SUMMARY
Our aim was to assess progress towards measles elimination from The Netherlands by studying humoral measles immunity in the Dutch population. A population-based seroepidemiological study was conducted in 2006–2007 (N = 7900). Serum samples were analysed by a bead-based multiplex immunoassay. IgG levels ⩾0·2 IU/ml were considered protective. The overall seroprevalence in the Dutch population was 96%. However, 51% of socio-geographically clustered orthodox Protestant individuals aged <10 years were susceptible. Infants might be susceptible to measles between ages 4 months and 14 months, the age at which maternal antibodies have disappeared and the first measles, mumps, rubella (MMR) vaccination is administered, respectively. Waning of antibody concentrations was slower after the second MMR vaccination than after the first. The Netherlands is at an imminent risk of a measles outbreak in the orthodox Protestant minority. To prevent subsequent transmission to the general population, efforts to protect susceptible age groups are needed.
Key words: Infectious disease epidemiology, measles (rubeola), MMR vaccination, serology, vaccine preventable diseases
INTRODUCTION
Measles is one of the most infectious diseases of childhood and remains an important cause of morbidity and mortality in developing countries. In 2010, about 85% of the world's children received at least one dose of measles vaccine in their first year of life. Measles vaccination successfully reduced global measles mortality by 74% between 2000 and 2010 [1]. However, coverage with two doses of measles-containing vaccine (MCV) of >95% is required to sustain the elimination of measles virus. Providing high quality vaccination services is essential to reach this goal [2]. For several reasons regarding economics, politics, safety concerns, philosophy or religion, many countries in Europe have experienced several setbacks in their individual measles vaccination programmes between 1990 and 2012, leading to the accumulation of susceptible persons and subsequent large-scale nationwide measles outbreaks [3]. Recently, an upsurge in the incidence of measles was reported in Romania, France, Spain, Italy, UK, Germany and Belgium [4]. At present, measles in Europe is no longer just a childhood disease, it also affects older children and adults. Public settings for transmission include mostly educational and healthcare facilities [5].
In The Netherlands, in 1976, monovalent measles vaccination was introduced for infants aged 14 months (birth cohorts 1975–1985). Since 1987, children have been offered a combination vaccine against measles, mumps and rubella (MMR vaccine) at ages 14 months and 9 years. A 3-year catch-up programme for pre-school children accompanied the start of the MMR programme (birth cohorts 1983–1985). The different MCVs that were used in the national immunization programme all contained the Moraten vaccine virus strain. Vaccination coverage in The Netherlands is high (96%) for one dose of MMR (cohort 2009; reporting year 2012) and 93% for two doses of MMR (cohort 2001; reporting year 2012). However, in some municipalities with a high number of socio-geographically clustered orthodox Protestant individuals (OPIs) the vaccination coverage is <60% [6].
Before the introduction of the separate measles vaccination, national measles epidemics occurred every other year. After introduction of measles vaccination, epidemics were primarily restricted to the orthodox Protestant minority, consisting of about 213 000 persons in January 2007 (1·3% of the Dutch population) [7]. Those epidemics occurred once every 4–7 years until 2000 (i.e. 1983, 1987–1988, 1992–1993, 1999–2000) [8–11]. Since the early 2000s, the annual incidence of reported cases of measles has generally been below the WHO elimination target level of 1 per million. Exceptions to this were in 2008 and 2011. In 2008, a small and relatively restricted outbreak of 99 cases occurred in unvaccinated persons because of their anthroposophic beliefs or critical attitude towards vaccination [12]. In 2011, increased incidence (50 cases) reflected outbreaks in other European countries.
To assess the progress towards measles elimination from The Netherlands, we studied the measles immunity in the Dutch population by using a population-based seroepidemiological study, performed in 2006–2007. In addition, we aimed to gain insight into the long-term protection following MMR vaccination and into risk factors for measles susceptibility in individuals that had received one and two doses of MMR vaccine. We compared results with those from a seroprevalence study performed 11 years before the current study (i.e. 1996) [13].
METHODS
Survey design and study population
We conducted a cross-sectional population-based seroepidemiological study in The Netherlands between February 2006 and June 2007. The study was approved by the Medical Ethics Testing Committee of the Foundation of Therapeutic Evaluation of Medicines (METC-STEG) in Almere, The Netherlands (clinical trial number: ISRCTN 20164309). All participants provided signed informed consent for blood sampling and/or questionnaire. If participants were minors consent was obtained from two parents or guardians.
The study design was similar to that of the population-based seroepidemiological study performed in 1995–1996 [14] and has been described previously [15]. In short, a two-stage cluster sample was drawn from the Dutch population. First, The Netherlands was divided into five geographical regions of approximately equal population size. In each of the five regions eight municipalities were sampled with a probability proportional to their size. Within each of these 40 municipalities an age-stratified sample (0, 1–4, 5–9, … , 75–79 years) of 380–500 individuals, was randomly drawn from the municipal population register. In addition to the nationwide sample (NS), eight municipalities with low vaccination coverage (LVC) were included. In these municipalities reside many OPIs who decline vaccination for religious reasons. Within 12 of the 40 municipalities from the NS non-Western migrants were over-sampled.
Participants were asked to attend a clinic for venepuncture, to complete a questionnaire at home and to bring their vaccination certificates. If the latter were not available, a copy was retrieved from the local authority for registration of vaccination. The questionnaire contained questions about, among others, demographic information, e.g. age, gender, ethnicity, religion.
Laboratory methods
A bead-based multiplex immunoassay (MMRV-MIA) was used for the simultaneous quantitative detection of antibodies against measles, mumps, rubella and varicella zoster as described previously [16]. In brief, serum samples were diluted 1/200 and 1/4000 in phosphate-buffered saline containing 0·1% Tween-20 and 3% bovine serum albumin. A reference, controls and blanks were included on each plate. Antibody concentrations were obtained by interpolation of the mean fluorescent intensity (MFI) in the reference serum curve and for measles expressed in international units per ml (IU/ml). The lower limit of quantification was 0·015 mIU/ml. Antibody concentrations of ⩾0·2 IU/ml were considered protective [17].
Data analyses
Seroprevalence and geometric mean concentrations (GMCs)
Data were analysed using SAS v. 9.1.3 (SAS Institute Inc., USA) and R [18]. All analyses took account of the survey design. Over-sampled migrants were included in the analyses of the nationwide sample. Seroprevalence and GMCs in the NS were estimated by weighting by age, gender, ethnicity and degree of urbanization. This was done to match the Dutch population distribution to that of 1 January 2007 [19] and to take into account the over-sampling of migrants. Seroprevalence and GMCs in the LVC sample were weighted by age and gender. Participants were stratified into five countries/regions of birth: (1) The Netherlands; (2) other Western countries; (3) Morocco + Turkey; (4) Dutch Antilles + Aruba + Suriname; (5) other non-Western countries. Dutch participants were those born in The Netherlands with both parents born in The Netherlands. Furthermore, we distinguished first- and second-generation migrants. A first-generation migrant was defined as a participant who was born abroad of whom one or both parents were born in the same country. A second-generation migrant was defined as a participant born in The Netherlands of whom one or both parents were born abroad. Depending on the municipality, each participant was assigned a degree of urbanization score (e.g. 1, very high (⩾2500 addresses per km2); 2, high (1500–2500 addresses per km2); 3, moderately high (1000–1500 addresses per km2); 4, low (500–1000 addresses per km2); 5, very low (<500 addresses per km2) based on data from Statistics Netherlands [20]. For the weighting procedure, we combined age groups with ethnic groups and degrees of urbanization 2–5 were combined. Denominations in the LVC sample were classified into two groups based on vaccination coverage defined by Ruijs et al. [21]. The first group (i.e. OPIs) consisted of the following denominations with low or intermediate vaccination coverage: the Protestant Congregations in The Netherlands, the Old Protestant Congregations, the Restored Protestant Church and the Protestant Congregations. The second group (i.e. non-OPIs) consisted of the following denominations/groups with relatively high vaccination coverage: Protestant Bond, Christian Protestant Churches, other Protestant Christians, other or no religion and unknown religion.
Differences in seroprevalence between years or age groups were determined as follows. First, the parameters of the beta distribution for both seroprevalences were estimated using the method of moments [22]. Next, the risk ratios, their corresponding 95% confidence intervals, and P values were estimated by Monte Carlo simulations of both seroprevalences. Differences in the GMC between years or age groups were identified by calculating differences in log titres and tested by using the t test. P values of <0·05 were considered statistically significant.
We used the target levels for susceptibility as defined by Gay for the WHO strategy to eliminate measles in the European Region [23]. These levels were based on a heterogeneous mixing model assuming a basic reproduction number (R0) of 11 and accepting a maximum value of the reproduction number (Rmax) of 0·7. The target levels for susceptibility were: <15% for the 1–4 years age group, <10% for the 5–9 years age group and <5% for the older 5-year age groups [23].
Persistence of antibodies after first and second MMR vaccination
We performed log-linear regression to study measles concentrations by time since MMR vaccination in our cross-sectional sample. Persistence of measles ln IgG antibody concentrations after the first MMR vaccination was studied in participants of Dutch origin, aged 2–8 years, who had received one MMR vaccination at the age of 13–16 months. Persistence of measles ln IgG antibody concentrations after the second MMR vaccination was studied in participants of Dutch origin, aged 9–20 years, who had received their MMR vaccinations at the ages of 13–16 months and at 8 or 9 years, respectively.
Risk factors for measles susceptibility in individuals vaccinated once and twice
We used logistic regression to study risk factors for measles susceptibility in individuals of Dutch origin aged 2–8 and 9–20 years, who received one or two MMR vaccinations, respectively. The following determinants were studied: age at first vaccination (i.e. 13/14–16 months and 13/14/15–17 months for 2–8 and 9–20 years age groups, respectively), age at second MMR vaccination (i.e. 8/9 years), time since first MMR vaccination (i.e. 0–3/4–7 years), time since second MMR vaccination (i.e. 0–4/5–8/9–12 years) and gender. Age was not included as this was highly correlated with the time since first/second MMR vaccination.
Backward selection was used to identify determinants of measles susceptibility. A determinant remained in the model if the P value was <0·1. The crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated.
RESULTS
The response was 32% (N = 6383) and 35% (N = 1517) in the NS and LVC sample, respectively.
Seroprevalence and GMC in the NS
The overall measles IgG seroprevalence was 95·7% (95% CI 95·1–96·2) with an overall GMC of 1·8 IU/ml (95% CI 1·8–1·9). The youngest age groups were sufficiently protected (seroprevalence was 92% and 94% for the 1–4 and 5–9 years age groups, respectively) according to the age-specific susceptibility targets. However, the following age groups were insufficiently protected: 20–24 (94%), 25–29 (91%) and 30–34 (94%) years. In general, males had a slightly lower seroprevalence and GMC than females (95·1% vs. 96·2%, P = 0·02 and 1·75 IU/ml vs. 1·93 IU/ml, P = 0·03).
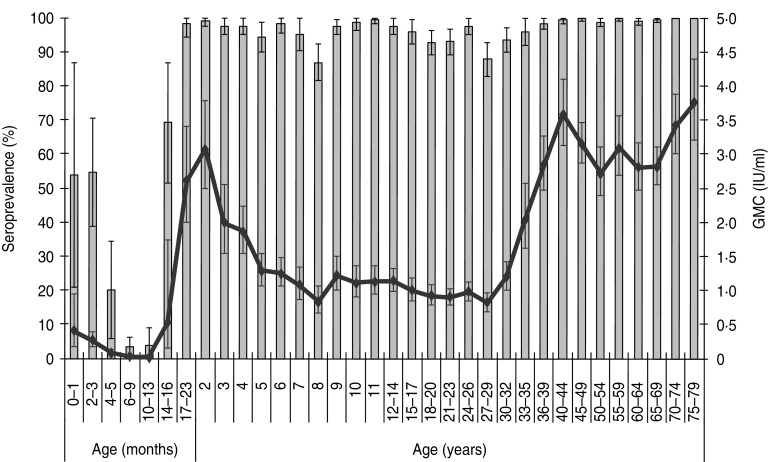
Figure 1 shows the seroprevalence and GMC per age group. The seroprevalence decreased from 54% for infants aged 0–1 month to 3·7% for infants aged 10–13 months.
Fig. 1.
Weighted age-specific seroprevalence and geometric mean concentrations (GMC) of measles IgG antibody (with 95% confidence intervals) in the general population.
For those aged 4–5 months the GMC was already below the cut-off level of 0·2 IU/ml, and decreased further to 0·01 IU/ml at age 10–13 months. Subsequent to the increase after the first MMR vaccination at 14 months, the GMC rapidly decreased from 3·1 IU/ml at age 2 years to 0·8 IU/ml at age 8 years. Note, the seroprevalence at age 8 years was 87%, but as was stated earlier the seroprevalence for the 5–9 years age group was above the target level of susceptibility (>90%). The seroprevalence after the second MMR vaccination at age 9 years increased from 87% at age 8 years to 97% at age 9 years, with a corresponding increase in the GMC from 0·8 to 1·2 IU/ml, respectively. Thereafter the seroprevalence and GMC gradually decreased to 88% and 0·8 IU/ml, respectively, until reaching the 27–29 years age group. The 27–29 years age group belongs to a cohort that was born just after introduction of monovalent measles vaccine in The Netherlands. The seroprevalence and GMC were higher for those born before the introduction of monovalent measles vaccine and reached seroprevalence and GMC levels over 96% and 2·0 IU/ml, respectively for the ⩾33–35 years age group. These older individuals were probably naturally infected with measles.
Persistence of antibodies after first and second MMR vaccination
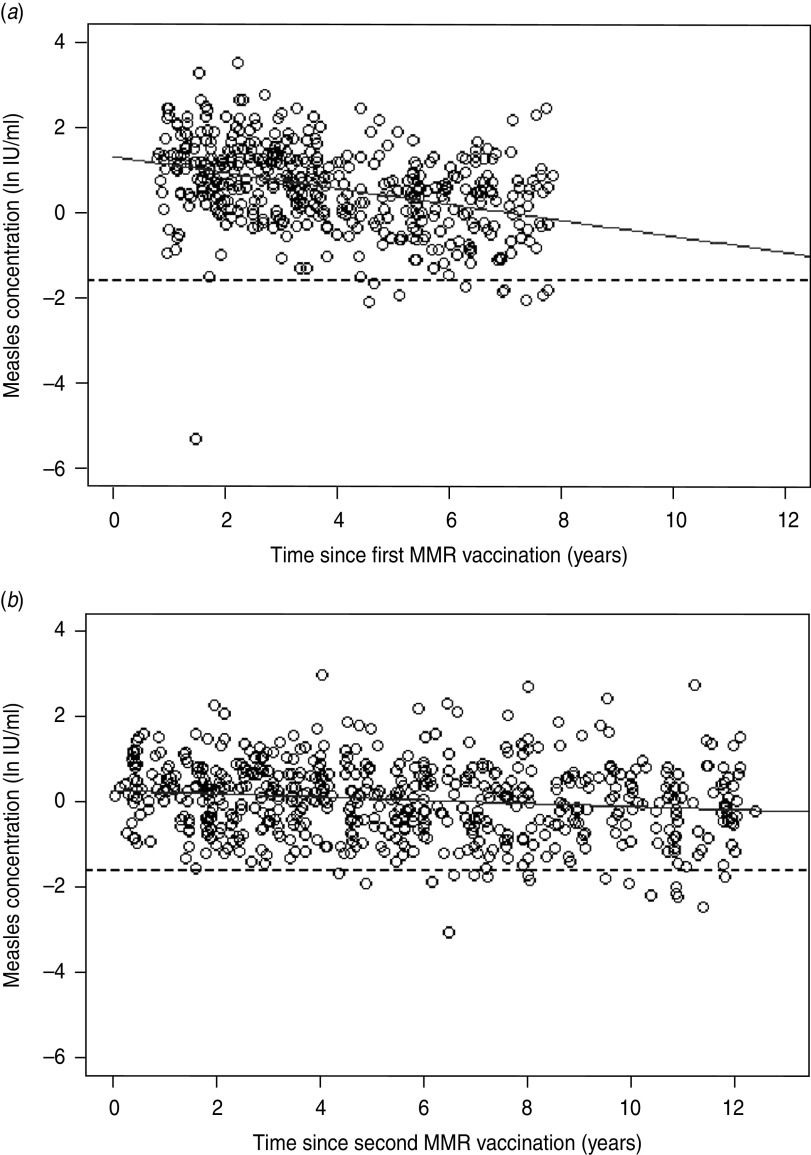
In individuals receiving both one and two MMR vaccinations (N = 444 and 582, respectively) a significant decline in measles IgG antibody concentrations per year since last vaccination was observed (Figure 2a, b). The slope in individuals receiving one and two doses was −0·19 ln IU/ml per year (95% CI −0·23 to −0·14, P < 0·0001) and −0·04 ln IU/ml per year (95% CI −0·06 to −0·02, P = 0·0005), respectively. The R2 of both models was 0·14 and 0·02, respectively.
Fig. 2.
Persistence of measles IgG antibodies after MMR vaccination. (a) The effect of time on the antibody concentrations (i.e. y in ln IU/ml) induced by the first MMR vaccination (i.e. x in years) (y = 1·3−0·2x). (b) The effect of time on the antibody concentrations induced by the second MMR vaccination (y = 0·3−0·04x). The solid line represents the fitted model and the dotted line represents the cut-off for seropositivity (ln(0·2) IU/ml).
Risk factors for measles susceptibility in individuals with one and two doses
We included 444 individuals of Dutch origin aged 2–8 years with one MMR vaccination, of whom 2·3% were seronegative, and 596 individuals of Dutch origin aged 9–20 years with two MMR vaccinations, of whom 2·9% were seronegative in risk factor analyses. Those vaccinated 4–7 years ago (vs. 0–3 years ago) were at increased risk (OR 12·8, 95% CI 1·6–102·1) of being seronegative in the analyses for once-vaccinated individuals. Age at first MMR vaccination and gender were not significant by univariate analysis. Those vaccinated 5–8 or 9–12 years ago (vs. 0–4 years ago) were at increased risk (OR 5·2, 95% CI 1·1–25·4 and OR 9·6, 95% CI 2·0–45·7, respectively) of being seronegative in the analyses for twice-vaccinated individuals. Age at first/second MMR vaccination and gender were not significant by univariate analysis.
Seroprevalence and GMC in the LVC sample
The seroprevalence in OPIs and non-OPIs was 90·6% (95% CI 83·7–97·5) and 96·2% (95% CI 94·8–97·5) with GMCs of 2·2 IU/ml (95% CI 1·4–3·4) and 2·3 IU/ml (95% CI 2·0–2·5), respectively. The following age groups were insufficiently protected according to the age-specific susceptibility targets: 1–4 years (36%) and 5–9 years (63%) for OPIs and 25–29 years (94%) for non-OPIs.
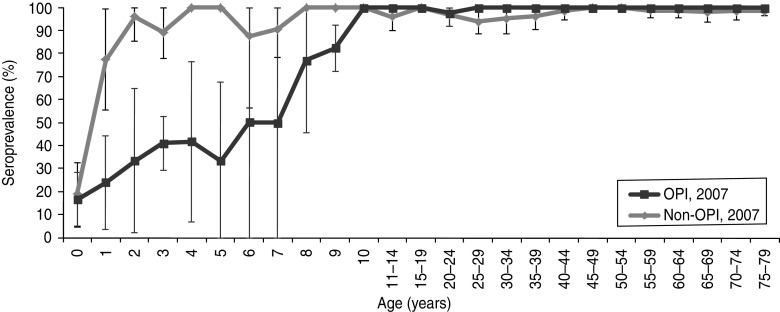
Up to the age of 9 years in 2007 the seroprevalence was much lower in OPIs compared to non-OPIs (Fig. 3). The age (from 8 years on) at which the seroprevalence started to increase was consistent with the last measles outbreak in The Netherlands (1999–2000). The increase before the age of 8 years might be explained by an increasing vaccination coverage as the OPIs consist of low (16%) and intermediate (59%) vaccination coverage denominations [21]. The seroprevalence in non-OPIs of the LVC sample did not differ from the NS.
Fig. 3.
Weighted age-specific seroprevalence of measles IgG antibody concentrations (with 95% confidence intervals) in orthodox Protestant individuals (OPIs) and non-OPIs in the low-vaccination coverage sample.
Population size at risk
The total orthodox Protestant minority consist of about 213 000 persons (as of January 2007, 1·3% of the Dutch population) [7]. If we assume that of each age cohort 1·3% are OPIs, the total number of susceptible individuals in this group would amount to 14 000 based on our seroprevalence results by age cohort. The fraction of susceptible individuals in OPIs would then be 0·067, which is above the epidemic threshold level Wallinga et al. [24] estimated, i.e. 0·043.
DISCUSSION
The overall measles seroprevalence in the Dutch population was high (96%). However, a large proportion of the socio-geographically clustered OPIs aged <10 years was found to be susceptible. We estimated that the fraction of susceptible individuals in the OPI group was above the epidemic threshold estimated by Wallinga et al. [24] for the Dutch situation. This was also the case for the 1996 study (0·049) and a few years later (i.e. 1999–2000) a large measles epidemic occurred. It is therefore surprising that no measles outbreak has yet occurred in The Netherlands in view of the large measles outbreaks that have occurred recently in neighbouring countries.
Because of the relatively large socio-geographically clustered group of unvaccinated individuals, it is likely that measles epidemics will occur in The Netherlands in the future. However, this group is probably not large enough for measles to remain endemic in the country. Bartlett [25] estimated a critical community size for measles to be between 250 000 and 300 000, below which measles is likely to fade out after a major epidemic until reintroduced from outside.
Ruijs et al. demonstrated that large differences exist in vaccination coverage (12–75%) between the various denominations within the OPI group [21]. For orthodox Protestant parents, including those accepting vaccination, religious arguments are decisive in their choice [26]. Therefore, the opportunities for healthcare professionals to positively influence vaccination coverage in this minority are limited, except for parents who refuse vaccination and merely follow the non-vaccination tradition in their environment. For this group healthcare professionals may stimulate deliberate decision-making [27].
Young infants aged between 4 and 14 months in the general population may be at risk of measles infection as maternal antibody levels had already fallen below the level of protection and the first vaccination is not administered earlier than age 14 months. Compared to the 1996 study, both the seroprevalence and GMC of maternal antibodies were lower in our study [13]. This is most likely due to a larger proportion of children born from mothers that had been vaccinated instead of being naturally infected, resulting in lower GMC of maternal antibodies. In 2011, two measles cases in persons aged <14 months in the general population were reported in The Netherlands [28]. To better protect infants prior to the first dose of MMR when the risk exposure is high, an extra measles vaccination could be offered at age 6 months. Van den Hof et al. [29] studied the measles vaccination schedule for the Netherlands that would provide optimal protection to the vaccinated population in case of a measles epidemic. The estimated percentage of susceptibles and the estimated rate at which new cases are reported in an epidemic year would be lowest when administering a measles vaccination at age 6 months and a MMR vaccination at ages 14 months and 4 years [29]. These results were based on the measles outcomes of the 1996 study [13] and the last measles outbreak in 1999–2000 [11]. With the results from our study it would probably be even more efficient to start earlier with vaccination at 6 months due to the fact that maternal antibody levels had disappeared sooner. However, it also has been suggested that measles vaccination before age 12 months is an important risk of primary, and possibly secondary, vaccine failure [30]. When deciding the optimal age for the first MMR, cellular and humoral immunological factors and the local epidemiology of measles need to be taken into account.
In the general population the 20–24, 25–29 and 30–34 years age groups (e.g. birth cohorts 1972–1986) and the 25–29 years age group (in the non-OPI group) did not reach the WHO target level for measles susceptibility. In the 25–29 years age group, measles cases were observed in 2007 [31] and 2011 [32]. A possible reason for the lower susceptibility for the 20–34 years age group is that these individuals were born around the time of the introduction of monovalent measles vaccination in 1976 when there was less circulation of wild measles virus and the coverage was sub-optimal. To prevent import of measles virus and transmission of the virus to vulnerable and susceptible individuals, incompletely vaccinated individuals (birth cohorts 1972–1986) who travel to measles-endemic regions and/or work in high-risk settings such as healthcare should be vaccinated.
Consistent with other studies [30, 33–39] our results from analysing both the persistence of antibodies and risk factors for susceptibility suggest waning of antibody concentrations after occurrence of the first and second vaccination. Importantly, GMCs remained well above the level of protection. In the 1996 study, similar results were found for individuals with one MMR vaccination [13], while no estimate was available at that time for twice-vaccinated individuals. Waning of antibody concentrations after the second MMR vaccination was much slower than after the first vaccination, indicating longer lasting immunological memory. A slightly longer half-life of measles IgG levels for two-dose vaccinees compared to one-dose vaccinees has been observed previously [30], while another study [33] did not find such a difference 3 years after the last vaccination. However, the proportion of seronegative individuals in that study was lower after the two-dose vaccination schedule than after the single-dose schedule. The low predictive values of both models did not allow projecting at what age the protective threshold of 0·2 IU/ml would be reached as the confidence intervals would be very large.
Strengths and limitations
The main strength of our study is that it used a large-scale population-based sample with a sufficient number of participants to allow (age)group-specific analyses. Furthermore, the availability of vaccination certificates made it possible to study persistence of measles IgG antibody levels and possible risk factors for measles seronegativity in once- and twice-vaccinated individuals.
Due to the relatively low response (32%), non-response bias might be present. We partly corrected for this and for the over-sampling of non-Western migrants by weighting the seroprevalence and GMC for several factors to achieve representativeness for the general population.
Based on international agreement, the cut-off value of 0·2 IU/ml was used as an indicator for protection against measles virus infection. However, other studies have shown that this value might differ between groups of individuals depending on their immune status [39, 40–41]. Our mixture modelling results (data not shown) did not show evidence for this in our study population. Last, to assess waning immunity, a cohort design would have been preferable as the results of our cross-sectional sample lacked precision.
CONCLUSION
The Netherlands is at high risk of a large measles outbreak in the orthodox Protestant minority. As religious arguments are decisive regarding vaccination decisions in this specific minority, it is unlikely that public health efforts will be successful in promoting an increase in vaccination coverage to an adequate level to prevent an outbreak. To prevent subsequent transmission to the general population, efforts to protect susceptible age groups in the population may be needed. These groups include infants aged between 4 and 14 months and incompletely vaccinated individuals born around the time of the introduction of monovalent measles vaccine (i.e. 1976).
ACKNOWLEDGEMENTS
We thank the participating Public Health Services, the municipalities involved in this study, the PIENTER project team, and all participants for their contributions. Furthermore, we also thank Helma Ruijs for her critical reading and input on the orthodox Protestant minority and Jacco Wallinga for input on calculating the population size at risk.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Simons E, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet 2012; 379: 2173–2178. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Strategic plan for measles and congenital rubella infection in the WHO European Region. Copenhagen, Denmark: WHO Regional Office for Europe, 2003. [Google Scholar]
- 3.Khetsuriani N, et al. Supplementary immunization activities to achieve measles elimination: experience of the European region. Journal Infectious Diseases 2011; 204 (Suppl. 1): 343–352. [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (ECDC). European monthly measles monitoring (EMMO). Issue 10. Stockholm: ECDC, 2012. [Google Scholar]
- 5.Muscat M. Who gets measles in Europe? Journal Infectious Diseases 2011; 204 (Suppl. 1): 353–365. [DOI] [PubMed] [Google Scholar]
- 6.Van Lier EA, et al. Vaccination coverage national immunization program the Netherlands [in Dutch]. Bilthoven, The Netherlands: National Institute for Public Health and the Environment, 2012. Report 201001001/2012. [Google Scholar]
- 7.Ruijs WL, et al. Religious subgroups influencing vaccination coverage in the Dutch Bible belt: an ecological study. BMC Public Health 2011; 11: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijkerk H, Bilkert-Mooiman MA, Houtters HJ. The immunization status of patients registered with measles during the 1987–88 epidemic [in Dutch]. Nederlands Tijdschrift voor Geneeskunde 1989; 133: 29–32. [PubMed] [Google Scholar]
- 9.Van der Zwan CW, et al. Measles in the Netherlands: epidemiology and the effect of vaccination [in Dutch]. Nederlands Tijdschrift voor Geneeskunde 1994; 138: 2390–2395. [PubMed] [Google Scholar]
- 10.Wallinga J, van den Hof S. Measles epidemiology in the Netherlands: an exploratory analysis of notification [in Dutch]. Nederlands Tijdschrift voor Geneeskunde 2000; 144: 171–174. [PubMed] [Google Scholar]
- 11.Van den Hof S, Conyn-van Spaendonck MAE, van Steenbergen JE. Measles epidemic in the Netherlands, 1999–2000. Journal Infectious Diseases 2002; 186: 1483–1486. [DOI] [PubMed] [Google Scholar]
- 12.Hahné S, et al. Measles outbreak, the Netherlands, 2008. Emerging Infectious Diseases 2010; 16: 567–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van den Hof S, et al. Sero-epidemiologic study of measles antibodies in the Netherlands, a cross-sectional study in a national sample and in municipalities with low vaccine coverage. Vaccine 2000; 18: 931–940. [DOI] [PubMed] [Google Scholar]
- 14.De Melker HE, Conyn-van Spaendonck MA. Immunosurveillance and the evaluation of national immunization programmes: a population-based approach. Epidemiology and Infection 1998; 121: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Klis FRM, et al. Second national serum bank for population-based seroprevalence studies in the Netherlands. Netherlands Journal of Medicine 2009; 67: 301–308. [PubMed] [Google Scholar]
- 16.Smits GP, et al. Development of a bead-based multiplex immunoassay for the simultaneously quantitative detection of IgG serum antibodies against measles, mumps, rubella and varicella zoster. Clinical Vaccine Immunology 2012; 19: 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen RT, et al. Measles antibody: reevaluation of protective titers. Journal Infectious Diseases 1990; 162: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 18.The R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2011. [Google Scholar]
- 19.Statistics Netherlands. Population on 1 January 2007 (http://www.cbs.nl). Accessed 1 January 2009.
- 20.Statistics Netherlands. Degree of urbaniziation per municipality (http://www.cbs.nl). Accessed 1 October 2007.
- 21.Ruijs WLM, et al. Measuring vaccination coverage in a hard to reach minority. European Journal of Public Health 2012; 22: 359–364. [DOI] [PubMed] [Google Scholar]
- 22.Bickel PJ, Doksum KA. Basic heuristics of estimation. In: Bickel PJ and Doksum KA, eds. Mathematical Statistics. Basic Ideas and Selected Topics. London: Prentice-Hall, 2001, pp. 101. [Google Scholar]
- 23.Gay NJ. The theory of measles elimination: implications for the design of elimination strategies. Journal Infectious Diseases 2004; 189 (Suppl. 1): 27–35. [DOI] [PubMed] [Google Scholar]
- 24.Wallinga J, Heijne JCM, Kretzschmar M. A measles epidemic threshold in a highly vaccinated population. PLoS Medicine 2005; 2: e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett MS. The critical community size for measles in the United States. Journal of the Royal Statistical Society. Series A (General) 1960; 123: 37–44. [Google Scholar]
- 26.Ruijs WL, et al. How orthodox protestant parents decide on the vaccination of their children: a qualitative study. BMC Public Health 2012; 12: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruijs WL, et al. How healthcare professionals respond to parents with religious objections to vaccination: a qualitative study. BMC Health Services Research 2012; 12: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van 't Klooster TM, de Melker HE. The national immunisation programme in the Netherlands: developments 2012. Bilthoven, The Netherlands: National Institute for Public Health and the Environment, 2012. Report 201001002/2012. [Google Scholar]
- 29.Van den Hof S, et al. Options for improvement of the Dutch measles vaccination schedule. Vaccine 2003; 21: 721–724. [DOI] [PubMed] [Google Scholar]
- 30.Lee MS, et al. Measles seroepidemiology and decay rate of vaccine-induced measles IgG titers in Taiwan, 1995–1997. Vaccine 2007; 19: 4644–4651. [DOI] [PubMed] [Google Scholar]
- 31.Wetsteyn JCFM, et al. Measles outbreak at the intensive care [in Dutch]. Nederlands Tijdschrift voor Geneeskunde 2008; 152: 2032–2036. [PubMed] [Google Scholar]
- 32.Van den Hoek A, et al. Two cases of mild IgM-negative measles in previously vaccinated adults, the Netherlands, April and July 2011. Eurosurveillance 2011; 16: pii = 20028. [PubMed] [Google Scholar]
- 33.Poethko-Müller C, Mankertz A. Sero-epidemiology of measles-specific IgG antibodies and predictive factors for low or missing titers in a German population-based cross-sectional study in children and adolescents (KiGGS). Vaccine 2011; 29: 7949–7959. [DOI] [PubMed] [Google Scholar]
- 34.Christenson B, Böttiger M. Measles antibody: comparison of long-term vaccination titres, early vaccination titres and naturally acquired immunity to and booster effects on the measles virus. Vaccine 1994; 12: 129–133. [DOI] [PubMed] [Google Scholar]
- 35.Amela C, Pachón I, de Ory F. Evaluation of measles, mumps and rubella immunisation programme in Spain by using a sero-epidemiological survey. European Journal of Epidemiology 2003; 18: 71–79. [DOI] [PubMed] [Google Scholar]
- 36.Mossong J, Putz L, Schneider F. Seroprevalence of measles, mumps and rubella antibodies in Luxembourg: results from a national cross-sectional study. Epidemiology and Infection 2003; 132: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davidkin I, et al. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. Journal of Infectious Diseases 2008; 197: 950–956. [DOI] [PubMed] [Google Scholar]
- 38.Rota MC, et al. Measles serological survey in the Italian population: interpretation of results using a mixture model. Vaccine 2008; 26: 4403–4409. [DOI] [PubMed] [Google Scholar]
- 39.Theeten H, et al. Are we hitting immunity targets? The 2006 age-specific seroprevalence of measles, mumps, rubella, diphtheria and tetanus in Belgium. Epidemiology and Infection 2011; 139: 494–504. [DOI] [PubMed] [Google Scholar]
- 40.Parker RA, Erdman DD, Anderson LJ. Use of mixture models in determining laboratory criterion for identification of seropositive individuals: application to parvovirus B19 serology. Journal of Virological Methods 1990; 27: 135–144. [DOI] [PubMed] [Google Scholar]
- 41.Vyse AJ, et al. Interpreting serological surveys using mixture models: the seroepidemiology of measles, mumps and rubella in England and Wales at the beginning of the 21st century. Epidemiology and Infection 2006; 134: 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]