SUMMARY
Recent much-publicized increases in pertussis case reports in some countries with high vaccine coverage have raised concerns about its current and future control. The ubiquity of this trend, however, remains unexamined. In an attempt to paint a global picture, we used case counts to determine which countries experienced statistically significant trends in incidence over the past two decades and to map changes in incidence during this period. These data reveal that pertussis resurgence is not a universal phenomenon. The heterogeneity in incidence trends, even in countries with superficially similar demography, socioeconomic conditions and vaccination programmes, is striking and requires explanation. In this opinion piece, we review and assess the multifaceted proposed explanations incorporating evolution, population dynamics, and the details of immunization programmes. While we do not solve the riddle that is pertussis epidemiology, we highlight critical aspects that are likely to hold the key to understanding its worldwide epidemiology.
Key word: Bordetella pertussis
INTRODUCTION
In the middle decades of the twentieth century, the prospects for control – even eradication – of pertussis seemed promising. As a consequence of widespread paediatric immunization using whole cell pertussis (wP) vaccines that began in the 1940s and 1950s, the global prevalence of pertussis was reduced substantially by the 1970s. As a typical example, mean incidence rates in Canada were brought down from an average of about 160 cases/100 000 people in 1934–1943 to roughly 11/100 000 by 1974–1983 [1]. Prior to the implementation of pertussis immunization programmes, this disease, caused primarily by the bacterium Bordetella pertussis, was a scourge of childhood, causing about 73 000 deaths from 1922 to 1931 in the USA, most of whom were infants; in contrast, the toll was reduced to 56 deaths between 1983 and 1992 [2]. As suggested by its common name, ‘whooping cough’, pertussis often manifests as violent paroxysms of coughing followed by a whooping noise as the patient struggles to catch their breath, commonly followed by post-tussive vomiting; mortality is typically associated with bronchopneumonia [3].
Despite the emphatic success of vaccination programmes in contributing to long-term, historical reductions in morbidity and mortality, two worrisome factors have led to increasing concern surrounding pertussis mitigation.
First, despite pertussis being ostensibly a vaccine-preventable disease, the annual burden of morbidity and mortality, particularly in the developing world, continues to be sobering: in 1999, there were an estimated 48·5 million cases in children and 295 000 deaths attributed to pertussis, with disability-adjusted life years exceeding that of lung cancer in 2000 [4].
Second, pertussis appears to be resurgent in a number of countries in the developed world that boast high immunization coverage. Starting in the 1980s, increased incidence rates were reported in the USA [5], Israel [6], Australia [7, 8], and Poland [9], along with other high vaccine coverage countries [10, 11]. In addition to increases in mean incidence rates, shifts in age-specific incidence have been observed, with a general trend towards higher representation by adolescents and adults [12].
The cost that pertussis continues to inflict, in terms of mortality and morbidity, provides sufficient reason for continuing to work to illuminate its epidemiology. Perhaps more compelling, however, is the possibility that apparent resurgences are harbingers of more severe upswings in incidence. Without a clear understanding of the nature and causes of the current epidemiology, we will be unable to anticipate and prevent future outbreaks. In addition, pertussis may serve as a useful case study to explore more general questions related to the control of vaccine-preventable diseases.
While a plethora of hypotheses have been proposed to explain increases in specific countries, evaluating their applicability and generality requires their confrontation with incidence data. Specifically, parsimonious explanations need to be consistent with large-scale pertussis epidemiology, rather than solely shedding light on patterns in one specific location. A comprehensive global picture of incidence trends may help identify whether increased reports in different countries share a common cause, or, alternatively, that there are unique drivers in each setting. Although the literature reflects a general sense that developed countries are experiencing a resurgence while developing countries continue to suffer high but declining incidences, more precisely defining the extent and magnitude of recent changes in prevalence may help to determine which hypothesis or combination of hypotheses best explains recent trends.
Here, we describe the changes in country-specific pertussis burdens on a worldwide basis during the past two decades. Using a combination of pertussis case data compiled by the World Health Organization (WHO) and the European Commission, we calculate absolute incidence rates (annual cases/100 000 individuals), and use these rates to detect significant trends in pertussis burden on a per-country basis over the past two decades. Although incidence data are known to be incomplete and subject to country-specific variations in reporting fidelity, these data allow for a first pass at characterizing the magnitude and extent of recent changes.
PERTUSSIS, 1990–2010
Country-specific pertussis case counts and vaccination coverage estimates for the third dose of diphtheria toxoid, tetanus toxoid, and pertussis vaccine (DTP3) for all years between 1990 and 2010 were obtained from the WHO database [13]. These data were reconciled where appropriate using supplemental information from the European Commission [14]. Incidences in cases/100 000 individuals were calculated using annual population data from The World Bank's World Development Indicators [15].
To detect statistically significant increases or decreases in pertussis incidence between 1990 and 2010, we calculated Kendall's tau rank correlation for high-coverage countries with nearly complete case count data, designated as countries with mean DTP3 vaccine uptakes of >80% and >80% complete case count data. For simplicity, we restricted our analysis to countries with populations >5 million individuals. The annual rate of change, defined as the ratio of the incidence in a given year divided by the incidence in the previous year, was also calculated; this value is a measure of the year-over-year incidence growth rate, with values >1 indicating positive growth and values <1 indicating a decrease in per capita incidence.
These data tell a story of substantial epidemiological heterogeneity across countries. Based on our trend analysis, 10 of the 54 countries that met our inclusion criteria had a significantly increasing trend in pertussis incidence during this period, 27 had a significantly decreasing trend, and 17 did not exhibit any significant trend (Fig. 1, Supplementary Tables S1 and S2). These results were robust to the inclusion or exclusion of the European Commission data (Supplementary Table S3). The qualitative result of a mix of increasing, stationary, and decreasing trends in high-coverage countries continues to hold if more stringent inclusion criteria are applied (Supplementary Figs S1, S2). Even in countries exhibiting a significant positive (negative) trend, there were many years in which the annual rate of change was negative (positive), and there was substantial variation in these rates. Although this is unsurprising given the known multiennial periodicity of pertussis [16–18], it highlights the fact that year-over-year changes in case counts can run substantially counter to long-term trends.
Fig. 1.
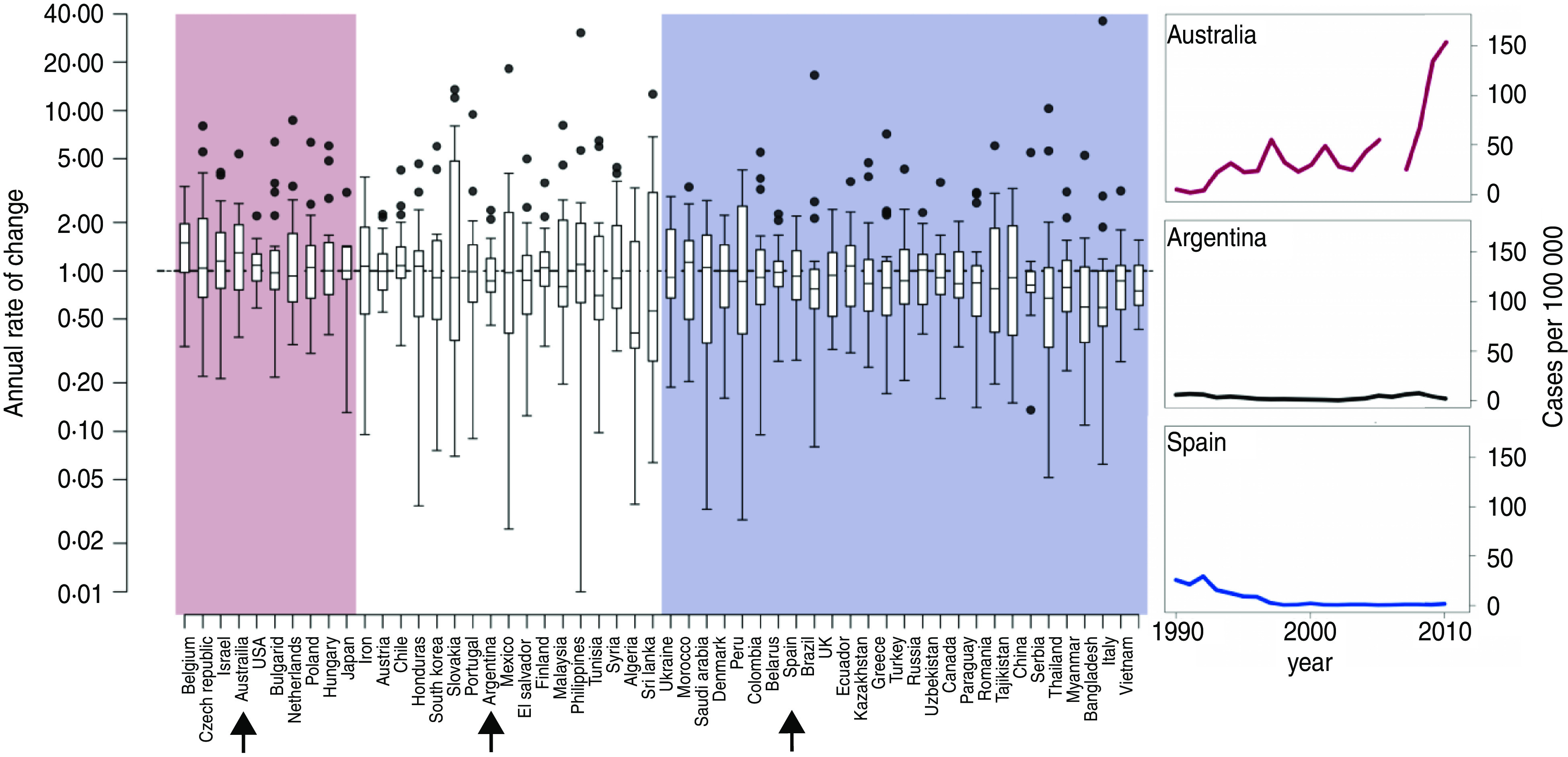
[colour online]. Boxplot of annual rates of change of pertussis incidence between 1990 and 2010 for countries that met our inclusion criteria: mean DTP3 vaccine uptake of >80% between 1990 and 2010; populations of >5 million individuals; and >80% complete case count records for this time period. The pink area indicates countries with significantly positive trends in incidence based on Kendall's tau rank correlation (P < 0·05), white indicates countries for which trends were not significant, and blue indicates countries with significantly decreasing trends. Note that rates of change are plotted on a log scale. Representative time-series of countries with increasing (Australia), stationary (Argentina), and decreasing (Spain) trends are shown on the right.
Another result that emerged from these data is a lack of a consistent geographical pattern in the trends. Countries with significantly increasing incidence were located in Asia (Japan), Europe (Belgium, Czech Republic, Bulgaria, The Netherlands, Poland, Hungary), the Middle East (Israel), North America (USA), and Oceania (Australia). Most of these same regions also contain countries that experienced significant decreases over this same time period: Asia (Vietnam, Thailand, China), Europe (e.g. Ukraine, Denmark, Belarus, Spain, UK, Greece, Romania, Italy), the Middle East (Saudi Arabia), and North America (Canada).
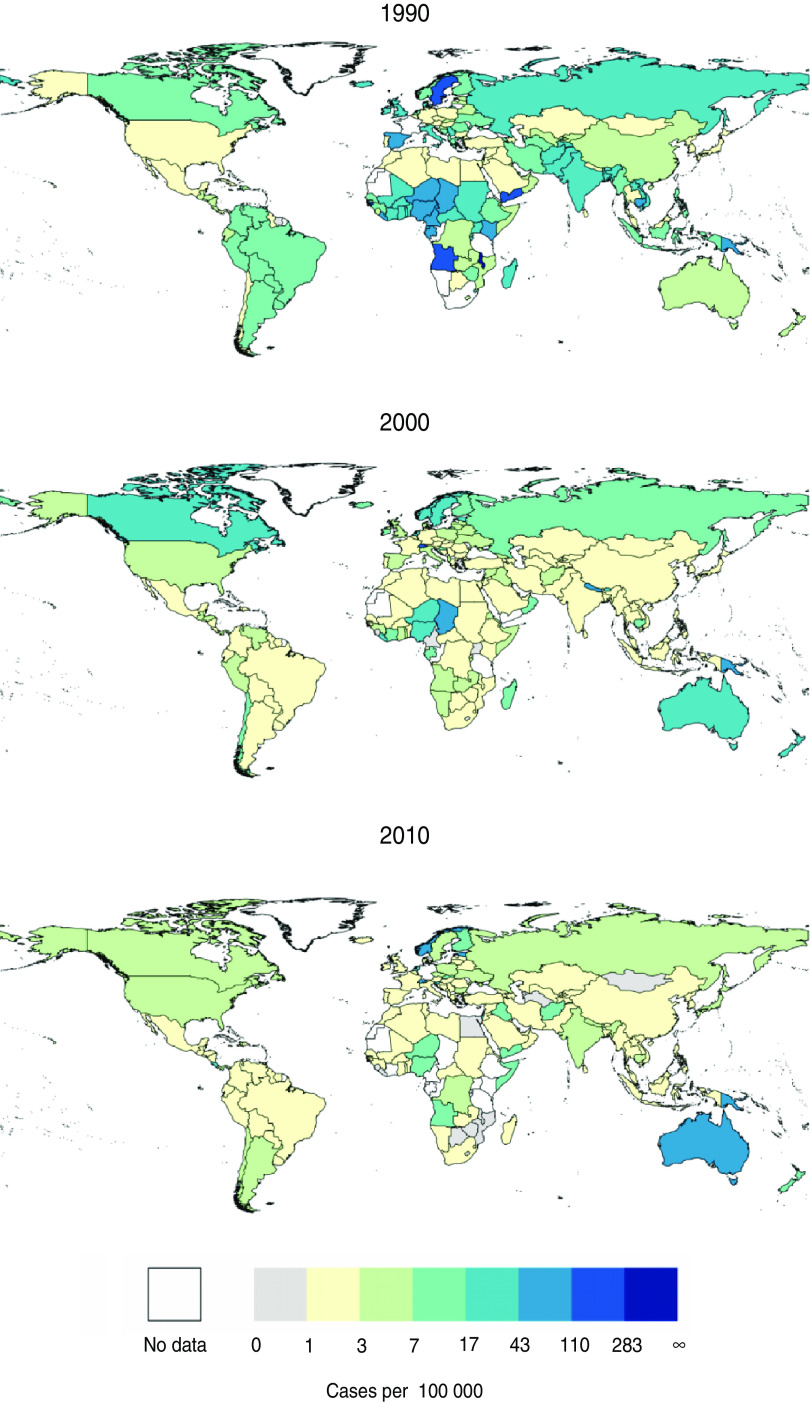
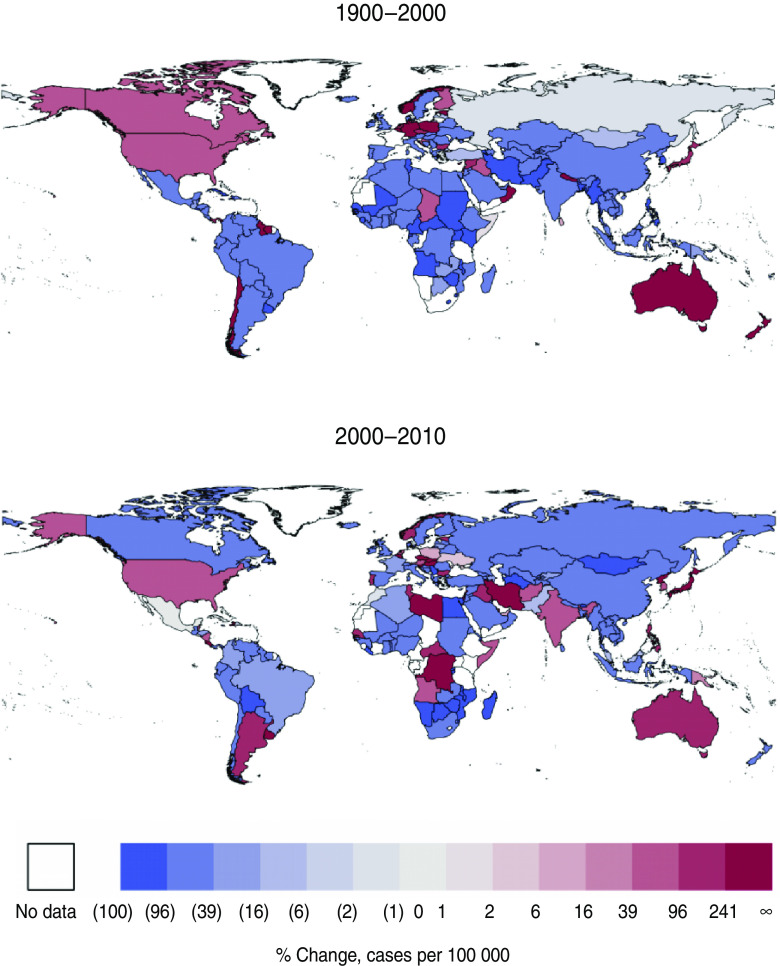
Spatial snapshots of incidence for all countries for which we have data, smoothed using a 5-year moving average to account for the known 2–4·6 year periodicity in pertussis incidence [17], reveal a similar qualitative picture: across much of the world, pertussis incidence fell over the past 20 years, but in some countries there is a clear resurgence (Figs 2, 3). The USA, Australia, and Poland exhibit clear, consistent increases in incidence throughout this period. A marked resurgence beginning in 1990 in Canada, which was shown to be due to a cohort of infants who received a poorly protective vaccine administered between 1985 and 1998 [19], is also apparent. Throughout much of the world, in contrast, the past two decades have seen continued, large decreases in incidence.
Fig. 2.
[colour online]. Absolute incidence of pertussis per country for the years 1990, 2000, and 2010, binned logarithmically. Data are smoothed using 5-year moving averages to account for the known periodicity of pertussis.
Fig. 3.
[colour online]. Changes in incidence of pertussis per country, 1990–2000 and 2000–2010, binned logarithmically. Data are smoothed using 5-year moving averages to account for the known periodicity of pertussis. Blue shading indicates decreases in incidence; maroon shading indicates increases in incidence.
CANDIDATE EXPLANATIONS FOR OBSERVED INCREASES
Although these data suggest that a resurgence of pertussis in developed countries is far from a universal phenomenon, the systematic rise in specific countries remains a cause for concern, and requires explanation. As with any observation, a possibility that must be considered is that the observed pattern is not real, but rather merely an artifact of improved reporting due to changes in awareness and diagnostic methodologies. Assuming there are genuine increases in incidence, a diversity of hypotheses have been proposed: changes in the composition of vaccines; differences in vaccine schedules; a change in immunity profiles in populations, e.g. due to waning immunity or changes in rates of natural boosting; and evolution of the bacterium, perhaps as a consequence of vaccination. We elaborate on each below.
Changes in reporting fidelity
Three factors contribute to suspicions that observed resurgences are not indicative of increased transmission. First, actual pertussis incidence has been shown to be greatly underreported [20, 21], particularly in adolescents and adults in whom clinical symptoms are much milder [3], resulting in a large potential for improved reporting fidelity to significantly elevate case notifications. Second, development of more sensitive detection methods, such as PCR and enzyme-linked immunoassay (ELISA) serology [12, 22–24] and an increased potential for media coverage to raise awareness of the disease have occurred in the putative resurgence era. Third, the possibility that vaccination serves to reduce clinical pertussis but not the actual transmission of the bacterium, as suggested by Fine & Clarkson [25], implies that immunization programmes may affect our perception of the prevalence of B. pertussis without affecting actual circulation rates. Taken in isolation, these factors make it reasonable to suspect that increased awareness and enhanced detection may play a major role in the apparent resurgences.
Direct evidence in support of this ‘perception-based resurgence’ hypothesis (that systemic changes in reporting fidelity lie at the root of the apparent upsurge in cases) has been difficult to obtain. It has been argued that pertussis in infants – too young to have received at least three doses of a vaccine – provides a reliable barometer of overall transmission [26]. Hence, some have interpreted the relative stability of cases in infants while increases were occurring in older age groups as potential evidence that changes in awareness and detection may be at play [27, 28].
In contrast, there are multiple lines of indirect evidence that suggest that the increases in reported incidence rates reflect real changes in the underlying epidemiology of pertussis. For instance, there was no consistent pattern of re-emergence of pertussis across the USA; trends in incidence point to a clearly identifiable turning point in each state, with the uptick in cases occurring sometime between 1970 and 1985 [5], substantially pre-dating either the increased awareness of pertussis in adolescents and adults [29] or the implementation of modern molecular diagnostic tools. Hence, in the USA at least, rising historical incidence is inconsistent with explanations based on changes in reporting alone.
A different line of reasoning has aimed to identify dynamic patterns in incidence reports, which have been shown to adhere to expectations derived from epidemiological theory to a degree that would be highly unlikely if they were simply reflecting changes in reporting of clinical pertussis. In a comparative study of 64 countries, Broutin et al. [17] demonstrated that the inter-epidemic period significantly increased, by an average of 1·27 years, after the introduction of national vaccination programmes (see also [30]). This is consistent with theory that predicts that a reduction in the effective replenishment rate of susceptibles as a consequence of vaccination should lead to reduced transmission of B. pertussis and hence less frequent outbreaks [31, 32]. The clear association between immunization effort and the inter-epidemic period reflects changes in pertussis circulation and not just the rate of clinical pertussis, in contrast with the oft-cited findings of Fine & Clarkson [25].
Changes in vaccination composition or schedule
Much of the large-scale dynamics of pertussis over the past 60 years, such as the pronounced decrease in incidence beginning in the middle of the twentieth century, have been driven by the roll-out of vaccination programmes, so changes in vaccination schedules and vaccine composition are obvious factors to consider.
The most prominent shift in the medical response amidst the resurgence was a change in the late 1990s to acellular pertussis (aP) vaccines in response to concerns about the reactogenicity of wP vaccines [3, 33]. In contrast to wP vaccines, which contain killed bacterial cells and hence the entire complement of ∼3000 antigens, the aP vaccines contain combinations of 1–5 antigens, including pertussis toxin, filamentous haemagglutinin, pertactin, and two fimbrial antigens [34]. Although aP vaccines contain only a subset of the antigens, the antigens they do contain are at higher concentrations. wP vaccines can be characterized as broad coverage with low titres, while aP vaccines provide narrow coverage but with higher titres [35].
There have been concerns that the immunity conferred by aP vaccines is not as complete or as long lasting as that obtained through wP vaccination [36–38] and that this could be at the root of increased incidences. However, the switch from a downward trend to an upward trend in the USA occurred as early as 1970 in some states, and by 1986 at the latest, which was a decade or more before the switch to an aP vaccine [5]. An upsurge in The Netherlands also began prior to the switch to aP vaccines [39]. However, considering just the countries that met our selection criteria, there was a significant association between the type of vaccine administered in the first dose of the primary series and the sign of the trends in incidence, with aP vaccines being associated with positive trends (Supplementary Table S5, P < 0·01). Countries using an aP vaccine also had a significantly higher Kendall's tau (P < 0·01). Both of these results are subject to the important caveat that these data only reflect the endpoint and not any changes that may have occurred in vaccine composition, type or schedule during the period of interest.
In addition to differences in vaccine composition, vaccination schedules vary among countries, and have also shifted over time. Most of the 17 countries represented in the Global Pertussis Initiative have adopted a schedule of three doses administered before age 6 months [40]. In considering vaccine schedules, both the straightforward, numerical effect of vaccinating a given fraction of an age group [41] and less obvious effects of vaccination timing on the immunological response of individuals should be considered. For example, maternal antibodies have been shown to block antibody responses to wP vaccines, but not aP vaccines [41]. Antibody responses also increase with longer intervals between injections [42, 43], and thus antibodies after three injections on a 3-, 5-, and 12-month schedule were higher than after three doses given at 2, 3, and 4 months [44]. A shift to an accelerated schedule of wP vaccination at ages 2, 3, and 4 months in the UK was shown in one laboratory study to result in lower antibody concentrations against pertussis toxin and fimbriae compared to the previous schedule of 3, 5, and 9 months [45], although this was not reflected in higher incidence at the population level in a surveillance study following the shift to the new schedule in 1990 [46]. Comparisons of a 2-, 4-, 6-month schedule and a 3-, 5-, 12-month schedule also found no difference [47]. In our analysis, the timing of the first dose (Supplementary Table S5) was not significantly associated with an increasing trend in incidence (P = 0·30); Kendall's tau was also not significantly higher for countries with an earlier onset of vaccination (P = 0·18).
Variability in vaccine composition and quality has also been considered as a potential contributing factor, with the case of Canada in the 1990s as a well-documented example [19]. Even in efficacious vaccines of a given type (wP or aP), there exists substantial heterogeneity in efficacy [47], which underscores the importance of not considering all countries with superficially similar vaccination programmes as equivalent.
Vaccine hesitancy has been another factor altering uptake rates in some populations, either through official changes in policy or through passive resistance. Higher incidences of pertussis have been linked to interruptions in vaccine coverage associated with anti-vaccine movements at the country level [11], the ease of obtaining non-medical exemptions at the state level in the USA [48], and spatial clustering of non-medical exemptions at the community level [49].
It is also important to bear in mind the ‘epidemiological arithmetic’; that is to say, in any population, over the long term, the unprotected sub-population and the group of individuals protected by vaccines must add up to the cumulative births. Hence, by way of example, consider a hypothetical country with a population of 100 million, a per capita annual birth rate of 2% and long-standing vaccination coverage of 90% with a vaccine that is 90% effective. Ignoring all other potential complexities, over a 10-year period, 10% of newborns, or 2 million people, remain unvaccinated. Even in the vaccinated population, 10%, or 1·8 million people, would remain susceptible due to primary failure of the vaccine. Thus, it should not be a surprise if, over this time span, hundreds of thousands of cases were observed in such a population. In light of this and the known effects of population size, growth rate, and demographics on epidemiological dynamics [50, 51], change in population structure is a potentially important axis of investigation.
Waning immunity and population-level dynamics
One of the most challenging aspects of understanding pertussis epidemiology is the need to resolve clinical observations made at the level of the patient with population-scale patterns. This is clearly manifested when considering immunity. There are numerous documented examples of individuals who have contracted clinical pertussis despite either past infection or full immunization. These observations raise significant questions: (i) how typical are these individuals? (ii) how long does immunity last, on average? (iii) is immunity protective against infection, or just disease? (iv) what is the transmission contribution of individuals infected after immunity has waned? Attempts to answer these questions using standard epidemiological methods have been informative but are often hampered by limitations in cohort size (e.g. [52]).
An alternative approach has been to employ mathematical models that integrate processes at the individual scale and translate them into predictions at the population level. When used in conjunction with methods of statistical inference, this approach can be a powerful means of unearthing intrinsic mechanisms [53]. For instance, by simply incorporating independently estimated age-specific contact rates into a basic transmission model, without resorting to complexities such as waning immunity, variability in vaccine uptake, changes in reporting fidelity, or antigenic divergence, it has been possible to make precise quantitative predictions regarding the epidemiological transition into the vaccine era in Sweden [54]. Specifically, Rohani et al.'s model successfully predicted the basic characteristics of a decrease in prevalence and a shift in age-specific incidence. These results highlight the potential for hidden details of the epidemiological dynamics to exert an influence that is as profound, or even more so, than more readily observable aspects of the system. In addition to these philosophical implications, this line of enquiry can be of practical value. Rohani et al. [54] demonstrated that because of the assortativity of the age-specific pattern of contacts, attempts to combat waning immunity via frequent adult booster shots [55] may have little effective impact on the total pertussis burden. This conclusion runs counter to the emerging consensus regarding the epidemiological role played by adults [56], but is supported by empirical data. In Sweden, for instance, vaccination of infants markedly reduced incidence in the 20–35 years age group [54], suggesting transmission from infants to adults (not vice versa). Similarly, it may be argued that because the administration of adolescent Tdap boosters in the USA has reduced incidence in the targeted age groups but not younger children [57], that the much-publicized transmission contribution of adolescents and adults may be markedly overstated.
In another example of this population-level dynamics approach, van Boven et al. [58] used a transmission model to demonstrate that vaccination may actually increase the infection pressure on the population if the infectiousness of secondary infections is sufficiently higher than that of primary infections (secondary infections being those of vaccinated individuals who have become susceptible due to waning immunity and subsequently infected, and primary infections being those affecting unvaccinated individuals). In this scenario, secondarily infected individuals are older and less likely to develop severe symptoms that lead to hospitalization – and are therefore less likely to be isolated or treated with antibiotics. Consequently, they are likely to remain more mobile and more infectious than younger, primarily infected individuals. Under this scenario, vaccination could serve to delay infection, shifting more cases from primary infections to more infectious secondary cases, with the result being that total incidence could counterintuitively increase with vaccination.
While an appealing possibility, key assumptions remain without support. First, in contrast to van Boven et al.'s assertion, it has been suggested that asymptomatic individuals contribute little to transmission [40, 59]. Second, analyses of age-stratified incidence reports identify the transmission impact of repeat infections (even when symptomatic) to be an order of magnitude less than primary infections [54]. These discrepancies illustrate a key benefit of models – that they make underlying assumptions explicit and transparent – while underscoring the need to confront existing models with empirical data.
Using a different modelling framework, Lavine et al. [60] found that increased vaccination could cause an uptick in incidence and a shift in age-specific incidence towards teenagers and adults. These results rely on the assumption that immune boosting occurs substantially more readily than primary infection, as has been suggested by studies of the sensitivity and speed of primed B and T cells. In this scenario, below a certain threshold of vaccination coverage the circulation of bacteria in the population is sufficient to repeatedly boost the otherwise waning immunity of individuals, thereby returning them firmly to the recovered class. Above this vaccination threshold, the force of infection is generally low enough to allow the population of susceptibles to be rapidly replenished, leading to recurrent epidemics and an increase in total incidence.
Boosting could also lead to a counterintuitive and nonlinear dependence on transmission rates [61]. If reinfection of vaccinated or previously infected individuals results in milder cases with lower transmissibility (relative to primary infections), decreased overall transmission rates could reduce boosting such that more individuals fall back into the fully susceptible class; these individuals are then prone to severe infections, which are more likely to be captured in incidence reports. Consequently, the net result of decreasing transmission rates below this reinfection threshold would be an increase in overall case counts, even though total incidence is reduced.
Attempts to use population-level incidence data to infer the mean duration of infection-derived immunity have arrived at estimates that are far in excess of figures obtained from cohort studies [52], or seroepidemiology [62]. When transmission models were challenged to explain the observed periodicity and extinction profile of pertussis in England and Wales, it was concluded that consensus was achieved when the duration of immunity was longer than 30 years, or if repeat infections contributed little to pertussis circulation [18]. Another strong conclusion of this study was the individual-level variability in the duration of immunity. Specifically, it was shown that if the fall off in immunity is exponential, a common assumption in epidemiological models, and the average duration of immunity is assumed to be 50 years, then about 25% of previously immune individuals will have lost immunity within just 15 years. This may provide some reconciliation between the long-lasting mean duration of immunity detected in population-level models and the observation of short-term immune loss in individual patients.
As the contrasting conclusions of these studies demonstrate, models are powerful heuristic devices that enable us to carry out thought experiments. Ultimately, their utility in shedding light on nature requires rigorous validation with data, the evaluation of testable hypotheses, and statistical inferential methodology [53].
Evolution of B. pertussis
As we have highlighted, a central puzzle is: why are countries with apparently similar vaccination policies (in terms of vaccine type and immunization schedule) experiencing markedly different pertussis trajectories? One potential factor may be genetic differences in the bacterium in these different locales. Specifically, Mooi et al. have argued that evolutionary shifts provide another avenue for hidden changes with the potential to substantially alter epidemiological characteristics [63]. Antigenic divergence from vaccine strains to strains with novel or previously rare alleles has been documented in a number of countries [64–69]. Shifts have been recorded in multiple genes, including: fim2 and fim3, which produce fimbriae that are involved in adherence and modulation of immune response; prn, which facilitates binding to eukaryotic cells; ptxA, the gene for the catalytic subunit of pertussis toxin (PT), which is involved in suppression of the host's immune response; and ptxP, the PT promoter [69]. Although definitive proof that these shifts have occurred as a consequence of vaccine-driven selection pressure would require the availability of contemporary, unvaccinated populations for comparison, substantial indirect evidence suggests that they have [69].
Whether the observed antigenic shifts have played a significant role in increasing incidence rates is still an open question. However, this phenomenon may explain sequential epidemics in Sweden and neighbouring Finland, which seem to have resulted from clonal expansion of certain B. pertussis strains [70]. Much attention has focused on the involvement of PT in increasing incidence. PT has been shown to suppress antibody response in mice, and a resurgence of pertussis in The Netherlands corresponded with an antigenic shift to a variant of the ptxP gene, ptxP3, which produces 1·6 times as much PT as the previously dominant, vaccine-type allele, ptxP1 [35, 69]. A similar shift to ptxP3 was noted in Australia [71]. Coupled with evidence that increased levels of PT lead to more severe infections (e.g. B. parapertussis, a congener of B. pertussis that causes milder infections, does not produce PT), there is some concern that vaccination has led to selection for strains that are both more able to suppress the host immune system and more virulent [69]. Vaccination appears to alter the B. pertussis niche, encouraging the selection of de novo genotypes and leading to a phylogenetic tree with a ladder-like structure [72]. By vaccinating naive hosts, we may have tipped the evolutionary scales in favour of more virulent strains that are able to more effectively elude the immunity of older, more intrinsically resistant individuals.
Despite their appeal, evolutionary explanations raise additional unanswered and puzzling questions. For instance, if differences in the genetic make-up of B. pertussis lie at the core of the contrasting national experiences of countries across the globe with otherwise similar immunization programmes, then it remains unclear why large-scale selective sweeps have not taken place. Perhaps this is due to strong regional genetic bottlenecks [73], although it would then be necessary to explain why the phylogeography of pertussis so starkly contrasts with the rapid global spread of other infectious agents, such as pandemic and seasonal influenza viruses [74, 75]. A systematic study of the evolutionary hypothesis would need to carefully consider the age distribution of pertussis cases and the potential limitations to geographical dispersal presented by the predominance of symptomatic infection in young children. Another enigmatic evolutionary observation comes from the documentation of multiple pertussis serotype replacement events in the USA over the past 75 years [73]. Curiously, however, the pace of these evolutionary changes appears slow, occurring over decades, compared to other well-studied examples, such as drug-resistant H1N1 viruses [76], HIV [77], or malaria [78], where spread has taken place over months or a few years. Uncovering the mechanisms behind these evolutionary time scales will necessitate quantifying the relative selective immune pressures exerted following infection and immunization, especially within the context of waning immunity.
CONCLUSIONS
In 1988, Noel Preston commented that there ‘must be few medical subjects that have generated so much controversy and even outright contradiction, as pertussis’ [79]. In the intervening 24 years since that statement, pertussis has become, if anything, more contentious, controversial and Balkanized.
When viewed from a global perspective, the history of pertussis in the recent past is marked by substantial heterogeneity (Figs 1–3). The variability within vaccines, within vaccination schedules, in demographic and social dynamics, and in the genetic make-up of the bacterium itself preclude easy classification or broad generalizations. The diversity in trends revealed by our analysis, even in countries that are superficially similar in terms of socioeconomic indicators and vaccination programmes, hints at tremendous underlying complexity and contingency that do not invite simple explanation. Rather, due to the nature of the human–B. pertussis system as a dynamic, evolving host–pathogen relationship, adequate explanations of its epidemiology are likely to require consideration of a combination of the factors discussed above, and of interactions between these influences.
Teasing apart and quantifying the effects of these multiple contributors will require evaluating the testable predictions arising from the various hypotheses. Some will be relatively straightforward to evaluate, and can be assessed using existing data. For example, the contributions of a switch from wP to aP vaccines or of a change in the vaccination schedule in a particular country can be evaluated using the timing and periodicity of case counts, provided that the effects of underreporting or changes in diagnostic methods are accounted for. Other hypotheses will require more sophisticated modelling of population-level [53] and evolutionary [58] dynamics or the collection of more and different data, ranging from serology, immunology, and epidemiology to genetic. In particular, the study of antigenic shifts would benefit greatly from additional data on the changes over time, space, and age in the genotypic composition of the bacterium. Combined with appropriate models, integrating within-host pathogenesis with between-host transmission, such data could provide powerful new insights into the evolutionary dynamics of pertussis and their influence on morbidity and mortality rates.
This review raises a number of questions of both scientific and practical importance. From a practical standpoint, the most obvious question is the appropriate vaccination policy in light of these putative explanations. For instance, if global trends in pertussis epidemiology are driven primarily by evolutionary changes in the bacterium, then perhaps, similar to seasonal influenza immunization, pertussis vaccines need to be regularly updated in an attempt to keep up with the contemporary composition of circulating serotypes. Similarly, if the observed patterns result from immune-boosting dynamics, the control implications are potentially complex. Any mitigation strategy will need to prioritize whether the ultimate goal is to minimize transmission of the bacterium in the population, reduce hospitalizations or to minimize the risk experienced by those most vulnerable to adverse health impacts. Regardless, determining the optimal control strategies is not straightforward, and lends an urgency to efforts to better understand evolutionary dynamics, shifting immunity profiles of populations, and transmission patterns within and between populations.
The epidemiology of pertussis is determined by a multifaceted confluence of social, biological, and historical factors. As such, it will continue to provide ample challenges for scientists, doctors, and policymakers alike, each of who brings their own unique perspective to the problem. Harmonizing these different interpretations presents a significant challenge, but also offers an opportunity to surmount the limitations of any single approach. For example, a spate of cases in recently vaccinated individuals may be puzzling when viewed from the perspective of a single practitioner, but may be well within the expected range of outcomes when viewed from a population-level perspective. Similarly, assumptions made in the interest of model tractability may have important implications for how results can be interpreted and applied; the input and expertise of doctors and policymakers is crucial in this regard.
This essential collaboration within and across disciplines depends crucially on the free exchange of data and isolates. It is increasingly recognized that lack of data sharing in the field of public health has stifled research, causing duplication of effort and impeding progress [80, 81]. Although the failure to converge on a set of rigorous explanations for the epidemiology of pertussis stems partly from the inherent complexity of the system, data withholding has also contributed to the perpetuation of divergent interpretations. Only by freely confronting all hypotheses with all the data can we hope to make urgently needed progress towards understanding how and why the epidemiology of pertussis has changed, is changing, and will continue to change.
Supplementary Material
Supplementary information supplied by authors.
ACKNOWLEDGEMENTS
P. R. was supported by the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health, the Vaccine Modeling Initiative of the Bill and Melinda Gates Foundation and by a grant from the National Institutes of Health (1R01AI101155).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812003093.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Varughese P. Incidence of pertussis in Canada. Canadian Medical Association Journal 1985; 132: 1041–1042. [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry JD. Pertussis in the preantibiotic and prevaccine era, with emphasis on adult pertussis. Clinical Infectious Diseases 1999; 28 (Suppl. 2): S107–111. [DOI] [PubMed] [Google Scholar]
- 3.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clinical Microbiology Reviews 2005; 18: 326–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowcroft NS, et al. How best to estimate the global burden of pertussis? Lancet Infectious Diseases 2003; 3: 413–418. [DOI] [PubMed] [Google Scholar]
- 5.Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics 2011; 3: 183–188. [DOI] [PubMed] [Google Scholar]
- 6.Moerman L, et al. The re-emergence of pertussis in Israel. Israel Medical Association Journal 2006; 8: 308–311. [PubMed] [Google Scholar]
- 7.Scheil W, et al. Pertussis in South Australia 1893 to 1996. Communicable Diseases Intelligence 1998; 22: 76–80. [PubMed] [Google Scholar]
- 8.Quinn H, McIntyre P. The impact of adolescent pertussis immunization, 2004–2009: lessons from Australia. Bulletin of the World Health Organization 2011; 89: 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gzyl A, et al. Pertussis in Poland. International Journal of Epidemiology 2004; 33: 358–365. [DOI] [PubMed] [Google Scholar]
- 10.Bamberger ES, Srugo I. What is new in pertussis? European Journal of Pediatrics 2008; 167: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangarosa EJ, et al. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet 1998; 351: 356–361. [DOI] [PubMed] [Google Scholar]
- 12.Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatric Respiratory Reviews 2008; 9: 201–211. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO). Immunization surveillance, assessment and monitoring (www.who.int/immunization_monitoring/data/data_subject/en/index.html). Accessed 27 August 2012.
- 14.European Commission. Health and Consumers Directorate-General Directorate C – Public Health and Risk Assessment C2 – Health information (http://ec.europa.eu/health/ph_information/dissemination/echi/docs/pertussis_en.pdf). Accessed 27 August 2012.
- 15.World Bank World Development Indicators (http://data.worldbank.org/data-catalog/world-development-indicators). Accessed 31 August 2012.
- 16.Rohani P, Earn DJD, Grenfell BT. Opposite patterns of synchrony in sympatric disease metapopulations. Science 1999; 286: 968–971. [DOI] [PubMed] [Google Scholar]
- 17.Broutin H, et al. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proceedings of the Royal Society of London, Series B: Biological Sciences 2010; 277: 3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wearing HJ, Rohani P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathogens 2009; 5: e1000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ntezayabo B, De Serres G, Duval B. Pertussis resurgence in Canada largely caused by a cohort effect. Pediatric Infectious Disease Journal 2003; 22: 22–27. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson JA, Fine PE. The efficiency of measles and pertussis notification in England and Wales. International Journal of Epidemiology 1985; 14: 153–168. [DOI] [PubMed] [Google Scholar]
- 21.Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. American Journal of Epidemiology 2002; 155: 866–874. [DOI] [PubMed] [Google Scholar]
- 22.Spokes PJ, Quinn HE, McAnulty JM. Review of the 2008–2009 pertussis epidemic in NSW: notifications and hospitalisations. New South Wales Public Health Bulletin 2010; 21: 167–173. [DOI] [PubMed] [Google Scholar]
- 23.Crowcroft NS. Whooping cough – a continuing problem. British Medical Journal 2002; 324: 1537–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q, Mertsola J. Factors contributing to pertussis resurgence. Future Microbiology 2008; 3: 329–339. [DOI] [PubMed] [Google Scholar]
- 25.Fine PE, Clarkson JA. The recurrence of whooping cough: possible implications for assessment of vaccine efficacy. Lancet 1982; 1: 666–669. [DOI] [PubMed] [Google Scholar]
- 26.Gay NJ, Miller E. Pertussis transmission in England and Wales. Lancet 2000; 355: 1553–1554. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, et al. Trends in pertussis among infants in the United States, 1980–1999. Journal of the American Medical Association 2003; 290: 2968–2975. [DOI] [PubMed] [Google Scholar]
- 28.Campbell H, et al. Accelerating control of pertussis in England and Wales. Emerging Infectious Diseases 2012; 18: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherry JD. Pertussis in adults. Annals of Internal Medicine 1998; 128: 64–66. [DOI] [PubMed] [Google Scholar]
- 30.Rohani P, Earn DJD, Grenfell BT. Pertussis transmission in England and Wales – reply. Lancet 2000; 355: 1553–1554. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen HTH, Rohani P. Noise, nonlinearity and seasonality: the epidemics of whooping cough revisited. Journal of the Royal Society Interface 2008; 5: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeling MJ, Rohani P. Modeling Infectious Diseases in Humans and Animals. Princeton, New Jersey: Princeton University Press, 2008. [Google Scholar]
- 33.Edwards KM, et al. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics 1995; 96: 548–557. [PubMed] [Google Scholar]
- 34.Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet 2006; 367: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 35.Mooi FR. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerging Infectious Diseases 2009; 15: 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark TA, Messonnier NE, Hadler SC. Pertussis control: time for something new? Trends in Microbiology 2012; 20: 211–213. [DOI] [PubMed] [Google Scholar]
- 37.Cherry JD. The present and future control of pertussis. Clinical Infectious Diseases 2010; 51: 663–667. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan SL, et al. Number and order of whole cell pertussis vaccines in infancy and disease protection. Journal of the American Medical Association 2012; 308: 454–456. [DOI] [PubMed] [Google Scholar]
- 39.Versteegh FGA, Edwards KM. How to fight whooping cough? Archives of Pediatrics and Adolescent Medicine 2012; 166: 389–391. [DOI] [PubMed] [Google Scholar]
- 40.Tan T, Trindade E, Skowronski D. Epidemiology of pertussis. Pediatric Infectious Disease Journal 2005; 24: S10–S18. [DOI] [PubMed] [Google Scholar]
- 41.Shinall MC, et al. Potential impact of acceleration of the pertussis vaccine primary series for infants. Pediatrics 2008; 122: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 42.Fecsik AI. Studies on antibody production: XI. Variation in the secondary response as a function of the interval between two antigenic stimuli. Journal of Experimental Medicine 1964; 120: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blennow M, et al. Primary immunization of infants with an acellular pertussis vaccine in a double-blind randomized clinical trial. Pediatrics 1988; 82: 293–299. [PubMed] [Google Scholar]
- 44.Taranger J, et al. Vaccination of infants with a four-dose and a three-dose vaccination schedule. Vaccine 1999; 18: 884–891. [DOI] [PubMed] [Google Scholar]
- 45.Booy R, et al. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet 1992; 339: 507–510. [DOI] [PubMed] [Google Scholar]
- 46.White JM, et al. The effect of an accelerated immunisation schedule on pertussis in England and Wales. Communicable Disease Report (CDR Review) 1996; 6: R86–91. [PubMed] [Google Scholar]
- 47.Jefferson T, Rudin M, DiPietrantonj C. Systematic review of the effects of pertussis vaccines in children. Vaccine 2003; 21: 2003–2014. [DOI] [PubMed] [Google Scholar]
- 48.Omer SB, et al. Nonmedical exemptions to school immunization requirements: Secular trends and association of state policies with pertussis incidence. Journal of the American Medical Association 2006; 296: 1757–1763. [DOI] [PubMed] [Google Scholar]
- 49.Omer SB, et al. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. American Journal of Epidemiology 2008; 168: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 50.Anderson RM, May RM, McLean A. Possible demographic consequences of AIDS in developing countries. Nature 1988; 332: 228–234. [DOI] [PubMed] [Google Scholar]
- 51.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford, New York: Oxford University Press, 1991. [Google Scholar]
- 52.Wendelboe AM, et al. Duration of immunity against pertussis after natural infection or vaccination. Pediatric Infectious Disease Journal 2005; 24: S58. [DOI] [PubMed] [Google Scholar]
- 53.Lavine JS, Rohani P. Resolving pertussis immunity and vaccine efficiency using incidence time series. Expert Review of Vaccines (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohani P, Zhong X, King AA. Contact network structure explains the changing epidemiology of pertussis. Science 2010; 330: 982–985. [DOI] [PubMed] [Google Scholar]
- 55.Cherry JD, Baraff LJ, Hewlett E. The past, present, and future of pertussis. The role of adults in epidemiology and future control. Western Journal of Medicine 1989; 150: 319–328. [PMC free article] [PubMed] [Google Scholar]
- 56.Cherry JD. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clinical Infectious Diseases 1999; 28 (Suppl 2): S112–117. [DOI] [PubMed] [Google Scholar]
- 57.Skoff TH, et al. Early impact of the US Tdap vaccination program on pertussis trends. Archives of Pediatrics and Adolescent Medicine 2012; 166: 344–349. [DOI] [PubMed] [Google Scholar]
- 58.van Boven M, et al. Pathogen adaptation under imperfect vaccination: implications for pertussis. Proceedings of the Royal Society of London, Series B: Biological Sciences 2005; 272: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schellekens J, von Konig C-HW, Gardner P. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatric Infectious Disease Journal 2005; 24: S19–S24. [DOI] [PubMed] [Google Scholar]
- 60.Lavine JS, King AA, Bjørnstad ON. Natural immune boosting in pertussis dynamics and the potential for long-term vaccine failure. Proceedings of the National Academy of Sciences USA 2011; 108: 7259–7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aguas R, Gonçalves G, Gomes MGM. Pertussis: increasing disease as a consequence of reducing transmission. Lancet Infectious Diseases 2006; 6: 112–117. [DOI] [PubMed] [Google Scholar]
- 62.Cattaneo LA, et al. The seroepidemiology of Bordetella pertussis infections: a study of persons ages 1–65 years. Journal of Infectious Diseases 1996; 173: 1256–1259. [DOI] [PubMed] [Google Scholar]
- 63.Mooi FR, van Loo IHM, King AJ. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerging Infectious Diseases 2001; 7: 526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fry NK, et al. Genotypic variation in the Bordetella pertussis virulence factors pertactin and pertussis toxin in historical and recent clinical isolates in the United Kingdom. Infection and Immunity 2001; 69: 5520–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mooi FR, et al. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infection and Immunity 1998; 66: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borisova O, et al. Antigenic divergence between Bordetella pertussis clinical isolates from Moscow, Russia, and vaccine strains. Clinical and Vaccine Immunology 2007; 14: 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Advani A, et al. Appearance of Fim3 and ptxP3-Bordetella pertussis strains, in two regions of Sweden with different vaccination programs. Vaccine 2011; 29: 3438–3442. [DOI] [PubMed] [Google Scholar]
- 68.Kodama A, et al. Antigenic divergence suggested by correlation between antigenic variation and pulsed-field gel electrophoresis profiles of Bordetella pertussis isolates in Japan. Journal of Clinical Microbiology 2004; 42: 5453–5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mooi FR. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infection, Genetics and Evolution 2010; 10: 36–49. [DOI] [PubMed] [Google Scholar]
- 70.Elomaa A, et al. Population dynamics of Bordetella pertussis in Finland and Sweden, neighbouring countries with different vaccination histories. Vaccine 2007; 25: 918–926. [DOI] [PubMed] [Google Scholar]
- 71.Octavia S, et al. Newly emerging clones of Bordetella pertussis carrying prn2 and ptxP3 alleles implicated in Australian pertussis epidemic in 2008–2010. Journal of Infectious Diseases 2012; 205: 1220–1224. [DOI] [PubMed] [Google Scholar]
- 72.van Gent M, et al. Small mutations in Bordetella pertussis are associated with selective sweeps. PLoS ONE 2012; 7: e46407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidtke AJ, et al. Population diversity among Bordetella pertussis isolates, United States, 1935–2009. Emerging Infectious Diseases 2012; 18: 1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bajardi P, et al. Human mobility networks, travel restrictions, and the global spread of 2009 H1N1 pandemic. PLoS ONE 2011; 6: e16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Russell CA, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320: 340–346. [DOI] [PubMed] [Google Scholar]
- 76.Shin SY, et al. Drug-resistant pandemic (H1N1) 2009, South Korea. Emerging Infectious Diseases 2011; 17: 702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wensing AM, et al. Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. Journal of Infectious Diseases 2005; 192: 958–966. [DOI] [PubMed] [Google Scholar]
- 78.Wongsrichanalai C, Pickard AL. Epidemiology of drug-resistant malaria. Lancet Infectious Diseases 2002; 2: 209–218. [DOI] [PubMed] [Google Scholar]
- 79.Preston NW. Pertussis today. In: Wardlow AC, Parton R, eds. Pathogenesis and Immunity in Pertussis. Chichester, UK: John Wiley & Sons Ltd, 1988, pp. 1–19. [Google Scholar]
- 80.Blumenthal D, et al. Data withholding in genetics and the other life sciences: prevalences and predictors. Academic Medicine 2006; 81: 137–145. [DOI] [PubMed] [Google Scholar]
- 81.Pisani E, et al. Time for fair trade in research data. Lancet 2010; 375: 703–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812003093.
click here to view supplementary material