SUMMARY
Serum samples collected during the National Health and Nutrition survey (ENSANUT 2006) were obtained from subjects aged 1–95 years (January–October 2010) and analysed to assess the seroprevalence of Bordetella pertussis (BP) in Mexico. Subjects' gender, age, geographical region and socioeconomic status were extracted from the survey and compiled into a subset database. A total of 3344 subjects (median age 29 years, range 1–95 years) were included in the analysis. Overall, BP seroprevalence was 47·4%. BP seroprevalence was significantly higher in males (53·4%, P = 0·0007) and highest in children (59·3%) decreasing with advancing age (P = 0·0008). BP seroprevalence was not significantly different between regions (P = 0·1918) and between subjects of socioeconomic status (P = 0·0808). Women, adolescents and young adults were identified as potential sources of infection to infants. Booster vaccination for adolescents and primary contacts (including mothers) for newborns and infants may provide an important public health intervention to reduce the disease burden.
Key words: Bordetella pertussis, cocoon strategy, Mexico, pertussis toxin, seroprevalence, waning immunity
INTRODUCTION
Bordetella pertussis (BP) is endemic globally and is the fifth leading cause of death due to a vaccine-preventable disease in children aged <5 years worldwide [1–3]. Global estimates by the World Health Organization (WHO) revealed 16 million pertussis cases, 95% of which occurred in developing countries and about 195000 child deaths due to the disease in 2008 [4]. The last decade has witnessed new preventive vaccination strategies against pertussis as recommended by the Global Pertussis Initiative (GPI) and other experts in the field [2].
In Mexico, the number of pertussis cases reported increased from 53 cases in 2000 to 559 cases in 2009 [5]. However, it is acknowledged that a substantial number of cases are underreported due to limitations in the diagnosis of pertussis [6]. Pertussis vaccination was introduced in Mexico between 1954 and 1955 using whole-cell pertussis vaccine combined with diphtheria and tetanus vaccines (DTPw); however, the vaccine coverage was poor. [7]. In 1973, DTPw was introduced into the universal mass vaccination programme and the vaccine coverage increased to 43% [7]. Since 2007, the routinely used vaccine against pertussis has been an acellular pertussis vaccine combined with diphtheria and tetanus vaccines (DTPa). It was combined with Haemophilus influenzae type b (Hib) and inactivated polio vaccine (IPV) to form a pentavalent vaccine (DTPa-Hib-IPV), administered at ages 2, 4, 6 and 18 months and an additional whole-cell component vaccine, DTPw given at age 4 years [8]. In 2010, the reported vaccine coverage was high (95%) in Mexico [5].
In many countries, despite high vaccination coverage in infants and children, an increase in reported pertussis cases has been observed [9]. Although there is under-reporting for older age groups to a large extent due to lack of typical pertussis symptoms, the frequency of infection is high in adolescents and young adults [9–11]. Waning vaccination- and infection-acquired immunity have been identified as significant contributors to the increase in pertussis morbidity in these populations [10]. These older age groups, in turn, have been recognized as sources of infection to non-immunized or partially immunized infants [6, 10]. This is also indicative of the fact that although pertussis is most commonly recognized in children, it has also been observed to affect other age groups [12]. Currently, there are two vaccines approved for adolescents and adults in Mexico; however, neither of them are included in the mandatory immunization schedules [13].
There is a need to understand the burden of disease and evaluate the impact of existing immunization programmes in order to envisage future vaccination strategies to prevent infection, especially in adolescents and adults, and subsequently reduce the chances of transmission to vulnerable infants. For this reason, nationwide epidemiological data is required. However, recent data on the epidemiology of BP in Mexico is limited. Therefore, in order to identify groups susceptible to the disease, and thereby provide evidence to recommend potential preventive strategies, this study aimed to assess the overall seroprevalence of BP in Mexico and categorize the disease by gender, age, region and socioeconomic status (SES).
METHODS
Study design and subjects
A cross-sectional, seroprevalence study was conducted in Mexico between January and October 2010 (NCT01160081) based on the data and serum samples obtained from subjects (corresponding to 47152 households visited) who participated in the National Health and Nutrition Survey (ENSANUT 2006), which was conducted between October 2005 and May 2006. Household characteristics such as number of residents, age and other related characteristics have been described in the ENSANUT 2006 report [14]. ENSANUT 2006 used a probabilistic multistage stratified cluster sampling method to collect representative health and nutrition data from the entire Mexican population to update the prevalence of infectious and chronic diseases and their associated risk factors. The national survey included children (<10 years), adolescents (10–19 years), and adults (⩾20 years) who were selected for interview; blood samples were collected from 30% of these. The Ethics Commission from the Mexican National Institute of Public Health approved the survey.
Based on unique identification numbers (assigned during ENSANUT 2006) for the subjects included in the present study, demographic data including age, gender, geographical location and SES were obtained from the structured qualitative questionnaires from ENSANUT 2006 and compiled in a subset database designed by the Mexican National Institute of Public Health. SES for participants was stratified in three levels: low, middle and high. These strata were defined by using an index with several components such as building materials, number of rooms, domestic property, electricity and electronic goods [14].
Subjects were excluded from the study in cases where information required for the study was not available or incomplete, quantity of serum sample was insufficient, or the serum sample was incorrectly identified.
Immunological assessment
As part of the survey, blood samples were collected, processed, stored in 2·5 ml aliquots and maintained under refrigeration at −150°C. The serum samples were processed at the Mexican National Institute of Public Health (a GSK-designated laboratory) in the present study. Antibodies against BP were determined quantitatively using an IgG pertussis toxin (PT) enzyme-linked immunosorbent assay (ELISA) method (Virotech®, Genzyme Corporation, Germany). Subjects with anti-PT antibody concentrations ⩾11 Virotech units (VE) (⩾45 FDA U/ml) were considered to be seropositive according to the manufacturer's specifications. The selection of this test kit was based on its sensitivity (84% and 80%) and specificity (87% and 93%) which has a linear correlation with the WHO reference preparation [15]. The first value corresponds to the working reference with anti-PT IgG content close to the suggested cut-off [WHO first reference reagent pertussis antiserum (human)] [15]. The second value corresponds to an in-house ELISA cut-off (>40 IU/ml). Additionally, the manufacturer indicated that a value >18 VE is equivalent to a conventional value of >125 IU/ml corresponding to an acute infection or vaccination received <12 months ago [15].
Statistical analyses
All statistical analyses were performed using Statistical Analysis Software version 9.1 (SAS Institute Inc., USA). The sample size of about 4000 subjects selected by random simple sampling categorized by age in the serum bank was estimated to provide 80% statistical power assuming an overall seroprevalence ranging from a minimum of 25% in children to a maximum of 95% seroprevalence in adults. To be able to estimate national seroprevalences, a post-stratification weight was obtained taking into consideration factors such as gender, age, area of residence and SES for each subject.
The overall BP seroprevalence and BP seroprevalence categorized by gender, age group (children, 1–9 years; adolescents, 10–19 years; adults, ⩾20 years), region (Central, Mexico City, Southern Mexico, Northern Mexico) and SES (low, medium, high) were tabulated with 95% confidence intervals (CIs). Two-sided χ2 testing was performed to compare BP seroprevalence differences between gender, age groups, regions and SES. P values < 0·05 were considered statistically significant.
All subjects who were enrolled in the study were included in the total cohort for analyses. Subjects who were compliant with protocol-defined procedures, and for whom sufficient and correctly identified serum samples were available were included in the analysis.
RESULTS
Demographic characteristics
A total of 3984 subjects were enrolled in the study of which 3344 subjects were included in the analysis. Subjects were eliminated from the analysis if they did not have the serum sample volume required for analysis (n = 636) and if information was missing (n = 4). Overall, the median age of subjects was 28 years (range 1–95 years) and 49·9% of subjects were female. The median age of children was 5 years (range 1–9 years); adolescents 15 years (range 10–19 years) and adults 38 years (range 20–95 years).
BP seroprevalence
Overall, 47·4% (95% CI 43·0–51·9) of subjects were seropositive for anti-BP antibodies. BP seroprevalence was observed to be higher in males (53·4%, 95% CI 47·1–59·6) than in females (41·4%, 95% CI 36·5–46·4) (P = 0·0007) (Table 1).
Table 1.
Seroprevalence of Bordetella pertussis
| Category | % | 95% CI | P value |
|---|---|---|---|
| Gender | 0·0007 | ||
| Female | 41·4 | (36·5–46·4) | |
| Male | 53·4 | (47·1–59·6) | |
| Region | 0·1918 | ||
| Centre | 46·5 | (40·0–53·1) | |
| Mexico City | 39·5 | (24·6–54·5) | |
| North | 54·1 | (48·1–60·1) | |
| South | 48·4 | (42·7–54·1) | |
| Socioeconomic status | 0·0808 | ||
| Low | 47·9 | (43·2–52·6) | |
| Medium | 48·1 | (41·2–54·9) | |
| High | 29·7 | (12·0–47·4) | |
CI, Confidence interval.
%, Percentage of subjects seropositive for anti-Bordetella pertussis antibodies.
P values (two-sided χ2 test) < 0·05 are statistically significant.
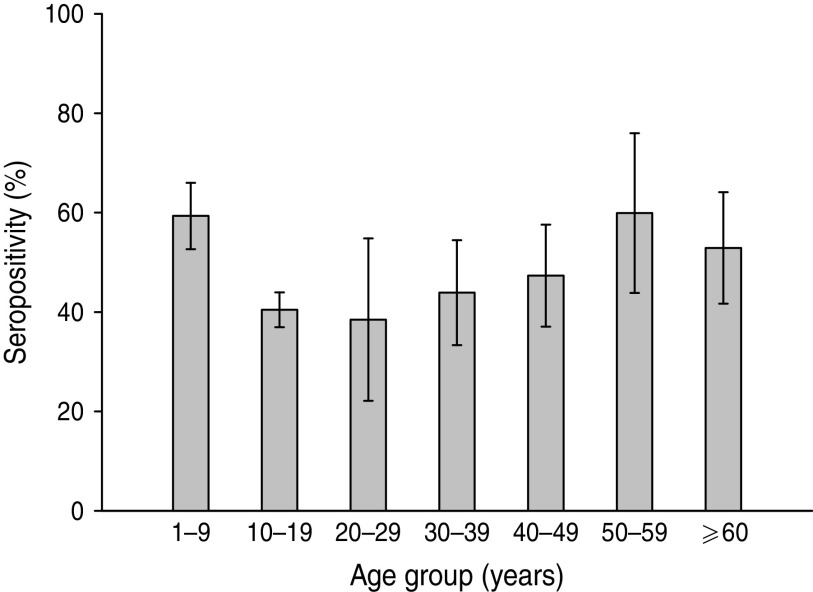
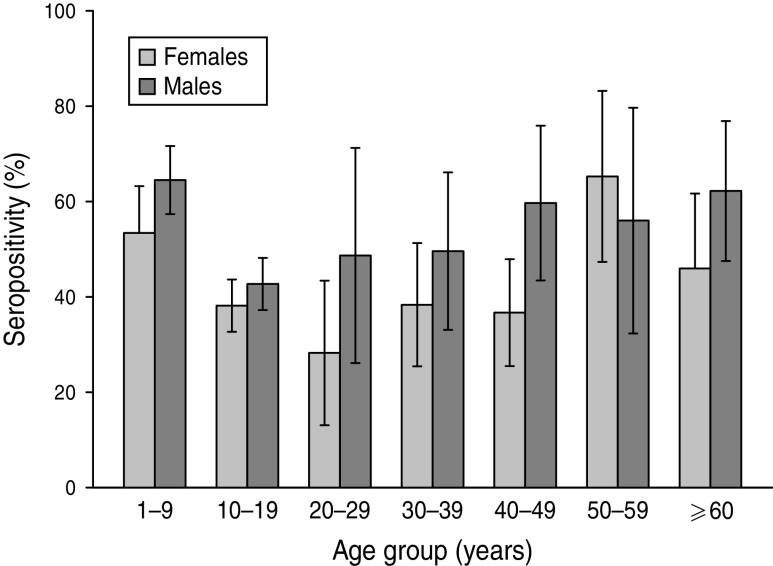
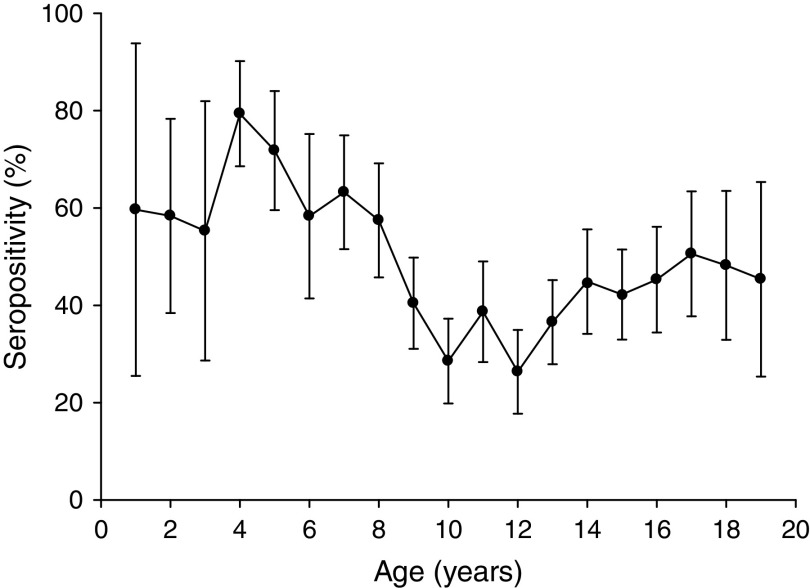
Analysis according to age revealed that the highest BP seroprevalence was observed in children (1–9 years) with 59·3% (95% CI 55·8–62·8) of this group being seropositive for anti-BP antibodies, while BP seroprevalence decreased further with age. Percentage of adolescents (10–19 years) and adults (⩾20 years) seropositive for anti-BP antibodies was 40·4% (95% CI 36·9–43·9) and 46·3% (95% CI 39·5–53·0), respectively. The difference in seroprevalence between the three age groups was statistically significant (P = 0·0008). The BP seroprevalence observed in age groups further stratified is shown in Figure 1. Regarding females, BP seroprevalence was higher in children and older adults compared to adolescent and young adult age groups: 10–19 years (38·2%), 20–29 years (28·2%), 30–39 years (38·3%) 40–49 years (36·7%) compared to 1–9 years (53·4%) and 50–59 years (65·3%) (Fig. 2). Across the age range 1–20 years, the results of the study revealed BP seroprevalence in children at age 4 years to be 79·3% (95% CI 68·5–90·1), following which, the BP seroprevalence was observed to decrease with age (Fig. 3).
Fig. 1.
Bordetella pertussis (BP) seroprevalence observed in age groups. Children aged 1–9 years presented with the highest prevalence with a decreasing trend in other strata. Some specific statistical differences were seen for this age group and adolescents and adults aged >20 years. Seropositivity rate (%) = percentage of subjects who were seropositive (anti-BP antibody concentrations >45 FDA U/ml). Error bars represent 95% confidence intervals.
Fig. 2.
Bordetella pertussis (BP) seroprevalence stratified by age and gender. This decreases in female and male adolescents and young adults between ages 10 and 30 years. Seropositivity rate (%) = percentage of subjects who were seropositive (anti-BP antibody concentrations >45 FDA U/ml). Error bars represent 95% confidence intervals.
Fig. 3.
Percentage of subjects seropositive for Bordetella pertussis (BP) across age range 1–20 years. The results of the study revealed BP seroprevalence in children aged 4 years to be 79·3% and decreasing with age. Seropositivity rate (%) = percentage of subjects who were seropositive (anti-BP antibody concentrations >45 FDA U/ml). Error bars represent 95% confidence intervals.
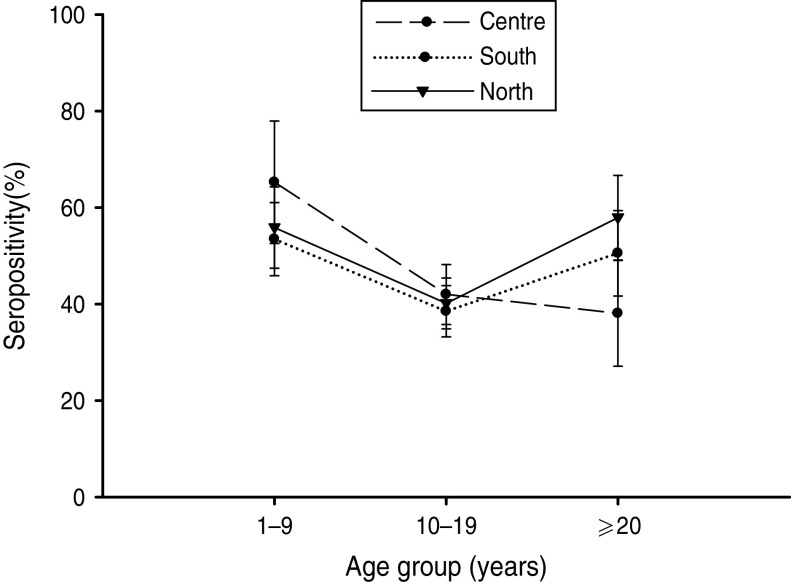
BP seroprevalence was observed to be 54·1% (95% CI 48·1–60·1) in Northern Mexico compared to 39·5% (95% CI 24·6–54·5) in Mexico City (Table 1). BP seroprevalence by age group across regions is shown in Table 2 and Figure 4. In the Central region, BP seroprevalence was highest in children (65·2%, 95% CI 52·5–77·9) with decreasing seroprevalence in adolescents (41·9%, 95% CI 35·7–48·1) and adults (38·0%, 95% CI 27·0–49·0). In Southern Mexico, the highest BP seroprevalence (53·4%, 95% CI 45·8–61·0) was observed in children, decreasing in adolescents (38·5%, 95% CI 33·2–43·8) and increasing in adults (50·5%, 95% CI 41·6–59·3). However, in the case of Northern Mexico, BP seroprevalence was observed to be comparable between children (55·8%, 95% CI 47·4–64·2) and adults (57·9%, 95% CI 49·1–66·6), while the seroprevalence in adolescents was low (40·1%, 95% CI 34·8–45·3).
Table 2.
Seroprevalence of Bordetella pertussis by age group and region
| Category | Centre | South | North | |||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Children (1–9 years) | 65·3 | (52·6–78·0) | 53·4 | (45·9–61·0) | 55·9 | (47·4–64·3) |
| Adolescents (10–19 yearss) | 42·0 | (35·8–48·2) | 38·5 | (33·2–43·8) | 40·1 | (34·9–45·4) |
| Adults (⩾20 years) | 38·1 | (27·1–49·0) | 50·5 | (41·7–59·4) | 57·9 | (49·1–66·7) |
CI, Confidence interval.
%, Percentage of subjects who were seropositive for anti-Bordetella pertussis antibodies.
Fig. 4.
Bordetella pertussis (BP) seroprevalence by age group across regions. In the Central region, seroprevalence was the highest in children, decreasing in adolescents and adults. In Southern Mexico, the highest BP seroprevalence was observed in children, decreasing in adolescents, and rising in adults. However, in the case of Northern Mexico, BP seroprevalence was comparable between children and adults, while in adolescents it was low. Seropositivity rate (%) = percentage of subjects who were seropositive (anti-BP antibody concentrations >45 FDA U/ml). Error bars represent 95% confidence intervals.
BP seroprevalence observed in subjects of low SES was 47·9% (95% CI 43·2–52·6) and in subjects of high SES it was 29·7% (95% CI 12·0–47·4) (Table 1).
A total of 729 (21·8%) subjects analysed had >18 VE corresponding to either current infection or a relatively recent vaccination. Of the total seropositive subjects (n = 1585), 46% (n = 729) had results consistent with an acute infection or had been recently immunized within the past year. Last, 90·7% of individuals with values >18 VE were aged ⩾5 years. There were no gender, geographical or socioeconomic differences in people with probable acute infection.
DISCUSSION
To our knowledge, this is the most recent population-based seroprevalence study conducted in Mexico. This study determined the nationwide seroprevalence of BP in Mexico and also assessed any possible correlation between BP seroprevalence and gender, age, region and SES. The findings of this study indicate that despite a high vaccination coverage rate in Mexican infants, the circulation of BP still exists due to possible waning immunity in older populations.
In this study, assessment by gender indicated a statistically significant difference in BP seroprevalence, observed to be higher in males compared to females (P = 0·0007). In contrast, a study conducted in Turkey showed a higher seroprevalence in females than in males (P = 0·03) indicating that females were more susceptible to pertussis [16]. However, in studies conducted in Taiwan and Italy, there were no significant differences in BP seroprevalence with respect to gender [17, 18]. The decreasing BP seroprevalence was observed in females of childbearing years. This is of note, given that most Mexican women give birth when they are in the 20–30 years age range [19]. This is consistent with studies that suggest that most women of childbearing age have low levels of antibodies to childhood diseases, including pertussis, and therefore are unable to transfer considerable antibodies to the newborn in the weeks following birth [20]. Although it is expected that passively acquired pertussis antibodies will protect newborn infants [21,22], low maternal antibody levels are not likely to provide protection to newborns. This lack of protection leaves the newborn susceptible to the disease. Therefore, there must be sufficient antibody titre (which has a half-life of only 36 days) in the mother's sera to provide protection at least until administration of vaccine to the young infant [21, 22]. The lower BP seroprevalence observed in females strongly supports the importance of vaccination strategies to protect newborns.
Age-specific BP seroprevalence suggest low BP seroprevalence in the population comprising adolescents and young adults (P = 0·0008), while also reflecting a possible waning immunity. Waning immunity may be related to the increase in pertussis cases as has been reported previously [23]. Evidence of an increase in pertussis cases in these age groups has been observed previously in public schools in Mexico City, which indicated low pre-existing immunity in adolescents (despite high immunization coverage during childhood) and reported a high pertussis infection incidence rate in adolescents [24]. This previous study conducted in 2009 highlighted the danger of an unrecognized risk of transmission to infants and suggested that the substantial disease burden of pertussis in Mexican adolescents should be considered when planning public health interventions, including an adolescent pertussis booster vaccination [24]. In the present study, a peak in anti-BP seropositivity was observed in children at age 4 years, probably due to the whole cellular pertussis booster dose administered at that age. Post-4 years of age, a decreasing trend in BP seroprevalence with increasing age was observed in adolescents and young adults, with the lowest seropositivity rates observed between ages 9 and 13 years. Similar findings were reported in an epidemiological study conducted by Cattaneo et al. where they analysed serum samples of healthy individuals aged 1–65 years [25]. The results of this previous study also showed a peak in anti-BP seropositivity at ages 4–6 years, which was most likely due to DTPa administered as a booster dose [25]. PT has been established as the only antigen specific to BP and is used as an estimate of BP circulation [22]. Vaccine-induced antibodies to PT are minimal and short-lived [26, 27]. It has also been suggested that whole cell vaccines rarely induce antibodies to PT [28, 29] as the amount of PT antigen in the whole cell vaccine itself is low, which could possibly be a reason for fewer adolescents and adults being seropositive for anti-BP antibodies in Mexico. Even in populations with whole cell pertussis vaccines containing moderate to high amounts of PT, the vaccine-induced antibodies decline and are barely detectable after 2–4 years [27]. The spike in seropositivity after age 50 years may be attributed to recent and natural BP infection [21, 30, 31], which suggests an increased circulation of BP in this age group [32]. Antibody responses to BP occur in most people with BP natural infection and although high antibody levels are observed, they persist only temporarily [26, 27]. Recently, it has been shown that age does not significantly influence the rise, peak and decline of antibody titres following infection [27, 33].
Small differences between regions of Mexico might be explained by regional disparities in terms of socioeconomic development. The Northern and Central states are more developed than the Southern states. Previous reports have indicated that Southern Mexico has limited resources, characterized by a strong indigenous heritage and is socioeconomically challenged, which suggests higher exposure in comparison to other states [34]. However, it is important to highlight that historically, individuals of low SES have a higher seroprevalence to BP than those of higher SES; a candidate explanation for this could be residential crowding for low SES individuals. In this study, however, the formal definition of SES for ENSANUT may be a potential confounding factor for the observed comparable seroprevalence rates in people from different SES levels [14].
As mentioned earlier, antibodies to PT can be used as an estimate of BP circulation [20]. The test used in the study, IgG PT ELISA is a validated assay that has been proven to show overall good sensitivity and specificity for measuring antibodies to PT [15]. This is because the assay contains only purified PT as an antigen which has been established as highly effective in contrast to other ELISA kits using a mixture of antigens [15, 22].
Latin American countries, such as Argentina and Panama, introduced adolescent immunization to reduce the potential source of infection from older age groups to infants [8]. The ‘cocoon strategy’, recommended by the GPI was implemented by Costa Rica in 2007 and by Panama in 2009, both in response to outbreaks [1, 8]. Despite the benefits associated with the cocoon strategy, cocooning may be insufficient to protect infants from pertussis during the first 1–2 weeks of life [20]. There has also been limited success in vaccinating fathers and other family members in close contact with newborns [35] and in reality, cocooning is expensive and logistically complicated [36, 37]. On the other hand, maternal immunization results in reduction of disease in women and provides potentially better protection of infants through passively acquired antibodies from the mother [38]. Furthermore, the Advisory Committee on Immunization Practices recommended a single dose of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) for unvaccinated pregnant mothers (or immediately postpartum) and for adults aged >65 years if they have or anticipate having close contact with an infant aged <12 months and previously have not received Tdap [39].
Important limitations need to be acknowledged in the present study. The risk factors related to BP and the BP vaccination history for adolescents and adults were not taken into consideration. In addition, the PT ELISA test did not differentiate between vaccine-induced antibodies and antibodies induced as a result of infection. Nonetheless, most of the subjects who reported higher values of IgG were older subjects with a very low chance of having been vaccinated recently and most likely corresponded to an acute pertussis infection. These limitations highlight the restrictive nature of the current surveillance and emphasize the need for improvement in order to suitably evaluate public health interventions considering the difference in disease and infection rates. Further areas of study needed in Mexico are the distinction between antibodies due to infection and antibodies due to immunization, the seasonality and increase in the number of pertussis cases, and documentation of the indication of vaccination in adolescents and young adults.
In conclusion, the results of this study indicate waning immunity against pertussis in adolescents and young adults and suggest that BP is widely circulating in these populations in Mexico. These populations serve as potential reservoirs of infection to infants and young children. Furthermore, the trends observed in the study appear to be consistent with the vaccine schedule and vaccine coverage in Mexico. In view of recognizing the importance of diminishing immunity with age in adolescents and adults and the convenience of a previous ad-hoc cost-effectiveness evaluation, booster vaccination in these populations including primary contacts (such as mothers) for newborns and infants may provide an important public health intervention to reduce the pertussis-related disease burden.
ACKNOWLEDGEMENTS
The authors thank the following from the Instituto Nacional de Salud Pública: María Olamendi-Portugal, M.Sc. and Santa García-Cisneros, Med.Tech., for their valuable support in running laboratory testing and preparing database results regarding Bordetella pertussis. Jacqueline Vargas, Claudia Cuevas and Claudia R. Rodriguez from GlaxoSmithKline Vaccines, Mexico for contributing to and supporting the coordination and monitoring activities for this study; and the following from GlaxoSmithKline Vaccines: Ashmita Ravishankar for medical writing; Dr Camilo Moreno and Jessica Mattos for editorial assistance and coordination in the development of this paper.
This study was sponsored and funded by the GlaxoSmithKline Biologicals SA, Rixensart, Belgium. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with the development and the publishing of this paper.
DECLARATION OF INTEREST
Rodrigo DeAntonio, Luis Romano-Mazzotti, Yolanda Cervantes and Eduardo Ortega-Barria are employed by the GlaxoSmithKline group of companies. Yolanda Cervantes, Rodrigo DeAntonio and Luis Romano-Mazzotti declare GlaxoSmithKline stock options; Eduardo Ortega-Barría declares GlaxoSmithKline stock ownership.
REFERENCES
- 1.Guiso N, Liese J, Plotkin S. The Global Pertussis Initiative: meeting report from the fourth regional roundtable meeting, France, April 14–15, 2010. Human Vaccines 2011; 7: 481–488. [DOI] [PubMed] [Google Scholar]
- 2.Ulloa-Gutierrez R, Carvajal-Riggioni D, Baltodano A. Pertussis: should we improve intensive care management or vaccination strategies? Expert Review of Vaccines 2011; 10: 49–53. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Pertussis vaccines: WHO position paper. Weekly Epidemiological Record 2010; 85: 385–400. [PubMed] [Google Scholar]
- 4.Black RE, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375: 1969–1987. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Immunization profile (http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileresult.cfm?C=mex). Accessed 10 February 2012.
- 6.Ulloa-Gutierrez R, Avila-Aguero ML. Pertussis in Latin America: current situation and future vaccination challenges. Expert Review of Vaccines 2008; 7: 1569–1580. [DOI] [PubMed] [Google Scholar]
- 7.Santos JI. El programa nacional de vacunacion: orgulla de Mexico (http://www.ejournal.unam.mx/rfm/no45-3/RFM45308.pdf). Accessed 8 February 2012.
- 8.Ulloa-Gutierrez R, et al. The Global Pertussis Initiative: meeting report from the regional Latin America meeting, Costa Rica, 5–6 December, 2008. Human Vaccines 2010; 6: 876–880. [DOI] [PubMed] [Google Scholar]
- 9.Tan T, Trindade E, Skowronski D. Epidemiology of pertussis. Pediatric Infectious Disease Journal 2005; 24: S10–S18. [DOI] [PubMed] [Google Scholar]
- 10.Guiso N, et al. The Global Pertussis Initiative: report from a round table meeting to discuss the epidemiology and detection of pertussis, Paris, France, 11–12 January 2010. Vaccine 2011; 29: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 11.Rendi-Wagner P, et al. The seroepidemiology of Bordetella pertussis in Israel – estimate of incidence of infection. Vaccine 2010; 28: 3285–3290. [DOI] [PubMed] [Google Scholar]
- 12.Heininger U, Cherry JD. Pertussis immunization in adolescents and adults – Bordetella pertussis epidemiology should guide vaccination recommendations. Expert Opinion on Biological Therapy 2006; 6: 685–697. [DOI] [PubMed] [Google Scholar]
- 13.Expert group on vaccinations for pertussis. Consensus on the clinical and microbiologic diagnosis of Bordetella pertussis and infection prevention. Salud Publica de Mexico 2011; 53: 57–65. [DOI] [PubMed] [Google Scholar]
- 14.Olaiz-Fernández G, et al. Encuesta Nacional de Salud y Nutrición 2006. Cuernavaca, México: Instituto Nacional de Salud Pública, 2006. (http://www.insp.mx/ensanut/ensanut2006.pdf). Accessed 2 April 2013.
- 15.Riffelmann M, et al. Performance of commercial enzyme-linked immunosorbent assays for detection of antibodies to Bordetella pertussis. Journal of Clinical Microbiology 2010; 48: 4459–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inandi T, Guraksin A, Hacialioglu N. Seroprevalence of pertussis among children in Eastern Turkey. Public Health 2005; 119: 550–555. [DOI] [PubMed] [Google Scholar]
- 17.Chiu TF, et al. Pertussis seroepidemiology in Taipei. Journal of the Formosan Medical Association 2000; 99: 224–228. [PubMed] [Google Scholar]
- 18.Giammanco A, et al. Seroepidemiology of pertussis in Italy. Reviews of Infectious Diseases 1991; 13: 1216–1220. [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Statistics, Geography and Informatics. Population and Household Census. Mexico, 2010. Census sample. Database. (http://www.censo2010.org.mx/). Accessed 2 April 2013.
- 20.Halperin BA, et al. Kinetics of the antibody response to tetanus-diphtheria-acellular pertussis vaccine in women of childbearing age and postpartum women. Clinical Infectious Diseases 2011; 53: 885–892. [DOI] [PubMed] [Google Scholar]
- 21.Esen B, et al. Prevalence of high antibody titers of pertussis in Turkey: reflection of circulating microorganism and a threat to infants. Journal of Clinical Laboratory Analysis 2007; 21: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. The immunological basis for immunization series: module 4: pertussis – update 2009 (http://whqlibdoc.who.int/publications/2010/9789241599337_eng.pdf). Accessed 10 February 2012.
- 23.Libster M, Edwards KM. Re-emergence of pertussis: what are the solutions? Expert Review of Vaccines 2012; 11: 1331–1346. [DOI] [PubMed] [Google Scholar]
- 24.Guerrero ML, et al. Pertussis in adolescents in Mexico City: a prospective cohort study. Presented at the 7th World Congress of the World Society for Pediatric Infectious Diseases (WSPID), Melbourne, Australia, 16–19 November 2011 (http://www.kenes.com/wspid2011/abstracts/pdf/235.pdf). Accessed 16 March 2012.
- 25.Cattaneo LA, et al. The seroepidemiology of Bordetella pertussis infections: a study of persons aged 1–65 years. Journal of Infectious Diseases 1996; 173: 1256–1259. [DOI] [PubMed] [Google Scholar]
- 26.De Melker HE, et al. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. Journal of Clinical Microbiology 2000; 38: 800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Melker HE, et al. The incidence of Bordetella pertussis infection estimated in the population from a combination of serological surveys. Journal of Infection 2006; 53: 106–113. [DOI] [PubMed] [Google Scholar]
- 28.Olin P, et al. Randomized controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Ad Hoc Group for the study of pertussis vaccines. Lancet 1997; 350: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 29.De Greef SC, et al. Seroprevalence of pertussis in the Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS ONE 2010; 5: e14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pebody RG, et al. The seroepidemiology of Bordetella pertussis infection in Western Europe. Epidemiology and Infection 2005; 133: 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks DA, Clover R. Pertussis infection in the United States: role for vaccination of adolescents and adults. Journal of the American Board of Family Medicine 2006; 19: 603–611. [DOI] [PubMed] [Google Scholar]
- 32.Hallander HO, et al. Seroprevalence of pertussis toxin (anti-PT) in Sweden before and 10 years after the introduction of a universal childhood pertussis vaccination program. Acta Pathologica, Microbiologica et Immunologica Scandinavica 2009; 117: 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Versteegh FGA, et al. Age-specific long-term course of IgG antibodies to pertussis toxin after symptomatic infection with Bordetella pertussis. Epidemiology and Infection 2005; 133: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valdespino JL, et al. Seroepidemiology of hepatitis A in Mexico. A detector of social inequity and monitor of immunization policies. Salud Publica de Mexico 2007; 49 (Suppl. 3): S377–S385. [Google Scholar]
- 35.Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clinical Infectious Diseases 2011; 52: 157–162. [DOI] [PubMed] [Google Scholar]
- 36.Castagnini LA, et al. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clinical Infectious Diseases 2012; 54: 78–84. [DOI] [PubMed] [Google Scholar]
- 37.Skowronski DM, et al. The number needed to vaccinate to prevent infant pertussis hospitalization and death through parent cocoon immunization. Clinical Infectious Diseases 2012; 54: 318–327. [DOI] [PubMed] [Google Scholar]
- 38.Munoz F, Englund J. Infant pertussis: Is cocooning the answer? Clinical Infectious Diseases 2011; 53: 893–896. [DOI] [PubMed] [Google Scholar]
- 39.CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months – Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2011; 60: 1424–1426. [PubMed] [Google Scholar]