SUMMARY
Helicobacter pylori culture on gastric biopsy was performed on 4964 subjects aged <18 years from 1988 to 2007 at a central laboratory in Brussels. The total number of biopsies increased markedly from 941 in 1988–1993 to 1608 in 2004–2007. Biopsies were repeated at least once for 922 subjects (603 initially negative and 319 initially positive for H. pylori). Persistence rate of H. pylori at 1 year after initial positive biopsy was greater in the 1998–2007 cohort than in the 1988–1997 cohort (72·7% vs. 45·8%, P = 0·002), suggesting a tailored selection of candidates for biopsy with non-invasive tests (13C urea breath test). Of 68 subjects initially positive and re-examined subsequently after a documented cure, re-infection/relapse rate was 48·6% within 5 years post-elimination of H. pylori. Acquisition rate over 10 years follow-up in the initially negative cohort (603 patients) was 38·7% (re-infection/relapse vs. acquisition: P < 0·001). Multivariate analysis showed a fourfold greater risk of H. pylori acquisition in children of non-European origin vs. European origin (P < 0·001). Clarithromycin and metronidazole susceptibility were determined in 226 and 223 paired positive cultures in cases of re-infection/relapse or persistence. An initial non-susceptibility profile was highly predictive of a subsequent non-susceptibility profile, and the non-susceptible proportion increased markedly from 13·3% to 21·2% for clarithromycin (P < 0·001) and from 27·3% to 35·0% for metronidazole (P = 0·014), with no difference regarding European or non-European origin.
Key words: Epidemiology, Helicobacter pylori, laboratory tests, modelling
INTRODUCTION
Helicobacter pylori, a Gram-negative bacterium found on the luminal surface of the gastric epithelium, induces chronic inflammation of the underlying mucosa. The infection is usually contracted during childhood or adolescence [1] and tends to persist lifelong unless treated. Its prevalence increases with older age and lower socioeconomic status and varies markedly around the world. H. pylori is responsible for the development of duodenal and/or gastric ulcers (reported to develop in 1–10% of infected patients) as well as a co-factor of gastric cancer (in 0·1–3%) and gastric mucosa-associated lymphoid-tissue lymphoma (in <0·01%) [2]. However, the majority of patients with H. pylori infection will not have any clinically significant complication.
A minority of patients with dyspepsia, infected with H. pylori, have an underlying ulcer disease [3]. It is essential to determine the H. pylori status with regard to adequate therapy. Two types of tests are available: invasive (endoscopic) tests and non-invasive (non-endoscopic) tests.
In our previous paper [1], a large retrospective observational study was performed by extracting data from a laboratory database covering a third of Brussels and the surrounding population. The study included subjects attending several digestive endoscopy centres in Brussels during a 20-year period (1988–2007). The criterion for entry in the database was the initial screening of H. pylori infection by culture of gastric biopsy, performed at a central clinical microbiology laboratory. The higher prevalence in older age groups of immigrants or in children born to immigrant families compared to the population of Belgian origin is thought to reflect a cohort effect related to poorer living conditions of children in previous decades.
The same database was used to analyse the child population (aged <18 years at initial biopsy) whenever a subsequent endoscopy was recorded during the 20-year study period.
The main objectives are to describe:
-
•
The risk of persistence of H. pylori after a first positive result.
-
•
The risk of acquiring H. pylori colonization in subjects with an initial negative result.
-
•
The risk of re-infection/relapse in subjects with successful ‘eradication’ treatment.
-
•
The effect of age, gender, geographical origin, time period on the persistence, acquisition or re-infection/relapse risk of H. pylori colonization.
METHODS
Patients
The study group was derived from the initial database as published in our previous paper [1] and restricted to paediatric patients (<18 years). Briefly, the source of data collection was the medical laboratory of Brugmann University Hospital in Brussels. Since 1984, the hospital has served as the H. pylori reference centre for a large number of institutions (3147/9175 hospital beds in Brussels) [4]. Patients originated from several Belgian cities, with the majority (80%) from Brussels. Administrative (including geographical origin) and microbiological data were collected for each patient. The indication for gastrointestinal endoscopy included various gastrointestinal complaints not necessarily associated with H. pylori. Only patients with at least one interpretable result (either positive or negative) by H. pylori culture were included.
Data on the evolution of antibiotic treatment over this lengthy period have been published previously in 2001, 2007 and 2011 [5–7]. Before 1995, treatments were usually dual therapies [amoxicillin + (clarithromycin or metronidazole)], tailored to the antimicrobial susceptibility from the early 1990s. Proton pump inhibitors (omeprazole) were then added to the regimens before switching to sequential treatments gradually from 2007 [7].
Biopsy specimens and H. pylori isolation
The Brussels laboratory receives gastric (antrum±fundus) biopsy specimens for culturing of H. pylori. Briefly, each biopsy specimen was ground in sterile distilled water. The final suspension was inoculated on in-house selective agar plates. The plates were then incubated for 3–7 days at 37°C under a humid, micro-aerobic atmosphere. The culturing was extended to 10 days if there was a known positive urea test. The typical growth of H. pylori was checked after 3 days and on a daily basis thereafter. Susceptibility testing was performed mainly using the disc diffusion method for several antibiotics including clarithromycin and metronidazole as described previously [8, 9].
Statistical analysis
All data related to the patients (demography, H. pylori culture) were transferred from the laboratory information system to Excel 2000 (Microsoft, USA). The database was analysed with the Statistical Package for the Social Sciences version 18.0.1 (PASW Statistics, USA).
Differences of medians for continuous variables were analysed by Mann–Whitney U test. Differences of ⩾2 proportions for categorical variables were analysed, respectively, with Fisher's exact test and Pearson's χ2 test.
Survival function: a database for survival analysis was constructed by aggregating all subjects monitored at least once after their initial screening biopsy.
Persistence rate was measured in subjects with an initial positive H. pylori result. Time to event was defined as the interval between initial biopsy and the timing of the first negative H. pylori culture. Subjects remaining positive were censored at their last positive H. pylori culture.
Acquisition and re-infection/relapse rates of H. pylori infection were measured, respectively, in subjects having an initial negative H. pylori result (acquisition) and in subjects initially infected after a first documented cure (re-infection/relapse). Time to event (diagnosis of H. pylori infection) was defined as the interval between initial biopsy (acquisition) or first cure (re-infection/relapse) and the timing of first subsequent positive H. pylori culture. Subjects remaining negative were censored at their last negative H. pylori culture.
Comparison of survival tables was performed with the Kaplan–Meier method and the P value was determined by log-rank test.
Cox regression analysis [10] with proportional hazards assumption was performed to measure the effect of variables on time-dependent risk of H. pylori infection in the three groups mentioned above (Persistence, Acquisition, and Re-infection/relapse).
All statistical tests were two-sided P values without correction for multiplicity of tests.
RESULTS
During the study period (Table 1), 4964 patients initially aged <18 years underwent an upper gastrointestinal endoscopy with gastric biopsy (antrum±fundus) and H. pylori culture. Median age was 7·9 years with an interquartile range of 3·3–12·0 years. Slightly more than a third (39·9%) of subjects were aged <6 years and a quarter (24·9%) were aged 12–17 years. Most patients (70·3%) were of non-European origin and sex ratio was close to unity. The data collection covered the period between January 1988 and December 2007, with a steady increase in the number of subjects during each 5-year period. Out of the initial group of 4964 patients, at least one subsequent upper gastrointestinal endoscopy with gastric biopsy and H. pylori culture was obtained in 922 subjects (18·6%). The majority (586/922, 65·6%) were examined twice while repeated biopsies with H. pylori culture reaching ⩾7 were performed for 25 patients.
Table 1.
Characteristics
| Patients, N | 4964 | |||
| Age (years) at initial biopsy, median (IQR) | 7·85 (3·25–11·98) | |||
| <6, n (%) | 1982 (39·9%) | |||
| 6–11, n (%) | 1743 (35·1%) | |||
| 12–17, n (%) | 1236 (24·9%) | |||
| Missing values, n (%) | 3 (0·1%) | |||
| M/F ratio (% males) | 2551/2413 (51·4%) | |||
| Time span of the collection of data, range | Jan. 1988 | Dec. 2007 | ||
| Period, n (%) | ||||
| 1988–1993 | 941 | (19·0%) | ||
| 1994–1998 | 1086 | (21·9%) | ||
| 1999–2003 | 1329 | (26·8%) | ||
| 2004–2007 | 1608 | (32·4%) | ||
| Without follow-up, n (%) | 4042 | (81·4%) | ||
| Repeated biopsy, n (%) | ||||
| 1 | 586 | }922 | (11·8%) | }18.6% |
| 2 | 153 | (3·1%) | ||
| 3 | 82 | (1·7%) | ||
| 4 | 46 | (0·9%) | ||
| 5 | 18 | (0·4%) | ||
| 6 | 12 | (0·2%) | ||
| ⩾7 | 25 | (0·5%) | ||
| Non-European, n (%) | 3489 | (70·3%) | ||
IQR, Interquartile range.
The comparison between the patients stratified according to their initial H. pylori status shows that patients initially non-infected were 3·7 years younger than those with an initial positive H. pylori culture (Table 2). The proportion of infected children increased with age (18·2% in children aged <6 years and 49·3% in adolescents aged 12–17 years) (P < 0·001). Girls were more often infected than boys (57% vs. 43%) (P < 0·001). The prevalence of H. pylori infection in the investigated children strongly decreased over the different time periods. Non-European origin was documented in 86·5% of children with an initial positive H. pylori result and in 60·7% of children with an initial negative result (P < 0·001). Antibiotic resistance was documented in 12·2% of cases for clarithromycin and in 20·9% of cases for metronidazole.
Table 2.
Baseline characteristics in 922 patients with repeated gastric biopsies and Helicobacter pylori culture
| Initial result of Helicobacter pylori culture | P value | ||
|---|---|---|---|
| Negative (n = 603) | Positive (n = 319) | ||
| Age (years), median (IQR) | 7·46 (3·01–11·61) | 11·13 (7·56–14·17) | <0·001 |
| <6, n/N (%) | 251/307 (81·8%) | 56/307 (18·2%) | <0·001 |
| 6–11, n/N (%) | 214/343 (62·4%) | 129/343 (37·6%) | |
| 12–17, n/N (%) | 138/272 (50·7%) | 134/272 (49·3%) | |
| M/F ratio (% male) | 340/263 (56·4%) | 137/182 (42·9%) | <0·001 |
| Period, n/N (%) | |||
| 1988–1993 | 150/258 (58·1%) | 108/258 (41·9%) | <0·001 |
| 1994–1998 | 160/253 (63·2%) | 93/253 (36·8%) | |
| 1999–2003 | 160/240 (66·7%) | 80/240 (33·3%) | |
| 2004–2007 | 133/171 (77·8%) | 38/171 (22·2%) | |
| Non-European (%) | 366 (60·7%) | 276 (86·5%) | <0·001 |
| Clarithromycin R/total (% R) | n.a. | 29/238 (12·2%) | n.a. |
| Metronidazole R/total (% R) | n.a. | 57/273 (20·9%) | n.a. |
IQR, Interquartile range; n.a., not applicable.
R/total = ratio of number of antibiotic non-susceptible strains and total number of antibiograms.
% R = per cent non-susceptible.
The effect of four variables (age, gender, period, geographical origin) on the time course of H. pylori acquisition, persistence and re-infection/relapse during follow-up was analysed with Cox proportional hazards regression (Table 3). Non-European origin had a marked effect on the hazard of H. pylori acquisition (P < 0·001). None of the four variables had a significant effect on persistence rate after an initial positive biopsy. Female gender had a significant decreasing effect on the relapse/re-infection hazard rate (P = 0·028). Antibiotic resistance (clarithromycin and metronidazole) was also introduced in the multivariate analysis: neither had a significant effect on persistence or re-infection/relapse of H. pylori infection (data not shown).
Table 3.
Time-dependent multivariate analysis of Helicobacter pylori risk
| Nature of event … | H. pylori acquisition after initial negative H. pylori biopsy | H. pylori persistence after initial positive H. pylori biopsy | H. pylori re-infection or relapse after cure |
|---|---|---|---|
| No. events/initial no. at risk (%) … | 56/603 (9·3%) | 195/319 (61·1%) | 20/68 (29·4%) |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age at initial biopsy | 1·013 (0·996–1·030) | 1·001 (0·995–1·007) | 0·945 (0·853–1·047) |
| Female sex | 0·816 (0·475–1·403) | 0·891 (0·866–1·192) | 0·343 (0·132–0·892)* |
| Yearly period at initial biopsy | 0·988 (0·936–1·044) | 0·979 (0·952–1·007) | 0·983 (0·879–1·100) |
| Non-European origin | 3·686 (1·805–7·525)† | 0·830 (0·564–1·221) | 0·527 (1·189–1·465) |
HR, Hazard ratio; CI, confidence interval.
P < 0·001.
P = 0·028.
Comparison of H. pylori antibiotic susceptibility profile at initial and last biopsy demonstrated a strong association of initial susceptibility with last susceptibility, with a pattern independent of geographical origin (European vs. non-European) (Table 4). In paired biopsy samples of the same subject, when initial biopsies contained non-susceptible H. pylori strains to clarithromycin or metronidazole, 83.3% and 82.0% of H. pylori obtained at last biopsy, respectively, were also non-susceptible to either antibiotic. By contrast, when initial biopsies contained susceptible H. pylori strains to either antibiotic, 10·2% strains for clarithromycin and 12·6% strains for metronidazole became non-susceptible at last biopsy. Within the 226 paired clarithromycin susceptibility tests, it can be calculated (by rearranging the numbers in last column of Table 4) that the number of non-susceptible H. pylori increased from 30 (13·3%) initial positive results to 48 (21·2%) last positive results (P = 5·08 × 10−4). In parallel, within the 223 paired metronidazole susceptibility tests, the number that were non-susceptible increased from 61 (27·3%) to 78 (35·0%) (P = 1·42 × 10−2).
Table 4.
Comparison of Helicobacter pylori non-susceptibility in paired samples at initial and at last biopsies in subjects stratified by geographical origin
| Clarithromycin non-susceptibility | |||||
|---|---|---|---|---|---|
| Initial biopsy | Last biopsy | Total | |||
| European | Non-European | ||||
| Non-susceptible | 100·0% (4/4) | 80·8% (21/26) | 83·3% (25/30) | ||
| Susceptible | 6·7% (2/30) | 10·7% (21/196) | 10·2% (23/226) | ||
| Fisher's exact test | P < 0·001 | P < 0·001 | P < 0·001 | ||
| OR (95% CI) | Indeterminate | 35·0 (11·9–102·6) | 44·1 (15·4–126·4) | ||
| Metronidazole non-susceptibility | |||||
| Initial biopsy | European | Non-European | Total | ||
| Non-susceptible | 80·0% (8/10) | 82·4% (42/51) | 82·0% (50/61) | ||
| Susceptible | 17·9% (5/28) | 11·8% (23/195) | 12·6% (28/223) | ||
| Fisher's exact test | P = 0·001 | P < 0·001 | P < 0·001 | ||
| OR (95% CI) | 18·4 (3·0–114·3) | 34·9 (15·0–80·9) | 31·7 (14·8–67·9) | ||
OR, Odds ratio; CI, confidence interval.
Percentages of susceptibility and of non-susceptibility to either antibiotic at initial biopsy did not differ in European children and in non-European children (P > 0·10).
In the group of 319 subjects initially positive for H. pylori and biopsied at least once subsequently, the risk of persistent H. pylori infection at 1 year after an initial positive biopsy was greater in the last period 1998–2007 (72·7%) than in the preceding period 1988–1997 (45·8%) (P = 1·96 × 10−3). The proportion of persistence decreased markedly to 16·5% at 10 years (Fig. 1), with the curves of both periods overlapping.
Fig. 1.
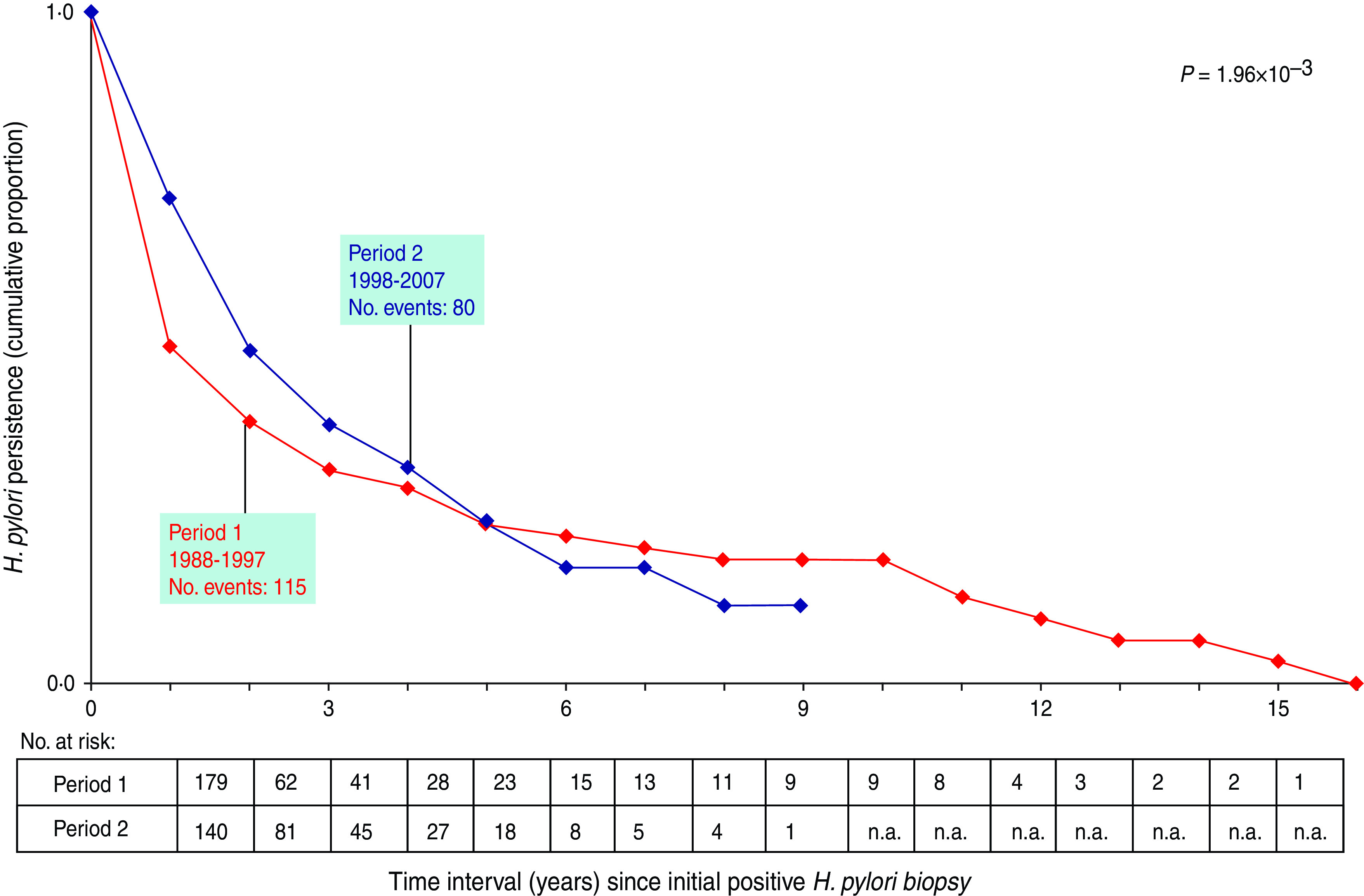
Comparison of Helicobacter pylori persistence in children (<18 years) according to the period of initial biopsy. Event = first negative H. pylori biopsy after an initial positive biopsy. n.a., Not available.
Of the 603 subjects initially free of H. pylori infection, 38·7% became infected within 12 years (Fig. 2, acquisition group). In 68 subjects with a documented cure of H. pylori infection, 48·6% relapsed within 5 years of follow-up (Fig. 2, re-infection/relapse) (P = 3·8 × 10−6).
Fig. 2.
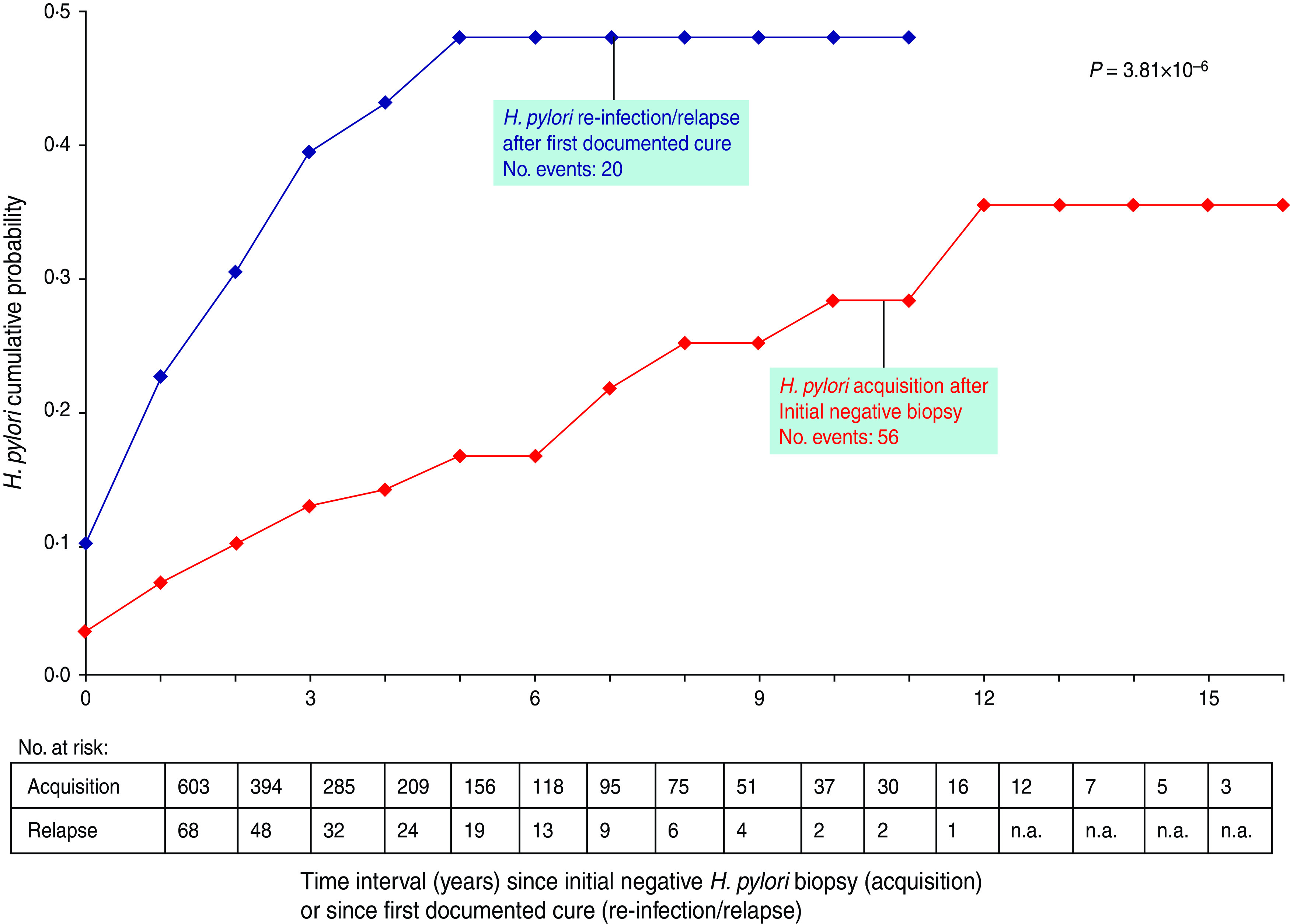
Comparison of Helicobacter pylori acquisition in a cohort of 603 subjects with an initial negative biopsy and of H. pylori re-infection/relapse in a cohort of 68 subjects with an initial positive biopsy after documented cure. Event = first positive H. pylori biopsy after initial negative biopsy (acquisition) or after first documented cure (re-infection/relapse). n.a., Not available.
DISCUSSION
Persistence of H. pylori infection after an initial positive biopsy
In clinical practice, the term ‘eradication’ is used for negative H. pylori tests at least 4–8 weeks after treatment [2]. In fact, many studies document persistence of H. pylori infection in the follow-up of patients even after appropriate treatment [11–16]. In our clinical team, the antibiotic protocol evolved according to successive guidelines, and remained based on a combination of amoxicillin+clarithromycin or metronidazole+omeprazole. The proportion of efficient treatment increased markedly in our last data, 81·9% being adequately treated as documented by a negative 13C breath test 8 weeks after treatment [7]. Other large series show that over one third of patients do not eliminate their H. pylori infection with antibiotic treatment [17].
The apparent greater risk of persistence of H. pylori infection in the present analysis during the more recent period (1998–2007 vs. 1988–1997) was, therefore, not an effect of decreased antibiotic efficacy, but probably resulted from a modification of patient selection. With the introduction of non-invasive testing such as the 13C-urea breath test [18], the indication of H. pylori culture on gastric biopsy is more restricted, with a better tailoring of patients in the recent period (1998–2007) vs. the preceding period (1988–1997). Our data should not be considered representative either of the general population or of the subpopulation infected by H. pylori, but only of the subgroup of subjects exposed to gastric biopsy and H. pylori culture in our Brussels series. Only a small percentage (18·6%) of subjects were monitored with repeated endoscopies after the first diagnosis of H. pylori and the majority of the initial cases were not followed up by this invasive technique. A non-invasive technique such as the 13C-urea breath test is preferred since its validation in the last decade of the 20th century.
Our patient group is representative of everyday clinical practice. H. pylori initially isolated at biopsy remained positive in many cases (18·1% as estimated by the 13C-urea breath test [7]), and this persistence is confirmed in most cases when H. pylori culture on gastric biopsies are performed. Despite a clinical follow-up of patients according to current international guidelines, it appears that around a fifth of patients remain infected, at least after the first antibiotic therapy. More encouraging is when some of these subjects are re-evaluated later (i.e. after subsequent antibiotic therapies), the proportion of positive H. pylori cultures decreased markedly. The cumulative proportion of elimination of the pathogen as documented by gastric biopsy and H. pylori culture reached 84% at 10 years. Spontaneous cure of H. pylori infection – without antibiotic therapy – is rare.
Compared to the urea breath test and histology, our in-house culture method yielded 98% sensitivity and 100% specificity [8] and, moreover, it was recently found to be equally efficient and more selective than two commercially available media [9]. The performance of these diagnostic tests strongly supports the view that the data are expected to be similar whenever invasive or non-invasive tests are performed.
Acquisition and relapse rates of H. pylori infection
H. pylori infection is mainly acquired during childhood and adolescence. In our previous analysis [1] based on initial results of H. pylori culture, an annual incidence of H. pylori of 15% per person-year was estimated in subjects of non-European origin in whom there was an indication for gastric biopsy and H. pylori culture. A fourfold lower incidence was calculated in subjects of European origin. The present data, based on follow-up (time 0 and subsequent H. pylori culture), show that an initial negative gastric biopsy does not predict a long-term absence of infection: 39% became H. pylori positive during a 10-year follow-up (Fig. 2). This result corresponds to ∼4% annual incidence rate of infection [1], close to the incidence reported in a recent review [11]. The multivariate analysis shows that the risk of acquisition is 3·7 times greater in the population of non-European origin: this result confirms strongly the fourfold greater risk of H. pylori infection previously calculated in non-European subjects [1).
After a documented cure of infection, about half of the subjects relapsed within 5 years of follow-up. Again, due to type of selection of patients, the extrapolation of this percentage should be limited to the subjects for whom there was a clinical indication for biopsy and H. pylori culture. In these two groups, the pattern of acquisition or re-infection/relapse was similar in both periods (1988–1997 vs. 1998–2007), with no statistically significant difference (data not shown).
Effect of age, gender, time period, geographical origin
In our previous analysis, the risk of H. pylori-positive biopsy was associated with geographical origin (non-European origin – immigrants mainly from Maghreb in Brussels). The same association was observed in the present analysis: non-European origin had a significant effect on the acquisition rate of H. pylori infection. Besides this association, a decreased risk of re-infection/relapse in females was documented, with a modest P value (P = 0·028). Due to the multiplicity of association tests, this result requires further confirmation and should not be considered definitive.
Limitations of the study and new questions
Our analysis clearly belongs to descriptive epidemiology and, consequently, is limited to generation of a hypothesis not to allowing definitive conclusions. The analysis of data was based on a laboratory information system database: it is not possible to infer to what extent this population is representative of the entire group of patients infected with H. pylori. Clinical outcomes other than those linked to H. pylori infection clearly influence the recruitment in the database.
Nevertheless, the main message seems sufficiently strong to be accepted: if elimination of H. pylori by current treatment is the objective, eradication of this pathogen over the long term remains uncertain.
One of the long-term complications of H. pylori infection is gastric carcinoma [19]. The elevated long-term recurrence of infection might explain the paradoxically low preventive effect of H. pylori elimination on gastric carcinoma [20]. Even if it is well established that antimicrobial therapy reduces markedly the recurrence of duodenal ulcer in H. pylori-positive subjects [21], our data suggest that repeated follow-up testing over the long term is indicated in order to detect the persistence or recurrence of the pathogen after several years. Non-invasive tests (13C-urea breath test and faecal antigen) allow easier regular repeated long-term follow-up of patients after a successful treatment of H. pylori. Furthermore, a multi-centre prospective study should be organized to confirm these results.
Our data cannot distinguish whether the infection is due to the persistence or recurrence of the initial strain, or to a re-infection with a different one. As there was an elevated risk association of antibiotic resistance in the first biopsy and in the last one, these data suggest strongly that a relapse occurred most of the time rather than a re-infection.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Miendje Deyi VY, et al. Marching cohort of Helicobacter pylori infection over two decades (1988–2007): combined effects of secular trend and population migration. Epidemiology and Infection 2011; 139: 572–580. [DOI] [PubMed] [Google Scholar]
- 2.McColl KE. Clinical practice. Helicobacter pylori infection. New England Journal of Medicine 2010; 362: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 3.Perri F, Andriulli A. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. New England Journal of Medicine 2000; 342: 589–590. [PubMed] [Google Scholar]
- 4.Taymans M. Zone of Influence of Hospitals in the Region of Brussels. IRIS editions, Brussels, Economy and Social (BRES), 1991, pp. 53. [Google Scholar]
- 5.Bontems P, et al. Twelve year observation of primary and secondary antibiotic-resistant Helicobacter pylori strains in children. Pediatric Infectious Disease Journal 2001; 20: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 6.Cadranel S, et al. Improvement of the eradication of Helicobacter pylori gastritis in children by adjunction of omeprazole to a dual antibiotherapy. Acta Paediatrica 2007; 96: 82–86. [DOI] [PubMed] [Google Scholar]
- 7.Bontems P, et al. Sequential therapy versus tailored triple therapies for Helicobacter pylori infection in children. Journal of Pediatric Gastroenterology and Nutrition 2011; 53: 646–650. [DOI] [PubMed] [Google Scholar]
- 8.Miendje Deyi VY, Van Den Borre C, Fontaine V. Comparative evaluation of 3 selective media for primary isolation of Helicobacter pylori from gastric biopsies under routine conditions. Diagnostic Microbiology and Infectious Disease 2010; 68: 474–476. [DOI] [PubMed] [Google Scholar]
- 9.Miendje Deyi VY, et al. Multicenter survey of routine determinations of resistance of Helicobacter pylori to antimicrobials over the last 20 years (1990 to 2009) in Belgium. Journal of Clinical Microbiology 2011; 49: 2200–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinbaum DG, Klein M. Survival Analysis. A Self-learning Text. New York: Springer-Verlag, 2011, pp. 324. [Google Scholar]
- 11.Moya DA, Crissinger KD. Helicobacter pylori persistence in children: distinguishing inadequate treatment, resistant organisms, and reinfection. Current Gastroenterology Reports 2012; 14: 236–242. [DOI] [PubMed] [Google Scholar]
- 12.Maconi G, et al. Predictors of long-term outcome of functional dyspepsia and duodenal ulcer after successful Helicobacter pylori eradication – a 7-year follow-up study. European Journal of Gastroenterology and Hepatology 2009; 21: 387–393. [DOI] [PubMed] [Google Scholar]
- 13.Luther J, Chey WD, Saad RJ. A clinician's guide to salvage therapy for persistent Helicobacter pylori infection. Hospital Practice (Minneapolis) 2011; 39: 133–140. [DOI] [PubMed] [Google Scholar]
- 14.Silva FM, et al. Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: a 5-year follow-up. Helicobacter 2010; 15: 46–52. [DOI] [PubMed] [Google Scholar]
- 15.Mansour-Ghanaei F, et al. Recurrence of Helicobacter pylori infection 1 year after successful eradication: a prospective study in Northern Iran. Medical Science Monitor 2010; 16: CR144–CR148. [PubMed] [Google Scholar]
- 16.Ryu KH, et al. Reinfection rate and endoscopic changes after successful eradication of Helicobacter pylori. World Journal of Gastroenterology 2010; 16: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oderda G, et al. Results from the pediatric European register for treatment of Helicobacter pylori (PERTH). Helicobacter 2007; 12: 150–156. [DOI] [PubMed] [Google Scholar]
- 18.Cadranel S, et al. Detection of Helicobacter pylori infection in children with a standardized and simplified 13C-urea breath test. Journal of Pediatric Gastroenterology and Nutrition 1998; 27: 275–280. [DOI] [PubMed] [Google Scholar]
- 19.Peeters M, et al. Guideline for the management of oesophageal and gastric cancer: scientific information to the Oncology College. Federal Centre of Public Health, 2008, Report KCE No. 75B.
- 20.Mazzoleni LE, Francesconi CF, Sander GB. Mass eradication of Helicobacter pylori: feasible and advisable? Lancet 2011; 378: 462–464. [DOI] [PubMed] [Google Scholar]
- 21.Hentschel E, et al. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. New England Journal of Medicine 1993; 328: 308–312. [DOI] [PubMed] [Google Scholar]


