study of hepatitis A, B and C virus using oral fluid in Flanders, Belgium. European Journal of Epidemiology 2007; 22: 195–202. [DOI] [PubMed] [Google Scholar]
- 18.Theeten H, et al. Are we hitting immunity targets? The 2006 age-specific seroprevalence of measles, mumps, rubella, diphtheria and tetanus in Belgium. Epidemiology and Infection 2011; 139: 494–504. [DOI] [PubMed] [Google Scholar]
- 19.Kafatos G, Andrews N, Nardone A. Model selection methodology for inter-laboratory standardisation of antibody titres. Vaccine 2005; 23: 5022–5027. [DOI] [PubMed] [Google Scholar]
- 20.Kafatos G, et al. The European Sero-Epidemiology Network 2: standardization of assay results for hepatitis B virus. Journal of Viral Hepatitis 2007; 14: 260–268. [DOI] [PubMed] [Google Scholar]
- 21.Anon. Total and Belgian population. Belgian General Directorate Statistics and Economic Information (http://statbel.fgov.be/nl/modules/publications/statistiques/bevolking/downloads/structuur_bevolking_leeftijd_geslacht.jsp). Accessed 15 February 2011.
- 22.Van Damme P, et al. Hepatitis B vaccines. In: Plotkin S, Orenstein WA, Offit P, eds. Vaccines, 6th edn. Philadelphia: Saunders, Elsevier, 2013, pp 205–234. [Google Scholar]
- 23.Zanetti AR, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet 2005; 366: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 24.Gilca V, et al. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine 2013; 31: 448–451. [DOI] [PubMed] [Google Scholar]
- 25.Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine 2006; 24: 572–577. [DOI] [PubMed] [Google Scholar]
- 26.Anon. Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet 2000; 355: 561–565. [PubMed] [Google Scholar]
- 27.Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clinical Infectious Diseases 2011; 53: 68–75. [DOI] [PubMed] [Google Scholar]
- 28.Anon. VENICE individual country profiles. ECDC's bi-weekly newsletter on vaccines and immunization, 12 July 2006–7 March 2007 (http://www.ecdc.europa.eu/en/healthtopics/immunisation/whats_new/Pages/archive_newsletter_vaccines_immunisation.aspx). Accessed 18 July 2012.
- 29.Mereckiene J, et al. Hepatitis B immunisation programmes in European Union, Norway and Iceland: where we were in 2009? Vaccine 2010; 28: 4470–4477. [DOI] [PubMed] [Google Scholar]
- 30.Mackroth MS, et al. Immunizing school-age children and adolescents: experience from low- and middle-income countries. Vaccine 2010; 28: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 31.Cawley J, Hull HF, Rousculp MD. Strategies for implementing school-located influenza vaccination of children: a systematic literature review. Journal of School Health 2010; 80: 167–175. [DOI] [PubMed] [Google Scholar]
- 32.Gidding HF, et al. The impact of a new universal infant and school-based adolescent hepatitis B vaccination program in Australia. Vaccine 2007; 25: 8637–8641. [DOI] [PubMed] [Google Scholar]
- 33.Mele A, et al. Acute hepatitis B 14 years after the implementation of universal vaccination in Italy: areas of improvement and emerging challenges. Clinical Infectious Diseases 2008; 46: 868–875. [DOI] [PubMed] [Google Scholar]
- 34.Bonanni P, et al. Impact of universal vaccination programmes on the epidemiology of hepatitis B: 10 years of experience in Italy. Vaccine 2003; 21: 685–691. [DOI] [PubMed] [Google Scholar]
- 35.Anon. Surveillance and prevention of vaccine preventable hepatitis. Data on surveillance and prevention of hepatitis A and B in 22 countries 1990s-2001 (www.eurohep.net). Accessed 30 August 2010. Antwerp, Belgium: Centre for the Evaluation of Vaccination, University of Antwerp. [Google Scholar]
- 36.Cai W, et al. Hepatitis B virus infections among children and adolescents in Germany: migration background as a risk factor in a low seroprevalence population. Pediatric Infectious Disease Journal 2010; 30: 19–24. [DOI] [PubMed] [Google Scholar]
- 37.Hahne SJ, et al. Prevalence of hepatitis B virus infection in The Netherlands in 1996 and 2007. Epidemiology and Infection 2012; 140: 1469–1480. [DOI] [PubMed] [Google Scholar]
- 38.Merrill RM, Hunter BD. Seroprevalence of markers for hepatitis B viral infection. International Journal of Infectious Diseases 2011; 15: e78–121. [DOI] [PubMed] [Google Scholar]