SUMMARY
The oncogenic potential of human papillomaviruses (HPV) is well known in the context of cervical carcinoma; however, their role in the development of oesophageal squamous cell carcinoma (OSCC) is less clear. We aimed to determine the extent of the association between HPV infection and OSCC. A comprehensive literature search found 132 studies addressing HPV and OSCC in human cases, and a meta-analysis was performed using a random-effects model. There was evidence of an increased risk of OSCC in patients with HPV infection [odds ratio (OR) 2·69, 95% confidence interval (CI) 2·05–3·54]. The prevalence of HPV in OSCC was found to be 24·8%. There was an increased risk associated with HPV-16 infection (OR 2·35, 95% CI 1·73–3·19). Subgroup analyses showed geographical variance, with Asia (OR 2·94, 95% CI 2·16–4·00), and particularly China (OR 2·85, 95% CI 2·05–3·96) being high-risk areas. Our results confirm an increase in HPV infection in OSCC cases.
Key word: Human papilloma virus (HPV)
INTRODUCTION
Globally, cancer of the oesophagus is the eighth leading cause of cancer mortality [1]. The US government spends an estimated $1.1 billion [2] on treating over 30 000 [3] Americans suffering from the disease each year. Barrett's oesophagus, the premalignant metaplasia of oesophageal tissue related to gastro-oesophageal reflux disease, is a major risk factor for oesophageal adenocarcinoma [4]; however, the causes of squamous cell carcinoma are less clear.
Oesophageal squamous cell carcinoma (OSCC) has traditionally been associated with risk factors such as tobacco and alcohol [5], although given the vast geographical differences in incidence, the aetiology of the disease remains poorly understood. Countries such as the People's Republic of China (China), Singapore, Iran, Chile, Brazil, South Africa and France have all been identified as high-risk regions for the development of OSCC where incidence rates are markedly higher than in other areas [6]. Given this variance, many possible risk factors have been studied in an attempt to determine the cause of OSCC – including nutritional deficiencies (e.g. vitamins A, B, C) [6], physical factors (e.g. poor oral hygiene, hot drinks) [7], chemicals (e.g. nitrosamines, tobacco, alcohol, opium) [7], and viruses [e.g. Epstein–Barr virus, herpes simplex virus, human papillomavirus (HPV)] [6].
HPV was first described in 1949 in skin warts; however, it was not until the mid-1970s that it was linked to cervical cancer [8]. The link between HPV and cervical cancer has since been well established, given global detection of HPV DNA in over 90% of cervical cancers and the lack of geographical variation [9]. Meanwhile, research was also being conducted into the malignant conversion of other anogenital as well as extragenital papillomatous lesions to squamous cell carcinomas [8]. In 1985, specific HPV types were first found in oropharyngeal carcinomas and it is now thought that over 25–30% of these cancers may be caused by high-risk HPV types [8]. In 1982, Syrjänen et al. published the first study into the histological presence of known HPV patterns in 60 Finnish patients with established OSCC and found positive results in 40% [10]. Since then, research has continued into the possible causative role of HPV infection in OSCC pathogenesis.
The aim of this meta-analysis is to combine, examine and quantify the results from studies addressing the association between HPV infection and the development of OSCC on a global scale.
METHODS
Study protocol
We followed the PRISMA guidelines where possible in performing our systematic review [11]. A systematic search was undertaken through Medline (from 1950), PubMed (from 1946), EMBASE (from 1949) and Current Contents Connect (from 1998) and Google Scholar to 3 December 2012, to identify relevant articles where possible. The search used the terms ‘oesophageal cancer’ OR ‘esophageal cancer’ AND ‘human papillomavirus’, which were searched as text words and as exploded medical subject headings. The reference lists of relevant articles were also searched for appropriate studies. No language restrictions were used in either the search or study selection. There were four studies included which were not published in English [12–15]. A search for unpublished literature was not performed.
Study selection
We included cross-sectional, case-control and cohort studies that met the following inclusion criteria: (1) all cases and controls were human and adult; (2) the oesophageal cancer was squamous cell carcinoma; (3) all patients had no other health conditions. We excluded studies that did not meet the inclusion criteria.
Data extraction
The data extraction was performed via a standardized data extraction form, collecting information on publication years, study designs, numbers of cases, numbers of controls, total sample sizes, countries, continents, development statuses, control sources, mean ages, percentage male of total samples, the risk estimates or data used to calculate the risk estimates, confidence intervals (CIs) or data used to calculate CIs, the types of oesophageal carcinoma investigated, the methods of detection of HPV in the samples and the specific types of HPV detected. Where no data was available on the specific types of HPV detected, studies were included in the Non-specific analysis. Where no control group was used, studies were included in the Prevalence analysis. Quality of the studies was not assessed and authors were not contacted for missing data. Adjusted odds ratios (ORs) were extracted in preference to non-adjusted ORs; however, where ORs were not provided, unadjusted ORs and CIs were calculated. Where more than one adjusted OR was reported, we chose the OR with the highest number of adjusted variables. A sample which was found to be HPV-16 and HPV-18 positive was included in both subgroup analyses but in the overall analysis only once. However, a number of studies did not distinguish in their results between HPV-16 and HPV-18 positive samples and instead gave combined results as samples being positive for HPV-16/18. As such these studies were included in pooled HPV-16/18 analysis.
Four studies did not distinguish between histological types of oesophageal carcinoma and, in order to include them in the meta-analysis, they were assumed to be squamous cell carcinoma (Table 1). Where multiple control groups were studied, we selected the group of separate people over normal mucosal biopsies taken from areas adjacent to the tumour site.
Table 1.
Summary of studies included in the meta-analysis
| Author | Country | Mean age | Case % male | Data-collection year | Cases | Controls | Detection method | Control source | Non-specific HPV | HPV-16 | HPV-18 | HPV-16/18 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV +ve cases | HPV +ve controls | OR (95% CI) | Prev (%) | HPV +ve Cases | HPV +ve Controls | OR (95% CI) | Prev (%) | HPV +ve Cases | HPV +ve Controls | OR (95% CI) | Prev (%) | HPV +ve cases | HPV +ve controls | OR (95% CI) | Prev (%) | |||||||||
| Abdirad, 2011 [79] | Iran† | 58·84 | 57 | 1991–2005 | 93 | PCR | 8·6 | 3·22 | 1·07 | 4·3 | ||||||||||||||
| Acevedo-Nuno, 2004 [80]* | Mexico† | 63·06 | 88 | 17 | PCR | 88·24 | ||||||||||||||||||
| Akutsu, 1995 [81] | Japan | 1989–1993 | 29 | PCR + SBH | 0 | |||||||||||||||||||
| Antonsson, 2010 [22] | Australia | 65·2 | 56 | 2002–2005 | 222 | 55 | PCR | Other | 8 | 0 | 2·02 (0·25–16·49)‡ | 3·6 | 6 | 0 | 1·5 (0·18–12·72)‡ | 2·7 | 6 | 0 | 1·5 (0·18–12·72)‡ | 2·7 | ||||
| Astori, 2001 [23] | Italy | 67 | 71 | 14 | 16 | PCR + SBH | ANM | 6 | 4 | 2·25 (0·48–10·6) | 42·86 | 3 | 3 | 1·18 (0·2–7·08) | 21·43 | 3 | 3 | 1·18 (0·2–7·08) | 21·43 | |||||
| Awerkiew, 2003 [82] | Germany | 69 | 23 | PCR | 0 | |||||||||||||||||||
| Ayshamgul, 2011 [83] | China† | 50·5 | 2007–2009 | 50 | PCR | 38 | 38 | 38 | ||||||||||||||||
| Bahnassy, 2005 [24] | Egypt | 61·3 | 68 | 1996–1998 | 47 | 50 | PCR + IHC | ANM | 24 | 12 | 3·3 (1·39–7·85) | 51·06 | 25·53 | 12·77 | 38·3 | |||||||||
| Baines, 2005 [84] | USA | 16 | PCR | 0 | ||||||||||||||||||||
| Benamouzig, 1992 [26] | France | 62 | 92 | 1990 | 12 | 17 | ISH + DBA +histological diagnosis | Other | 1 | 0 | 1·45 (0·08–25·81)‡ | 8·33 | ||||||||||||
| Benamouzig, 1995 [25] | France | 64 | 75 | 75 | 49 | PCR+ DBA | ANM | 0 | 0 | 0 | ||||||||||||||
| Bjorge, 1997 [27] | Norway | 123 | ELISA | Other | 2·2 (0·9–5·3)§ | 21 | 2·9 (0·8–10) | 2·2 (0·7–6·7) | ||||||||||||||||
| Bognar, 2008 [85] | Germany | 1999–2000 | 16 | PCR + SBH | 31·25 | 31·25 | 31·25 | |||||||||||||||||
| Brandsma, 1989 [28] | USA | 1984–1987 | 3 | 3 | SBH | Other | 0 | 0 | 0 | |||||||||||||||
| Cao, 2005 [29] | China† | 50·12 | 58 | 1999–2003 | 265 | 357 | PCR | Other | 207 | 203 | 2·17 (1·89–3·88) | 78·11 | 182 | 179 | 2·18 (1·56–3·04) | 11 | 5 | 3·05 (1·05–8·88) | 186 | 181 | 2·29 (1·64–3·2) | |||
| Castillo, 2006 [86] | Colombia and Chile† | 63·6–72·2 | 53 | 1996–2001 | 73 | PCR + SBH | 28·77 | 15·07 | 47·62 | 28·77 | ||||||||||||||
| Chang, 1992 [90] | China† | 54·6 | 51 | ISH + PCR +SBH | 49·02 | |||||||||||||||||||
| Chang, 1993 [89] | China† | 53·3(M) 57·2(F) | 59 | 1989–1990 | 363 | ISH | 22·31 | 0·28 | ||||||||||||||||
| Chang, 1997 [31] | China† | 51–57 | 50 | 36 | 21 | ISH | ANM | 4 | 2 | 1·82 (0·18–18·69) | 8·33 | |||||||||||||
| Chang, 2000 [91] | China† | 55·3–57·4 | 62 | 101 | ISH + PCR | 16·83 | ||||||||||||||||||
| Chang, 2000 [87] | China† | 55·1(M) 57·3(F) | 58 | 1989–1990 | 700 | ISH | 16·57 | 2 | ||||||||||||||||
| Chang, 1990 [30] | China† | 6 | 9 | FISH | Other | 3 | 0 | 7 (0·69–70·75)‡ | 66·67 | |||||||||||||||
| Chang, 1990 [88] | China† | 52·3 | 51 | 51 | ISH | 3·92 | 1·96 | |||||||||||||||||
| Chen, 1994 [92] | China† | 58·2 | 88 | 40 | PCR + SBH | 60 | 15 | |||||||||||||||||
| Damin, 2006 [32] | Brazil† | 58·4 | 70 | 1989–1996 | 165 | 26 | PCR | Other | 26 | 0 | 4·68 (0·61–36·04)‡ | 15·76 | 25 | 0 | 4·46 (0·58–34·46)‡ | 15·15 | 2 | 0 | 0·31 (0·03–3·51)‡ | 1·21 | 26 | 0 | 4·68 (0·61–36·04)‡ | 15·76 |
| de Villiers, 1999 [94] | China† | 53·9–57·8 | 49 | 117 | PCR | 17·09 | ||||||||||||||||||
| de Villiers, 2004 [93] | Germany | 60 | 81 | 21 | PCR | 66·67 | 47·62 | 4·76 | 52·38 | |||||||||||||||
| Dillner, 1995 [33] | Finland | 1968–1991 | 39 | 78 | ELISA | Other | 13·1 (1·6–108)§ | 24·14 | 24·14 | 24·14 | ||||||||||||||
| Ding, 2010 [95] | China† | 58 | 71 | 2005–2008 | 17 | PCR | 47·06 | |||||||||||||||||
| Dreilich, 2006 [96] | Sweden | 1990–2000 | 71 | PCR | 14·08 | 14·08 | 14·08 | |||||||||||||||||
| Emadian, 2011 [12] | Iran† | 2001–2008 | 40 | 40 | PCR | ANM | 15 | 5 | 4·2 (1·35– 13·06) | 37·5 | ||||||||||||||
| Erol, 2009 [13] | Hungary | 57 | 56 | 4 | PCR | 25 | ||||||||||||||||||
| Far, 2007 [97] | Iran† | 49 | 140 | PCR | 23·57 | |||||||||||||||||||
| Farhadi, 2005 [34] | Iran† | 54·2 | 58 | 1996–2001 | 38 | 38 | PCR | Other | 14 | 5 | 3·85 (1·22–12·14) | 36·84 | 5 | 0 | 5·61 (0·62–50·48)‡ | 13·16 | 3 | 5 | 0·57 (0·12–2·56) | 7·89 | 8 | 5 | 1·67 (0·52–5·97) | 21·05 |
| Fidalgo, 1995 [35] | Portugal | 62 | 76 | 16 | 8 | PCR | ANM | 9 | 8 | 0·18 (0·02–1·86) | 56·25 | 8 | 5 | 0·37 (0·07–1·95) | 15 | 3 | 5 | 0·28 (0·05–1·56) | 18·75 | 9 | 8 | 0·48 (0·09–2·52) | 56·25 | |
| Furihata, 1993 [98] | Japan | 79 | 1981–1992 | 38 | ISH | 36·84 | 13·16 | 23·68 | 36·84 | |||||||||||||||
| Furihata, 1993 [99] | Japan | 87 | 1981–1992 | 71 | ISH | 33·8 | 14·08 | 19·72 | 33·8 | |||||||||||||||
| Gao, 2006 [36] | China† | 40–50 | 2002 | 4 | 475 | Hybrid Capture II | Other | 2 | 373 | 0·27 (0·04–1·97) | 50 | |||||||||||||
| Goto, 2011 [100] | Japan, Korea, China, Singapore† | 62 | 181 | PCR + ISH | 9·39 | 7·18 | ||||||||||||||||||
| Hale, 1989 [101] | South Africa† | 45 | 1983–1988 | 20 | Modified Feulgen | 65 | ||||||||||||||||||
| Han, 1996 [37] | China† | 70 | 1995 | 90 | 121 | ELISA | Other | 4·5 (1·8–11·9) | 24 | |||||||||||||||
| Haswgawa, 2002 [102] | Japan | 48 | PCR + ISH | 41·67 | 35·42 | 20 | 41·67 | |||||||||||||||||
| He, 1997 [38] | China† | 88 | 1987–1994 | 152 | 152 | PCR + SBH | ANM | 37 | 0 | 48·58 (6·57–359·36)‡ | 24·34 | 31 | 0 | 38·69 (5·21–287·48)‡ | 20·39 | 8 | 0 | 8·39 (1·04–67·92)‡ | 5·26 | 37 | 0 | 48·58 (6·57–359·36)‡ | 24·34 | |
| Herrera-Goepfert, 2009 [103] | Mexico† | 62·7(M) 61·2(F) | 78 | 2000–2008 | 60 | PCR | 25 | 10 | 8·33 | 18·33 | ||||||||||||||
| Hille, 1985 [105] | South Africa† | 24 | Histological criteria | 33·33 | ||||||||||||||||||||
| Hille, 1986 [104] | South Africa† | 53 | 100 | 70 | Histological criteria | 33·86 | ||||||||||||||||||
| Hippelainen, 1993 [106] | Finland | 68 | 49 | 1979–1991 | 61 | Histological criteria | 18·03 | |||||||||||||||||
| Hussain, 2011 [39] | India† | 57·5 | 57 | 75 | 75 | PCR | ANM | 14 | 0 | 16·98 (2·17–132·84)‡ | 18·67 | 14 | 0 | 16·98 (2·17–132·84)‡ | 18·67 | 14 | 0 | 16·98 (2·17–132·84)‡ | ||||||
| Junquera, 2009 [14] | Spain | 62 | 80 | 2007–2008 | 11 | PCR | 54·54 | |||||||||||||||||
| Kamangar, 2006 [40] | China† | 54·8 | 49 | 1985–1991 | 99 | 381 | FISH | Other | 33 | 106 | 1·3 (0·18–2·08) | 33·33 | 12 | 29 | 1·67 (0·82–3·14) | 12·12 | 8 | 35 | 0·87 (0·39–1·94) | 8·08 | 20 | 64 | 1·25 (0·72–2·19) | 20·2 |
| Kamath, 2000 [107] | USA | 65 | 50 | 1988–1998 | 22 | PCR | 0 | |||||||||||||||||
| Katiyar, 2005 [41] | India† | 73 | 101 | 26 | PCR | Other | 19 | 1 | 5·79 (0·74–45·48) | 18·81 | 17 | 1 | 5·06 (0·64–39·92) | 16·83 | 2 | 0 | 0·5 (0·04–5·8)‡ | 1·98 | 19 | 1 | 5·79 (0·74–45·46) | 18·81 | ||
| Kawaguchi, 2000 [42] | China and Japan† | 1993–1998 | 75 | 75 | PCR | ANM | 17 | 17 | 1 (0·47–2·15) | 22·67 | 17 | 17 | 1 (0·47–2·15) | |||||||||||
| Khurshid, 1998 [43] | Japan | 66·2 | 74 | 27 | 12 | PCR | Other | 17 | 3 | 5·1 (1·11–23·37) | 62·96 | 3 | 0 | 1·37 (0·13–14·75)‡ | 11·11 | 4 | 0 | 1·91 (0·19–19·2)‡ | 14·81 | 7 | 0 | 7·7 (0·8–74·05)‡ | 25·93 | |
| Kiki, 2002 [44] | Turkey | 43 | 30 | 10 | PCR | Other | 10 | 0 | 3·1 (0·35–27·66)‡ | 33·33 | 9 | 0 | 3·86 (0·42–35·11)‡ | 30 | 7 | 0 | 2·74 (0·29–25·54)‡ | 23·33 | 10 | 0 | 4·5 (0·5–40·66)‡ | 33·33 | ||
| Kim, 1992 [15] | Korea† | 24 | 21 | PCR | ANM | 16 | 2 | 19 (3·52–102·58) | 66·67 | 16 | 2 | 19 (3·52–101·58) | 66·67 | 16 | 2 | 19 (3·52–101·58) | 66·67 | |||||||
| Kiyabu, 1989 [108] | USA | 1950–1987 | 13 | PCR + DBA | 0 | |||||||||||||||||||
| Koh, 2008 [45] | Korea† | 62·3 | 95 | 1998–1999 | 102 | 40 | PCR | ANM | 0 | 0 | 0 | |||||||||||||
| Kok, 1997 [109] | The Netherlands | 76 | 63 | PCR | 0 | |||||||||||||||||||
| Koshiol, 2010 [110] | China† | 60 | 2006 2007 | 267 | PCR | 0·37 | ||||||||||||||||||
| Kulski, 1986 [46] | Australia | 1973–1985 | 10 | 10 | FISH | ANM | 5 | 0 | 9 (0·81–100·14)‡ | 50 | ||||||||||||||
| Kulski, 1990 [111] | Australia | 39 | hisFISH | 23·08 | ||||||||||||||||||||
| Lagergren, 1999 [47] | Sweden | 64 | 74 | 1994–1997 | 121 | 302 | ELISA | Other | 20 | 62 | 0·77 (0·44–1·34) | 16·53 | 14 | 33 | 1·07 (0·55–2·07) | 11·57 | 6 | 29 | 0·49 (0·2–1·21) | 4·96 | 20 | 62 | 0·77 (0·44–1·33) | 16·53 |
| Lam, 1997 [112] | Hong Kong | 63 | 84 | 1992–1994 | 70 | PCR + SBH | 8·57 | |||||||||||||||||
| Lambot, 2000 [48] | Belgium | 21 | 19 | PCR | ANM | 1 | 0 | 0·9 (0·05–15·47)‡ | 4·76 | |||||||||||||||
| Lavergne, 1999 [113] | China, South Africa† | 57–61 | 63 | PCR | 30·16 | |||||||||||||||||||
| Lenhart, 1991 [49] | USA | 12 | 12 | ISH | Other | 4 | 0 | 5·5 (0·51–59·02)‡ | 33·33 | |||||||||||||||
| Lewensohn-Fuchs, 1994 [114] | Sweden | 10 | PCR | 0 | ||||||||||||||||||||
| Li, 2001 [50] | China† | 40 | 3 | 72 | PCR + ISH | Other | 2 | 43 | 0·67 (0·04–11·22) | 100 | 2 | 43 | 0·67 (0·04–11·22) | 2 | 43 | 0·67 (0·04–11·22) | ||||||||
| Li, 2002 [51]* | China† | 1999–2000 | 62 | 36 | PCR + ISH | Other | 39 | 17 | 1·9 (0·82–4·36) | 62·9 | 39 | 17 | 1·9 (0·82–4·36) | 62·9 | ||||||||||
| Liu, 2005 [52] | China† | 70 | 1997–2000 | 40 | 15 | PCR + IHC | ANM | 24 | 4 | 4·13 (1·12–15·25) | 60 | 24 | 4 | 4·12 (1·12–15·25) | 24 | 4 | 4·21 (1·12–15·25) | |||||||
| Liu, 2010 [53] | China † | 69 | 32 | PCR + IHC | ANM | 35 | 2 | 15·44 (3·42–69·7) | 35 | 2 | 15·44 (3·42–69·7) | 35 | 2 | 15·44 (3·42–69·7) | ||||||||||
| Loke, 1990 [54] | Hong Kong | 37 | 37 | ISH + SBA | ANM | 0 | 0 | 0 | ||||||||||||||||
| Lu, 2004 [56] | China† | 1999–2003 | 104 | 104 | PCR | ANM | 55 | 41 | 1·72 (0·99–2·99) | 52·88 | 55 | 41 | 1·72 (0·99–2·99) | 52·88 | 55 | 41 | 1·72 (0·99–2·99) | |||||||
| Lu, 2008 [55] | China† | 53 | 61 | 1999–2004 | 67 | 67 | PCR | ANM | 14 | 6 | 2·69 (0·69–7·48) | 20·9 | 5 | 3 | 1·72 (0·39–7·51) | 4·48 | 2 | 0 | 2·03 (0·18–22·95)‡ | 2·99 | 7 | 3 | 2·49 (0·61–10·07) | 7·46 |
| Lyronis, 2005 [58] | Greece | 67 | 87 | 1989–2002 | 30 | 27 | PCR | ANM | 17 | 6 | 4·58 (1·43–14·59) | 56·67 | 1 | 1 | 0·9 (0·05–15·07) | 3·33 | 15 | 5 | 4·4 (1·32–14·7) | 50 | 16 | 6 | 4 (1·26–12·72) | 53·33 |
| Lyronis, 2008 [57] | Greece | 67 | 87 | 1989–2002 | 30 | 32 | PCR | Other | 17 | 6 | 5·66 (1·8–17·79) | 56·67 | ||||||||||||
| Matsha, 2002 [115] | South Africa† | 57·5 | 58 | 1995–1998 | 50 | PCR | 46 | 4 | 4 | |||||||||||||||
| Matsha, 2007 [59] | South Africa† | 114 | 41 | PCR | Other | 52 | 3 | 10·62 (3·1–36·41) | 45·61 | 6 | 0 | 2·22(0·26–19·04)‡ | 5·26 | 6 | 0 | 2·22 (0·26–19·04)‡ | 5·26 | |||||||
| Miller, 1997 [116] | USA | 66 | 64 | 1958–1988 | 22 | PCR + ISH | 45·45 | 7 | 7 | |||||||||||||||
| Mir, 2007 [60] | India† | 2001–2005 | 62 | 62 | PCR | ANM | 0 | 0 | 0 | |||||||||||||||
| Mizobuchi, 1997 [61] | Japan | 41 | 41 | PCR + SBH | ANM | 3 | 3 | 1 (0·2–5·27) | 7·32 | 3 | 3 | 1 (0·19–5·27) | 7·32 | 3 | 3 | 1 (0·19–5·27) | 7·32 | |||||||
| Mohseni Mehran, 2010 [117] | Iran† | 64 | 2005–2007 | 45 | PCR | 37·78 | 4·44 | |||||||||||||||||
| Moradi, 2002 [20] | Iran† | 62 | 1997–1998 | 85 | PCR | 49·42 | 27·06 | 2·35 | 29·41 | |||||||||||||||
| Moradi, 2006 [21] | Iran† | 31 | PCR | Other | 42 | 18 | 0·71 (0·31–1·62) | 25 | 7 | 1·43 (0·55–3·74) | 8 | 0 | 3·12 (0·37–26)‡ | 31 | 7 | 1·97 (0·76–5·09) | ||||||||
| Morgan, 1997 [118] | UK | 68 | 34 | 22 | PCR + SBH | 0 | ||||||||||||||||||
| Mori, 1989 [62] | Japan | 46 | 11 | IHC | Other | 8 | 0 | 2·1 (0·23–18·86)‡ | 17·39 | |||||||||||||||
| Nakamura, 1995 [119] | Japan | 63 | 79 | 1983–1991 | 61 | IHC | 8·2 | |||||||||||||||||
| Ogura, 1993 [120] | Japan | 1981–1991 | 14 | PCR + SBH | 7·14 | |||||||||||||||||||
| Ono, 1994 [121] | Japan | 41–83 | 86 | 1982–1992 | 42 | ISH | 30·95 | 16·67 | 19·05 | 30·95 | ||||||||||||||
| Ostrow, 1987 [122] | USA | 5 | ISH | 0 | ||||||||||||||||||||
| Pantelis, 2007 [63] | Germany | 58 | 96 | 53 | 53 | PCR | ANM | 9 | 9 | 1 (0·36–2·76) | 16·98 | 6 | 6 | 1 (0·3–3·33) | 11·32 | 3 | 3 | 1 (0·19–5·19) | 5·66 | 9 | 9 | 1 (0·36–2·76) | 16·98 | |
| Paz, 1997 [123] | USA | 1982–1994 | 11 | PCR + SBH | 0 | |||||||||||||||||||
| Peixoto Guimaraes, 2001 [64] | China† | 57 | 47 | 1986 | 32 | 57 | PCR | Other | 2 | 4 | 0·88 (0·15–5·11) | 6·25 | 1 | 1 | 1·81 (0·12–29·9) | 3·12 | 0 | 1 | 1 | 1 | 1·81 (0·12–29·9) | 3·12 | ||
| Poljak, 1993 [124] | Slovenia | 58·8 | 100 | 20 | PCR | 0·1 | 10 | 10 | ||||||||||||||||
| Poljak, 1998 [125] | Slovenia | 59·6 | 1985–1995 | 117 | PCR | 0 | ||||||||||||||||||
| Qi, 2006 [65] | China† | 58·1 | 80 | 2004–2005 | 60 | 60 | ISH + IHC | ANM | 24 | 9 | 9·07 (3·48–23·64) | 40 | ||||||||||||
| Rakoczy, 1989 [126] | Australia | 5 | DBA | |||||||||||||||||||||
| Rugge, 1997 [66] | Italy | 61 | 100 | 18 | PCR | 0 | ||||||||||||||||||
| Saegusa, 1997 [127] | Japan | 1989–1994 | 92 | 18 | PCR | ANM | 0 | 0 | 0 | |||||||||||||||
| Shen, 2002 [67] | China† | 1994–1997 | 55 | 55 | PCR | ANM | 36 | 33 | 1·26 (0·58–2·74) | 65·45 | 19 | 6 | 4·31 (1·56–11·88) | 65·45 | ||||||||||
| Shibagaki, 1995 [128] | Japan | 72 | PCR + SBH | 20·83 | 20·83 | 20·83 | ||||||||||||||||||
| Shimada, 1997 [129] | Japan | 1981–1992 | 31 | PCR + SBH | 16·13 | |||||||||||||||||||
| Shuyama, 2007 [130] | China† | 61 | 81 | 1994–2005 | 59 | PCR + SBH | 32·2 | 25·42 | 5·08 | 30·51 | ||||||||||||||
| Si, 2003 [132] | Hong Kong and China† | 319 | PCR | 13·48 | 12·23 | 1·88 | 13·48 | |||||||||||||||||
| Si, 2004 [131] | China† | 87 | PCR | 20·69 | 20·69 | 3·45 | 20·69 | |||||||||||||||||
| Sitas, 2012 [149] | Australia, South Africa, Brazil, Iran, China† | 60 | 64 | 1561 | 2502 | ELISA | Other | 15·1 | 13·3 | 35·0 | 29·9 | |||||||||||||
| Smits, 1995 [133] | The Netherlands | 63 | PCR | 0 | ||||||||||||||||||||
| Sobti, 2001 [134] | India† | 52 | PCR + ELISA | 73·08 | ||||||||||||||||||||
| Strickler, 1998 [135] | USA | 62 | 88 | 1975–1991 | 19 | ELISA | 5·26 | |||||||||||||||||
| Suzuk, 1996 [136] | China and USA | 57·6–61·6 | 93 | PCR + ISH | 4·3 | 3·23 | 3·23 | |||||||||||||||||
| Syrjanen, 1982 [10] | Finland | 45 | 1969–1980 | 60 | Histological criteria | 40 | ||||||||||||||||||
| Takahashi, 1998 [137] | Japan | 1982–1996 | 123 | PCR + ISH | 30·08 | |||||||||||||||||||
| Talamini, 2000 [138] | Italy | 63 | 91 | 1989–1996 | 45 | PCR | 0 | |||||||||||||||||
| Togawa, 1994 [68] | Italy, France, Japan, Iran, USA, South Africa | 35–91 | 81 | 72 | 33 | PCR + SBA | ANM | 10 | 0 | 5·16 (0·63–42·13)‡ | 13·89 | 10 | 0 | 5·16 (0·63–42·13)‡ | 13·89 | |||||||||
| Toh, 1992 [139] | Japan | 1990–1990 | 45 | PCR | 6·67 | 2·22 | 4·44 | 6·67 | ||||||||||||||||
| Tornesello, 2009 [140] | Italy | 61·3 | 1999–2003 | 36 | PCR | 27·78 | 1 | 1 | ||||||||||||||||
| Turner, 1997 [141] | Canada and USA | 67 | 63 | 51 | PCR | 1·96 | 1·96 | 1·96 | ||||||||||||||||
| Van Doornum, 2003 [69] | The Netherlands | 64·9 | 61 | 1989–1999 | 56 | 100 | ELISA | Other | 8 | 18 | 0·76 (0·31–1·88) | 14·29 | 8 | 18 | 0·76 (0·31–1·88) | 14·29 | 8 | 18 | 0·76 (0·31–1·88) | 14·29 | ||||
| Van Rensburg, 1993 [70] | South Africa† | 10 | 10 | ISH | ANM | 3 | 5 | 0·43 (0·07–2·68) | 30 | |||||||||||||||
| Wang, 2010 [142]* | China and USA | 71 | 435 | PCR | 56·09 | |||||||||||||||||||
| Weston, 2003 [71] | Brazil† | 63 | 75 | 1999–2000 | 40 | 10 | Hybrid Capture II | Other | 1 | 1 | 0·23 (0·01–4·05) | 2·5 | ||||||||||||
| White, 2005 [143] | Kenya† | 58 | 69 | 29 | PCR | 0 | ||||||||||||||||||
| Williamson, 1991 [72] | South Africa† | 56 | 11 | 11 | PCR | ANM | 5 | 1 | 8·33 (0·78–89·47) | 45·45 | ||||||||||||||
| Woo, 1996 [144] | Korea† | 1991–1994 | 25 | ISH + histological criteria | 44 | |||||||||||||||||||
| Yang, 2008 [73] | China† | 59 | 2000–2005 | 435 | 550 | PCR | Other | 308 | 253 | 2·38 (2·18–3·72) | 70·8 | 308 | 253 | 2·85- (2·18–3·71) | 70·8 | |||||||||
| Yao, 2006 [74] | China† | 55·7–58 | 82 | 40 | ICH + ISH | ANM | 23 | 0 | 15·2 (1·97–117·23)‡ | 28·05 | 23 | 0 | 15·2 (1·97–117·23)‡ | |||||||||||
| Zhang, 2010 [75] | China† | 57·32 | 70 | 2002–2006 | 70 | 60 | PCR + IHC | ANM | 35 | 20 | 2 (0·98–4·08) | 50 | 21 | 13 | 1·55 (0·7–3·44) | 30 | 8 | 3 | 2·45 (0·62–9·69) | 11·43 | ||||
| Zhang, 2011 [76] | China† | 57·3 | 70 | 2000–2006 | 70 | 100 | PCR | Other | 28 | 8 | 7·67 (3·22–18·23) | 40 | 28 | 8 | 7·67 (3·22–18·23) | 40 | 28 | 8 | 7·67 (3·22–18·23) | 40 | ||||
| Zhang, 2011 [77]* | China† | 57·3 | 67 | 2000–2006 | 106 | 100 | PCR | Other | 82 | 33 | 6·94 (3·47–12·86) | 77·36 | 61 | 22 | 4·81 (2·61–8·85) | 77·36 | 77·36 | |||||||
| Zheng, 1999 [145] | China† | 46 | 67 | 3 | PCR | 0 | ||||||||||||||||||
| Zhou, 2003 [78] | China† | 57·4 | 75 | 48 | 23 | PCR + ISH + IHC | ANM | 31 | 8 | 3·42 (1·21–9·69) | 64·58 | 31 | 8 | 3·42 (1·21–9·69) | 64·58 | 31 | 8 | 3·24 (1·21–9·69) | 64·58 | |||||
ANM, Adjacent normal mucosa; Other, other patients; PCR, polymerase chain reaction; SBH, Southern blot hybridization; ISH, in-situ hybridization; ELISA, enzyme-linked immunosorbent assay; FISH, fluorescence in-situ hybridization; ICH, immunohistochemistry; DBA, dot blot analysis; SBA, slot blot analysis; hisFISH, histopathological-fluorescence in-situ hybridization.
All types of esophageal cancer.
Classified as a developing country [146].
Insufficient data to calculate an OR as the number of HPV positive controls was 0 therefore in order to be able to calculate an OR, we added one HPV positive control sample.
Statistical analysis
Pooled ORs and 95% CIs were calculated for the effect of HPV on the risk of developing OSCC using a random-effects model [16]. We tested heterogeneity with Cochran's Q statistic, with P < 0·10 indicating heterogeneity, and quantified the degree of heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I2 values of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity, respectively [17]. We quantified publication bias using Egger's regression model [18], with the effect of bias assessed using the fail-safe number method. The fail-safe number was the number of studies that we would need to have missed for our observed result to be nullified to statistical non-significance at the P < 0·05 level. Publication bias is generally regarded as a concern if the fail-safe number is less than 5n+10, with n being the number of studies included in the meta-analysis [19]. We performed a sensitivity analysis based on sample size using different sample sizes (n < 10, n < 20, n < 50) and calculated the respective ORs. All analyses were performed with Comprehensive Meta-analysis version 2.0 (Biostat, USA).
Population attributable risk percentage (PAR%) was calculated using the formula:
The input data was taken from this meta-analysis.
RESULTS
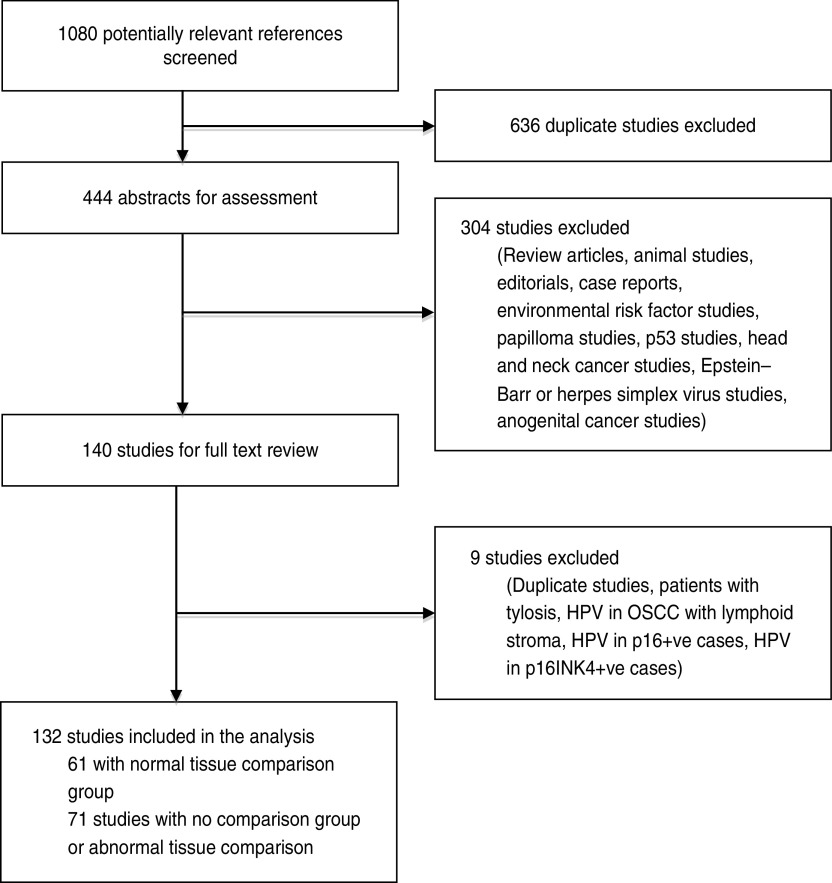
From the literature search, we screened 1080 potentially relevant references, of which 444 abstracts were extracted for assessment (Fig. 1). Of these, 140 were selected for a detailed full-text review, and four studies were excluded – one included patients with tylosis, one studied HPV DNA presence in only p16-positive cases, one studied HPV DNA presence in only p16INK4-positive cases and one studied HPV DNA presence in OSCC with lymphoid stroma. There were five duplicate publications, we used the most recent in each case and the others were excluded. In total, 132 studies were included for analysis. There were two studies published by Moradi and colleagues [20, 21] that included the same case group; however, one study was a case-control study and was included in the analysis of risk while the other was a cross-sectional study and was used in the prevalence analysis. We excluded the case-control study [21] from the prevalence analysis due to repetition of data. In the event that the same authors published multiple studies within a similar time period, we used information from the Materials, Methods and Results sections to confirm that these were from different sample populations. Sixty-one studies had control groups comprising of normal tissue samples and were used in both the risk and prevalence analyses [12, 15, 21–78]. The 71 other studies contained either no control group or samples from controls with benign or structural oesophageal disease and were used only in the analysis of the prevalence of HPV DNA in OSCC [10, 13, 14, 20, 79–145]. In total, 13 795 oesophageal samples were included in the analysis, with 9291 as cases with established OSCC and 4504 as controls with normal oesophageal mucosa. The mean age of the patients was 59·36 (range of study means 45–67 years) and 70·1% were male (although data was only available in 82 studies) (Table 1).
Fig. 1.
Results of the literature search.
Prevalence
From the 138 studies that assessed prevalence (n = 12 037 cases), 27·4% (95% CI 21·2–28·8) of OSCC samples were infected with HPV (Table 2). These studies contained cases from 30 countries and six continents (Table 1), which during subgroup analyses, demonstrated geographical differences.
Table 2.
Prevalence of human papillomavirus in oesophageal squamous cell carcinoma
| Factor | Prevalence, % (95% CI) |
|---|---|
| Global | |
| All studies | 24·8 (21·2–28·8) |
| Region | |
| Africa | 42·4 (34·0–51·3) |
| Asia | 27·9 (22·6–33·8) |
| Europe | 18·8 (12·8–26·7) |
| North America | 15·2 (6·5–31·7) |
| Oceania | 15·7 (3·6–48·2) |
| South America | 16·3 (7·4–32·2) |
| Socioeconomic status | |
| Developing | 31·1 (25·5–37·4) |
| Developed* | 18·9 (14·8–23·8) |
| Country | |
| Australia | 15·7 (3·6–48·2) |
| Brazil | 8·1 (1·3–36·9) |
| China | 32·8 (25·6–40·9) |
| Finland | 24·8 (12·9–42·3) |
| France | 2·8 (0·0–27·1) |
| Germany | 26·2 (8·0–59·1) |
| India | 26·6 (12·9–46·9) |
| Iran | 26·9 (13·2–47·2) |
| Italy | 16·6 (6·8–35·0) |
| Japan | 20·7 (14·2–29·2) |
| Korea | 26·5 (5·0–71·4) |
| Mexico | 59·4 (6·5–96·9) |
| The Netherlands | 2·9 (0·0–25·0) |
| South Africa | 40·4 (28·5–53·5) |
| USA | 8·7 (3·5–20·0) |
| HPV type | |
| 16 | 16·8 (12·9–21·7) |
| 18 | 8·1 (5·7–11·4) |
| 16/18 | 23·0 (17·9–28·9) |
| Detection method | |
| Polymerase chain reaction | 25·4 (20·9–30·4) |
| In-situ hybridization | 22·6 (16·7–29·9) |
CI, Confidence interval.
Countries classified as developing are identified in Table 1.
When comparing the prevalence according to economic development, developing countries had a 12·2% higher rate of HPV DNA in OSCC than developed countries [31·1 (95% CI 25·5–37·4) and 18·9 (95% CI 14·8–23·8), respectively]. We found that when analysing the prevalence by region, there was a 25·9% difference between the lowest, North America and the highest, Africa (Table 2). The largest population of people studied was Chinese (46 studies, n = 5859 cases), where the prevalence was found to be 32·8% (95% CI 25·6–40·9), ranking third highest globally behind only Mexico and South Africa (Table 2).
Of the 54 studies that reported data on the prevalence of HPV-16 (n = 4737 cases), 16·8% (95% CI 12·9–21·7) of cases contained HPV DNA, while of the 30 studies that reported data on HPV-18 (n = 2272 cases), only 8·1% (95% CI 5·7–11·4) of cases contained HPV DNA. However, 56 studies looked at high-risk HPV types, 16 and/or 18, (n = 4351 cases) and the combined prevalence was found to be 23·0% (95% CI 17·9–28·9).
There were 12 methods used to detect HPV in the samples (Table 1), the most commonly used being polymerase chain reaction (PCR) (94 studies) followed by in-situ hybridization (ISH) (24 studies), Southern blot hybridization (16 studies), immunohistochemistry (10 studies), enzyme-linked immunosorbent assay (8 studies), histological criteria (5 studies), dot blot analysis (4 studies), fluorescence ISH (3 studies), hybrid capture II (2 studies), slot blot analysis (2 studies), histopathological-fluorescence ISH (1 study), and the modified Feulgen technique (1 study). Thirty-seven studies used multiple detection methods, all of which included either PCR or ISH. In the 32 studies that included PCR, these were the results selected for analysis. In the remaining five studies, the ISH results were selected. The authors acknowledge that the use of histological criteria to diagnose HPV infection is a technique no longer employed. Two of the studies using histological criteria also utilized other detection methods in which cases the latter results were included; however, the remaining studies were included for a historical perspective.
Association between HPV and OSCC
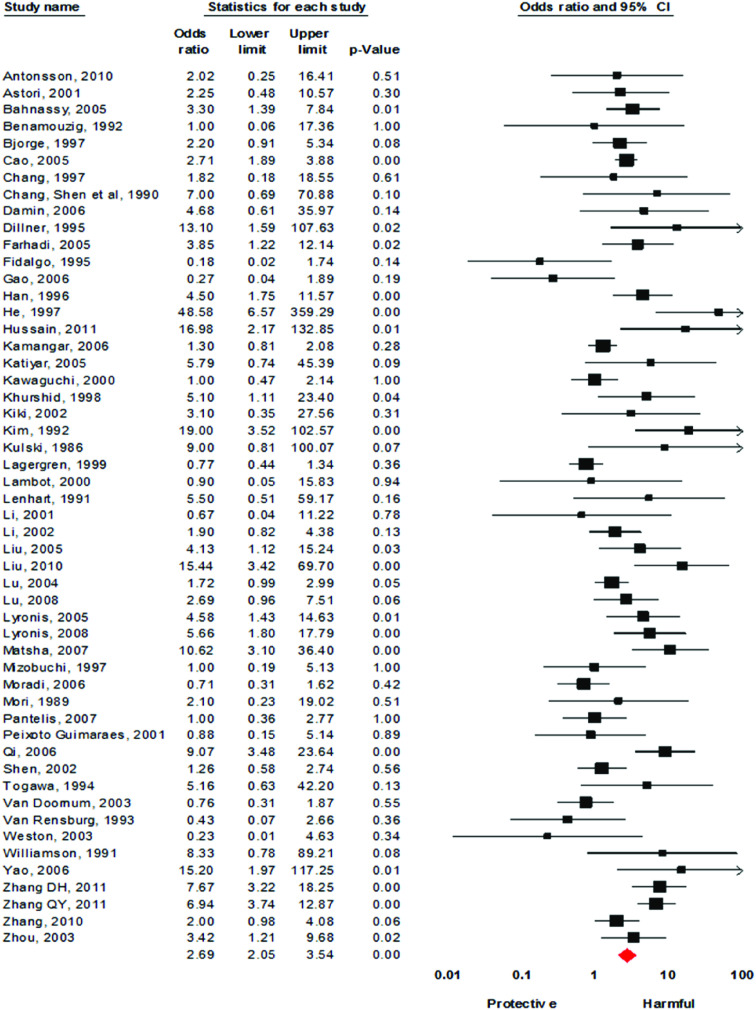
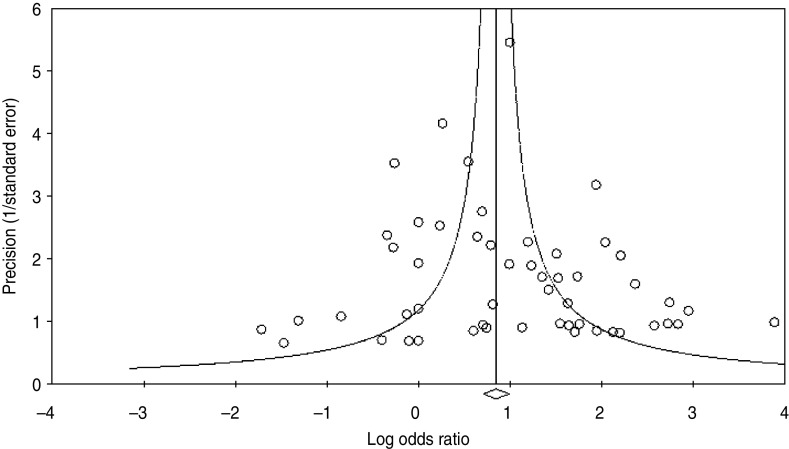
Using the 61 studies (n = 3970 cases) that contained a normal tissue comparison group, we calculated a pooled OR of 2·69 (95% CI 2·05–3·54) (Fig. 2), demonstrating an increased risk of developing OSCC in the presence of HPV infection. There was a moderate level of heterogeneity (I2 = 63·82%, P < 0·001), and with a fail-safe n of 1675 (P = 0·15) there was no evidence of publication bias (Fig. 3). We performed a number of subgroup analyses, looking at the risk of specific types of HPV, the effect of differing control sources, geographical variance, and the method of HPV detection (Table 3).
Fig. 2.
[colour online]. Forest plot of association between the presence of human papillomavirus DNA and the development of oesophageal squamous cell carcinoma. There were six case-control studies that had no positive samples in either the case or control groups. Therefore these are not included in the forest plot.
Fig. 3.
Funnel plot of precision by log odds ratio.
Table 3.
Subgroup analysis of case-control studies
| Factor | OR (95% CI) | I2 (P value) |
|---|---|---|
| All studies | 2·69 (2·05–3·54) | 63·82 (<0·001) |
| True Ors* | 2·44 (1·82–3·27) | 69·27 (<0·001) |
| Approximate ORs* | 5·12 (2·67–9·81) | 9·33 (0·35) |
| SES | ||
| Developed | 2·28 (1·40–3·73) | 57·73 (0·001) |
| Developing | 2·92 (2·08–4·11) | 67·77 (<0·001) |
| Detection method | ||
| Enzyme-linked immunosorbent assay | 2·23 (0·76–6·53) | 61·66 (0·02) |
| In-situ hybridization | 5·98 (3·46–10·33) | 0·00 (0·92) |
| Polymerase chain reaction | 2·05 (1·45–2·90) | 58·79 (<0·001) |
| HPV subtype | ||
| 16 | 2·35 (1·73–3·19) | 64·14 (<0·001) |
| 18 | 1·28 (0·87–1·88) | 31·35 (0·11) |
| True 18 (excl. approximate) | 1·14 (0·63–2·08) | 51·46 (0·04) |
| 16/18 | 2·46 (1·85–3·28) | 61·04 (<0·001) |
| Control source | ||
| Adjacent normal mucosa | 2·51 (1·72–3·68) | 65·47 (<0·001) |
| Other patients | 2·98 (1·95–4·57) | 64·17 (<0·001) |
| Region | ||
| Africa | 3·47 (1·02–11·84) | 65·46 (0·03) |
| Europe | 1·64 (0·90–2·99) | 60·44 (0·005) |
| Oceania | 3·84 (0·79–18·67) | 0 (0·36) |
| South America | 1·28 (0·07–23·82) | 62·23 (0·1) |
| Asian analysis | ||
| Asia | 2·94 (2·16–4·00) | 66·01 (<0·01) |
| All other continents | 2·20 (1·34–3·61) | 58·44 (<0·001) |
| China | 2·85 (2·05–3·96) | 67·20 (<0·001) |
OR, Odds ratio; CI, confidence interval.
Approximate ORs refers to case-control studies that had insufficient data to calculate an OR as the number of HPV-positive controls was 0 (Table 1). In order to be able to calculate an OR, we added one HPV-positive control sample. True ORs refers to case-control studies that had sufficient data to calculate an OR and therefore these are an absolute representation of the study.
There were 35 studies (n = 2942 cases) that provided information on specific types of HPV detected. We performed a subgroup analysis investigating the risk of HPV-16 and HPV-18, as well as combined HPV-16/18, in the development of OSCC. We found an increased risk of developing OSCC in patients with HPV-16 (OR 2·35, 95% CI 1·73–3·19) rather than HPV-18 (OR 1·28, 95% CI 0·87–1·88); however, a combined analysis of patients with HPV-16, HPV-18, or both resulted in a risk point estimate of 2·46 (95% CI 1·85–3·28). The heterogeneity was significantly high in all three analyses (Table 3).
Samples making up the control groups used in the studies were either biopsies of normal mucosa taken from an area of oesophgus adjacent to the tumour or from separate patients. We performed a subgroup analysis and found that studies that used separate patients as controls yielded a pooled OR of 2·98 (95% CI 1·95–4·57), which was higher than those studies that used adjacent samples (OR 2·51, 95% CI 1·72–3·68).
In concordance with the data assessing prevalence, there was a greater risk in developing countries of developing OSCC in the presence of HPV infection than in developed countries (Table 3).
Asia was the most predominantly studied region (36 studies, n = 2738 cases) and we calculated a risk point estimate of 2·94 (95% CI 2·16–4·0). There was a significant difference between this and the second most studied region, Europe (13 studies, n = 526), where the OR was found to be 1·64 (95% CI 0·90–2·99).
There were nine methods used in detecting HPV DNA in the samples (Table 1). PCR was the most commonly used method (41 studies), followed by ISH (10 studies), immunohistochemistry (8 studies), enzyme-linked immunosorbent assay (6 studies), Southern blot hybridization (4 studies), fluorescence ISH (2 studies), hybrid capture II (2 studies), dot blot analysis (2 studies) and slot blot analysis (2 studies). Sixteen studies used multiple detection methods, all of which included either PCR or ISH. In the 12 studies that included PCR, these were the results selected for analysis. In the remaining four studies, the ISH results were selected. We performed a subgroup analysis on the different detection methods, and studies using ISH resulted in the highest risk (OR 5·98, 95% CI 3·46–10·33), which was higher than the pooled risk of studies using PCR (OR 2·05, 95% CI 1·45–2·90).
PAR%
Using the formula discussed in the Methods section, the PAR% was calculated as a global figure as well as for differing regions. The results (Table 3) demonstrated that 15·7% of OSCC cases globally are attributable to being HPV positive. Regional analysis revealed a higher percentage in Asia (19·0%) than in Europe (6·3%). When analysed by socioeconomic status, developing countries were found to have a higher PAR than developed countries.
DISCUSSION
The results of our meta-analysis highlight an almost threefold increased risk of OSCC in the presence of HPV infection. The study population included cases from a range of geographical areas, high- and low-incidence areas for oesophageal cancer and high- and low-prevalence areas for HPV infection.
We found HPV-16 to be both the most prevalent type detected as well as the highest risk in terms of oncogenic potential. Of the studies with data on the various HPV types, we found HPV-16 to make up 65% of all HPV infections, and with an OR of 2·47 it was shown to be associated with the development of oesophageal cancer. HPV-16 infection was found to be twice as prevalent and almost double the risk for oesophageal cancer than HPV-18. Analysis of HPV-16 and/or HPV-18 showed that together these two types of HPV make up over 80% of HPV in infected samples, although the increase in risk is similar to that of HPV-16. These figures are in accordance with HPV infection in cervical squamous cell carcinoma, where HPV-16 is the most common type, followed by HPV-18 [9]. However, it has also been found that HPV-18 is the most predominant type found in cervical adenocarcinoma [146], and this may also be the case for oesophageal adenocarcinoma although more research is needed to explore this potential link.
Control samples were either taken from normal oesophageal mucosa adjacent to the tumour site or completely different participants. A pooled subgroup analysis found studies using other participants to be have a higher risk than studies with adjacent normal mucosa as the normal control. One study included in our analysis, published by Benamouzig et al. in 1992 [26], included two control groups, 17 separate patients with normal oesophageal musoca as well as 12 biopsies of normal mucosa taken from adjacent to the tumour site of the case patients. Out of the total 29 control biopsies, only one of the adjacent normal mucosa samples tested positive for HPV DNA by ISH. However, three such biopsies were found positive by histological diagnosis. This was hypothesized as possibly being due to paraneoplastic impairment of the local oesophageal mucosal immunity [26]. As this effect can not be excluded, theoretically, separate patients as controls should yield a truer estimate of the risk. However, further research should be undertaken to evaluate this.
While Oceania and Africa were found to have the highest ORs, both groups had small numbers of studies (n = 2 and n = 4, respectively) and in terms of both the risk and prevalence analyses, the CIs overlapped considerably. This could be due to inter-region differences in baseline HPV prevalence or small sample sizes within the analysis. Therefore, these results are difficult to compare with regions such as Asia and Europe, which had much larger numbers of studies and while the CIs again overlapped, it was to a smaller degree. With this in mind, comparison of the results from Asia and Europe still demonstrated geographical variance. The risk for Asian populations was found to be higher than European populations. However, sample size may also play an important role again as it is important to note that the average sample size of cases in Asian studies is 76, compared to the average 40 of European studies. Interestingly, the geographical variance is in direct opposition to that of cervical cancer, which has a higher HPV prevalence in Europe, North America and Australia than Africa and Asia [9]. Given the questionable validity of these results without further research into general population prevalence of HPV as well as the inter-region differences from larger studies, it is difficult to interpret the significance of the geographical differences noted.
Previous research has shown that OSCC is the second most common cause of death in Chinese males [38]. Chinese studies made up a large proportion of our meta-analysis (35 studies, n = 3861 cases) and were found to have a pooled OR of 2·85. This put China at a greater risk than the global estimate of 2·69. The prevalence of HPV in OSCC in China was also found to be greater than the global estimate by 8%. A previous meta-analysis published in 2009 on the prevalence of HPV in OSCC in China found an average prevalence of 46·9% (95% CI 43·8–50·0) [147], which is considerably higher than that of the present analysis, where the results showed a prevalence of 32·8%. However, the 2009 meta-analysis had a number of limitations – it included only 15 studies, compared to the 23 of this study, included studies only using PCR in the detection of HPV and excluded all studies that were not in Chinese.
This study attempted to analyse the general population prevalence utilizing the separate control group data; however, only 33 studies could be included (as opposed to normal mucosa adjacent to the tumour site) – less than a third of the included studies. On a global scale 22·8% of controls were HPV positive. Regional analysis demonstrated that the estimated general population prevalence in Asia was 35·2%, Europe 19·8%, Africa 7·7% and 0% in Oceania. Given the number of studies utilized to gain these results (Asia n = 19, Europe n = 3, Africa n = 2, Oceania n = 1), it is difficult to assess the reliability of these results. A wider study of the general population is required before an accurate prevalence of HPV can be determined.
Due to the multiple detection methods used, a subgroup analysis was also performed to evaluate for any differences. Previous research has shown that in terms of sensitivity of HPV detection, ISH is almost equivalent to PCR, and in terms of reproducibility it is the superior detection method [148]. Therefore it is interesting to note that subgroup analysis showed that studies using ISH resulted in a pooled OR of 5·98 (95% CI 3·46–10·33), with minimal heterogeneity (I2 = 0·00, P = 0·92); however, PCR studies resulted in a much lower risk (OR 2·05, 95% CI 1·45–2·90) with a much higher heterogeneity (I2 = 58·79, p < 0·001). A possible explanation for this is the individual results in the ISH analysis as there were only seven studies included in the group, compared to the 41 in the PCR group.
The PAR% was also calculated based on data from this meta-analysis. The results showed that globally 15·7% of OSCC cases can be attributed to HPV. A limitation of this analysis is the use of the data from studies in this meta-analysis and a truer PAR% would be gained from using continent-wide statistics. This would require data on the incidence of OSCC throughout each region as well as oesophageal HPV positivity, which is not available at the current time. However, as a preliminary measure, our results demonstrate that 19·0% of OSCC cases in Asia can be attributed to HPV compared to 6·3% in Europe. Furthermore, Africa was found to have the highest PAR% at 28·7%; however, given the small number of studies from this region it is difficult to determine the significance – similarly for Oceania.
This meta-analysis has several strengths. A broad literature search was conducted with no language restrictions, a point which has been a limitation of previous meta-analyses on this topic. The search was also geographically unrestricted, which allowed for a broader insight into the global prevalence and risk associated with HPV and OSCC, as well as enabling the analysis of geographical variances.
This study also had several limitations. First, many of the studies included had small sample sizes – only 22 out of the total 131 studies had sample sizes over 100 cases. However, the risk ratios for different sample sizes (n < 10, n < 20, n < 50) were calculated and there was not statistically significant difference between them, suggesting that this had little impact on the combined estimates. Some analyses showed heterogeneity, which may have been due to small sample size. The use of adjacent normal mucosa as a control group also limited our ability to compare the results throughout the analysis. Only two of the studies provided ORs that had been adjusted for known risk factors for oesophageal cancer (i.e. alcohol and smoking), while the others provided only the raw data and unadjusted ORs were calculated. Therefore while this study was able to demonstrate an increased risk of OSCC in the presence of HPV infection, its independence as a risk factor is yet to be determined.
These results have demonstrated a considerable global association between the presence of HPV infection and the development of OSCC. They have implications for future research into the transmission of the virus to oesophageal mucosa as well as possible future treatment regimens for oesophageal cancer. On a regional scale, in areas such as Asia, where the incidence of OSCC is high, these results may have implications for immunization schemes and programmes advocating for preventative measures to minimize the transmission of the virus.
In conclusion, this study has confirmed a relationship between OSCC and HPV infection. In addition, it has demonstrated an increased risk in developing countries, particularly China, as well as with HPV-16 infection compared to HPV-18. The prevalence of HPV DNA in OSCC was found to be higher in similar areas, and HPV-16 found to be the most prevalent globally, with the highest risk in comparison to other HPV types.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Ferlay J, et al. GLOBOCAN 2008 v. 1.2 International Agency for Research on Cancer (http://globocan.iarc.fr). Accessed 6 January 2012.
- 2.US DHHS. A snapshot of esophageal cancer. US Department of Health and Human Services, National Institutes of Health, 2011.
- 3.Fast Stats. (http://seer.cancer.gov/faststats/index.php). Accessed 6 January 2012.
- 4.Morgan RJ, et al. Investigation of oesophageal adenocarcinoma for viral genomic sequences. European Journal of Surgical Oncology 1997; 23: 24–29. [DOI] [PubMed] [Google Scholar]
- 5.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Seminars in Radiation Oncology 2007; 17: 2–9. [DOI] [PubMed] [Google Scholar]
- 6.Syrjänen KJ. HPV infections and oesophageal cancer. Journal of Clinical Pathology 2002; 55: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamangar F, et al. Esophageal cancer in Northeastern Iran: a review. Archives of Iranian Medicine 2007; 10: 70–82. [PubMed] [Google Scholar]
- 8.zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology 2009; 384: 260–265. [DOI] [PubMed] [Google Scholar]
- 9.Smith JS, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International Journal of Cancer 2007; 121: 621–632. [DOI] [PubMed] [Google Scholar]
- 10.Syrjänen KJ. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Archiv fur Geschwulstforschung 1982; 52: 283–292. [PubMed] [Google Scholar]
- 11.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009; 151: 264–269, W264. [DOI] [PubMed] [Google Scholar]
- 12.Emadian O, et al. Correlation of human papillomavirus infection with esophageal squamous cell carcinoma. Journal of Babol University of Medical Sciences 2011; 13. [Google Scholar]
- 13.Erol D, et al. Investigation of the presence of human papillomavirus DNA in various gastrointestinal carcinoma samples. Mikrobiyoloji bülteni 2009; 43: 259–268. [PubMed] [Google Scholar]
- 14.Junquera F, et al. High prevalence of human papillomavirus in a spanish population with esophageal cancer. Gastroenterology 2009; 136: A459. [Google Scholar]
- 15.Kim WH, et al. Detection of human papillomavirus DNA in esophageal cancer using polymerase chain reaction. Korean Journal of Gastroenterology 1992; 24: 207–215. [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, et al. Measuring inconsistency in meta-analyses. British Medical Journal 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orwin RG. A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics 1983; 8: 157–159. [Google Scholar]
- 20.Moradi A, et al. Detection of human papillomavirus DNA by PCR in esophageal squamous cell carcinoma from Turkmen Sahra, North-East of Iran. Iranian Biomedical Journal 2002; 6: 19–23. [Google Scholar]
- 21.Moradi A, Mokhtari-Azad T. Detection of HPV in cancerous and non-cancerous esophageal tissues from Turkmen-Sahra, Iran. International Journal of Cancer Research 2006; 2: 113–118. [Google Scholar]
- 22.Antonsson A, et al. High-risk human papillomavirus in esophageal squamous cell carcinoma. Cancer, Epidemiology, Biomarkers and Prevention 2010; 19: 2080–2087. [DOI] [PubMed] [Google Scholar]
- 23.Astori G, et al. Detection of human papillomavirus DNA and p53 gene mutations in esophageal cancer samples and adjacent normal mucosa. Digestion 2001; 64: 9–14. [DOI] [PubMed] [Google Scholar]
- 24.Bahnassy AA, et al. Human papillomavirus infection in Egyptian esophageal carcinoma: correlation with p53, p21, mdm2, C-erbB2 and impact on survival. Pathology International 2005; 55: 53–62. [DOI] [PubMed] [Google Scholar]
- 25.Benamouzig R, et al. Absence of human papillomavirus DNA detected by polymerase chain reaction in French patients with esophageal carcinoma. Gastroenterology 1995; 109: 1876–1881. [DOI] [PubMed] [Google Scholar]
- 26.Benamouzig R, et al. Human papillomavirus infection in esophageal squamous-cell carcinoma in western countries. International Journal of Cancer 1992; 50: 549–552. [DOI] [PubMed] [Google Scholar]
- 27.Bjorge T, et al. A prospective, seroepidemiological study of the role of human papillomavirus in esophageal cancer in Norway. Cancer Research 1997; 57: 3989–3992. [PubMed] [Google Scholar]
- 28.Brandsma JL, Abramson AL. Association of papillomavirus with cancers of the head and neck. Otolaryngology – Head & Neck Surgery 1989; 115: 621–625. [DOI] [PubMed] [Google Scholar]
- 29.Cao B, et al. LMP7/TAP2 gene polymorphisms and HPV infection in esophageal carcinoma patients from a high incidence area in China. Carcinogenesis 2005; 26: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 30.Chang F, et al. Detection of human papillomavirus DNA in cytologic specimens derived from esophageal precancer lesions and cancer. Scandinavian Journal of Gastroenterology 1990; 25: 383–388. [DOI] [PubMed] [Google Scholar]
- 31.Chang F, et al. p53 overexpression and human papillomavirus (HPV) infection in oesophageal squamous cell carcinomas derived from a high-incidence area in China. Anticancer Research 1997; 17: 709–715. [PubMed] [Google Scholar]
- 32.Damin APS, et al. Detection of human papillomavirus DNA in squamous cell carcinoma of the esophagus by auto-nested PCR. Diseases of the Esophagus 2006; 19: 64–68. [DOI] [PubMed] [Google Scholar]
- 33.Dillner J, et al. Prospective seroepidemiological evidence that human papillomavirus type 16 infection is a risk factor for oesophageal squamous cell carcinoma. British Medical Journal 1995; 311: 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farhadi M, et al. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World Journal of Gastroenterology 2005; 11: 1200–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidalgo PO, et al. High prevalence of human papillomavirus in squamous cell carcinoma and matched normal esophageal mucosa: assessment by polymerase chain reaction. Cancer 1995; 76: 1522–1528. [DOI] [PubMed] [Google Scholar]
- 36.Gao G-F, et al. No association between HPV infection and the neoplastic progression of esophageal squamous cell carcinoma: result from a cross-sectional study in a high-risk region of China. International Journal of Cancer 2006; 119: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 37.Han C, et al. Serologic association between human papillomavirus type 16 infection and esophageal cancer in Shaanxi Province, China. Journal of the National Cancer Institute 1996; 88: 1467–1471. [DOI] [PubMed] [Google Scholar]
- 38.He D, et al. Prevalence of HPV infection in esophageal squamous cell carcinoma in Chinese patients and its relationship to the p53 gene mutation. International Journal of Cancer 1997; 72: 959–964. [DOI] [PubMed] [Google Scholar]
- 39.Hussain S, et al. Methylation-mediated gene silencing of suppressor of cytokine signaling-1 (SOCS-1) gene in esophageal squamous cell carcinoma patients of Kashmir valley. Journal of Receptors and Signal Transduction 2011; 31: 147–156. [DOI] [PubMed] [Google Scholar]
- 40.Kamangar F, et al. Human papillomavirus serology and the risk of esophageal and gastric cancers: results from a cohort in a high-risk region in China. International Journal of Cancer 2006; 119: 579–584. [DOI] [PubMed] [Google Scholar]
- 41.Katiyar S, et al. p53 gene mutation and human papillomavirus (HPV) infection in esophageal carcinoma from three different endemic geographic regions of India. Cancer Letters 2005; 218: 69–79. [DOI] [PubMed] [Google Scholar]
- 42.Kawaguchi H, et al. p53 polymorphism in human papillomavirus-associated esophageal cancer. Cancer Research 2000; 60: 2753–2755. [PubMed] [Google Scholar]
- 43.Khurshid A, et al. Infection of human papillomavirus (HPV) and Epstein-Barr virus (EBV) and p53 expression in human esophageal carcinoma. Journal of the Pakistani Medical Association 1998; 48: 138–142. [PubMed] [Google Scholar]
- 44.Kiki I, et al. Detection of human papillomavirus infection in esophageal carcinomas by the histopathological method and polymerase chain reaction technique. Turkish Journal of Medical Sciences 2002; 32: 223–230. [Google Scholar]
- 45.Koh JS, et al. No association of high-risk human papillomavirus with esophageal squamous cell carcinomas among Koreans, as determined by polymerase chain reaction. Diseases of the Esophagus 2008; 21: 114–117. [DOI] [PubMed] [Google Scholar]
- 46.Kulski J, et al. Human papilloma virus DNA in oesophageal carcinoma. Lancet 1986; 2: 683–684. [DOI] [PubMed] [Google Scholar]
- 47.Lagergren J, et al. Human papillomavirus infection and esophageal cancer: a nationwide seroepidemiologic case-control study in Sweden. Journal of the National Cancer Institute 1999; 91: 156–162. [DOI] [PubMed] [Google Scholar]
- 48.Lambot MA, et al. Evaluation of the role of human papillomavirus in oesophageal squamous cell carcinoma in Belgium. Acta Gastroenterologica Belgica 2000; 63: 154–156. [PubMed] [Google Scholar]
- 49.Lenhart D, et al. Association of human papillomavirus with esophageal neoplasms. Diseases of the Esophagus 1991; 4: 123–128. [Google Scholar]
- 50.Li T, et al. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis 2001; 22: 929–934. [DOI] [PubMed] [Google Scholar]
- 51.Li T, et al. p53 codon 72 polymorphism (C/G) and the risk of human papillomavirus-associated carcinomas in China. Cancer 2002; 95: 2571–2576. [DOI] [PubMed] [Google Scholar]
- 52.Liu WK, et al. The relationship between HPV16 and expression of CD44v6, nm23H1 in esophageal squamous cell carcinoma. Virology 2005; 150: 991–1001. [DOI] [PubMed] [Google Scholar]
- 53.Liu WK, et al. The relationship between HPV16 and expression of cyclooxygenase-2, P53 and their prognostic roles in esophageal squamous cell carcinoma. European Journal of Gastroenterology and Hepatology 2010; 22: 67–74. [DOI] [PubMed] [Google Scholar]
- 54.Loke SL, et al. Human papillomavirus in oesophageal squamous cell carcinoma. Journal of Clinical Pathology 1990; 43: 909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu XM, et al. Human papillomavirus in esophageal squamous cell carcinoma of the high-risk Kazakh ethnic group in Xinjiang, China. European Journal of Surgical Oncology 2008; 34: 765–770. [DOI] [PubMed] [Google Scholar]
- 56.Lu X-M, et al. p53 polymorphism in human papillomavirus-associated Kazakh's esophageal cancer in Xinjiang, China. World Journal of Gastroenterology 2004; 10: 2775–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyronis ID, et al. K-ras mutation, HPV infection and smoking or alcohol abuse positively correlate with esophageal squamous carcinoma. Pathology and Oncology Research 2008; 14: 267–273. [DOI] [PubMed] [Google Scholar]
- 58.Lyronis ID, et al. Evaluation of the prevalence of human papillomavirus and Epstein-Barr virus in esophageal squamous cell carcinomas. International Journal of Biological Markers 2005; 20: 5–10. [DOI] [PubMed] [Google Scholar]
- 59.Matsha T, et al. Expression of p53 and its homolog, p73, in HPV DNA positive oesophageal squamous cell carcinomas. Virology 2007; 369: 182–190. [DOI] [PubMed] [Google Scholar]
- 60.Mir MM, et al. The Association of beta-catenin gene mutations and human papillomavirus in carcinoma of esophagus in a high-risk population of India. International Journal of Health Sciences 2007; 1: 177–183. [PMC free article] [PubMed] [Google Scholar]
- 61.Mizobuchi S, et al. Absence of human papillomavirus-16 and -18 DNA and Epstein-Barr virus DNA in esophageal squamous cell carcinoma. Japanese Journal of Clinical Oncology 1997; 27: 1–5. [DOI] [PubMed] [Google Scholar]
- 62.Mori M, et al. Papillomavirus and esophageal cancer in the Japanese and Chinese. American Journal of Gastroenterology 1989; 84: 1126–1127. [PubMed] [Google Scholar]
- 63.Pantelis A, et al. p53 Codon 72 polymorphism, loss of heterozygosity and high-risk human papillomavirus infection in a low-incidence German esophageal squamous cell carcinoma patient cohort. Oncology Reports 2007; 17: 1243–1248. [PubMed] [Google Scholar]
- 64.Peixoto Guimaraes D, et al. Absence of association between HPV DNA, TP53 codon 72 polymorphism, and risk of oesophageal cancer in a high-risk area of China. Cancer Letters 2001; 162: 231–235. [DOI] [PubMed] [Google Scholar]
- 65.Qi ZL, et al. Relationship between HPV16/18 E6 and 53, 21WAF1, MDM2, Ki67 and cyclin D1 expression in esophageal squamous cell carcinoma: comparative study by using tissue microarray technology. Experimental Oncology 2006; 28: 235–240. [PubMed] [Google Scholar]
- 66.Rugge M, et al. p53 alterations but no human papillomavirus infection in preinvasive and advanced squamous esophageal cancer in Italy. Cancer, Epidemiology, Biomarkers and Prevention 1997; 6: 171–176. [PubMed] [Google Scholar]
- 67.Shen Z-Y, et al. Detection of human papillomavirus in esophageal carcinoma. Journal of Medical Virology 2002; 68: 412–416. [DOI] [PubMed] [Google Scholar]
- 68.Togawa K, et al. Human papillomavirus DNA sequences in esophagus squamous cell carcinoma. Gastroenterology 1994; 107: 128–136. [DOI] [PubMed] [Google Scholar]
- 69.Van Doornum GJ, et al. Reactivity to human papillomavirus type 16 L1 virus-like particles in sera from patients with genital cancer and patients with carcinomas at five different extragenital sites. British Journal of Cancer 2003; 88: 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rensburg EJ, Venter EH, Simson IW. Human papillomavirus DNA in aerodigestive squamous carcinomas demonstrated by means of in situ hybridisation. South African Medical Journal 1993; 83: 516–518. [PubMed] [Google Scholar]
- 71.Weston AC, Prolla JC. Association between esophageal squamous cell carcinoma and human papillomavirus detected by Hybrid Capture II assay. Diseases of the Esophagus 2003; 16: 224–228. [DOI] [PubMed] [Google Scholar]
- 72.Williamson AL, Jaskiesicz K, Gunning A. The detection of human papillomavirus in oesophageal lesions. Anticancer Research 1991; 11: 263–265. [PubMed] [Google Scholar]
- 73.Yang WJ, et al. P53 codon 72 polymorphism and the risk of esophageal squamous cell carcinoma. Molecular Carcinogenesis 2008; 47: 100–104. [DOI] [PubMed] [Google Scholar]
- 74.Yao P-F, et al. Evidence of human papilloma virus infection and its epidemiology in esophageal squamous cell carcinoma. World Journal of Gastroenterology 2006; 12: 1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang D, et al. Comparison of prevalence, viral load, physical status and expression of human papillomavirus-16, -18 and -58 in esophageal and cervical cancer: a case-control study. BMC Cancer 2010; 10: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D-H, et al. Prevalence and association of human papillomavirus 16, Epstein-Barr virus, herpes simplex virus-1 and cytomegalovirus infection with human esophageal carcinoma: a case-control study. Oncology Reports 2011; 25: 1731–1738. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q-Y, et al. Infection and integration of human papillomavirus in esophageal carcinoma. International Journal of Hygiene & Environmental Health 2011; 214: 156–161. [DOI] [PubMed] [Google Scholar]
- 78.Zhou X-B, et al. Detection of human papillomavirus in Chinese esophageal squamous cell carcinoma and its adjacent normal epithelium. World Journal of Gastroenterology 2003; 9: 1170–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdirad A, et al. Human papillomavirus detected in esophageal squamous cell carcinoma in Iran. European Journal of Internal Medicine 2011. [DOI] [PubMed] [Google Scholar]
- 80.Acevedo-Nuno E, et al. Human papillomavirus DNA and protein in tissue samples of oesophageal cancer, Barrett's oesophagus and oesophagitis. Anticancer Research 2004; 24: 1319–1323. [PubMed] [Google Scholar]
- 81.Akutsu N, et al. Rare association of human papillomavirus DNA with esophageal cancer in Japan. Journal of Infectious Diseases 1995; 171: 425–428. [DOI] [PubMed] [Google Scholar]
- 82.Awerkiew S, et al. Esophageal cancer in Germany is associated with Epstein-Barr virus but not with papillomaviruses. Medical Microbiology and Immunology 2003; 192: 137–140. [DOI] [PubMed] [Google Scholar]
- 83.Ayshamgul H, et al. Association of defective HLA-I expression with antigen processing machinery and their association with clinicopathological characteristics in Kazak patients with esophageal cancer. Chinese Medical Journal (English) 2011; 124: 341–346. [PubMed] [Google Scholar]
- 84.Baines JE, et al. Consensus-degenerate hybrid oligonucleotide primers (CODEHOP) for the detection of novel papillomaviruses and their application to esophageal and tonsillar carcinomas. Journal of Virology Methods 2005; 123: 81–87. [DOI] [PubMed] [Google Scholar]
- 85.Bognar G, et al. Possible role of human papilloma virus infection in response to neoadjuvant therapy in patients with esophageal cancer. Hepatogastroenterology 2008; 55: 93–97. [PubMed] [Google Scholar]
- 86.Castillo A, et al. Human papillomavirus in esophageal squamous cell carcinoma in Colombia and Chile. World Journal of Gastroenterology 2006; 12: 6188–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang F, et al. Human papillomavirus involvement in esophageal carcinogenesis in the high-incidence area of China. A study of 700 cases by screening and type- specific in situ hybridization. Scandinavian Journal of Gastroenterology 2000; 35: 123–130. [DOI] [PubMed] [Google Scholar]
- 88.Chang F, et al. Human papillomavirus (HPV) DNA in esophageal precancer lesions and squamous cell carcinomas from China. International Journal of Cancer 1990; 45: 21–25. [DOI] [PubMed] [Google Scholar]
- 89.Chang F, et al. Screening for human papillomavirus infections in esophageal squamous cell carcinomas by in situ hybridization. Cancer 1993; 72: 2525–2530. [DOI] [PubMed] [Google Scholar]
- 90.Chang F, et al. Human papillomavirus involvement in esophageal precancerous lesions and squamous cell carcinomas as evidenced by microscopy and different DNA techniques. Scandinavian Journal of Gastroenterology 1992; 27: 553–563. [DOI] [PubMed] [Google Scholar]
- 91.Chang FJ, et al. Evaluation of HPV, CMV, HSV and EBV in esophageal squamous cell carcinomas from a high-incidence area of China. Anticancer Research 2000; 20: 3935–3940. [PubMed] [Google Scholar]
- 92.Chen B, Yin H, Dhurandhar N. Detection of human papillomavirus DNA in esophageal squamous cell carcinomas by the polymerase chain reaction using general consensus primers. Human Pathology 1994; 25: 920–923. [DOI] [PubMed] [Google Scholar]
- 93.de Villiers EM, et al. Esophageal squamous cell cancer in patients with head and neck cancer: Prevalence of human papillomavirus DNA sequences. International Journal of Cancer 2004; 109: 253–258. [DOI] [PubMed] [Google Scholar]
- 94.de Villiers EM, et al. An interlaboratory study to determine the presence of human papillomavirus DNA in esophageal carcinoma from China. International Journal of Cancer 1999; 81: 225–228. [DOI] [PubMed] [Google Scholar]
- 95.Ding GC, et al. Human papillomavirus DNA and P16(INK4A) expression in concurrent esophageal and gastric cardia cancers. World Journal of Gastroenterology 2010; 16: 5901–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dreilich M, et al. High-risk human papilloma virus (HPV) and survival in patients with esophageal carcinoma: a pilot study. BMC Cancer 2006; 6: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Far AE, et al. Frequency of human papillomavirus infection in oesophageal squamous cell carcinoma in Iranian patients. Scandinavian Journal of Infectious Diseases 2007; 39: 58–62. [DOI] [PubMed] [Google Scholar]
- 98.Furihata M, et al. The detection of either human papillomavirus genomes (types 16 and 18) or overexpressed p53 protein in esophageal cancers patients from Kochi, Japan [14]. American Journal of Gastroenterology 1993; 88: 471–472. [PubMed] [Google Scholar]
- 99.Furihata M, et al. Prognostic significance of human papillomavirus genomes (type-16, -18) and aberrant expression of p53 protein in human esophageal cancer. International Journal of Cancer 1993; 54: 226–230. [DOI] [PubMed] [Google Scholar]
- 100.Goto A, et al. Human papillomavirus infection in lung and esophageal cancers: analysis of 485 Asian cases. Journal of Medical Virology 2011; 83: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 101.Hale MJ, Liptz TR, Paterson AC. Association between human papillomavirus and carcinoma of the oesophagus in South African blacks. A histochemical and immunohistochemical study. South African Medical Journal 1989; 76: 329–330. [PubMed] [Google Scholar]
- 102.Hasegawa M, et al. Expression of p21/WAF-1, status of apoptosis and p53 mutation in esophageal squamous cell carcinoma with HPV infection. Pathology International 2002; 52: 442–450. [DOI] [PubMed] [Google Scholar]
- 103.Herrera-Goepfert R, et al. Human papilloma virus and esophageal carcinoma in a Latin-American region. World Journal of Gastroenterology 2009; 15: 3142–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hille JJ, et al. Human papillomavirus infection related to oesophageal carcinoma in black South Africans. A preliminary study. South African Medical Journal 1986; 69: 417–420. [PubMed] [Google Scholar]
- 105.Hille JJ, et al. Human papillomavirus and carcinoma of the esophagus. New England Journal of Medicine 1985; 312: 1707. [DOI] [PubMed] [Google Scholar]
- 106.Hippelainen M, et al. Mitotic activity index, volume corrected mitotic index and human papilloma-virus suggestive morphology are not prognostic factors in carcinoma of the oesophagus. Anticancer Research 1993; 13: 677–681. [PubMed] [Google Scholar]
- 107.Kamath, et al. Investigation of the association of esophageal carcinoma with human papillomaviruses. Diseases of the Esophagus 2000; 13: 122–124. [DOI] [PubMed] [Google Scholar]
- 108.Kiyabu MT, et al. Detection of human papillomavirus in formalin-fixed, invasive squamous carcinomas using the polymerase chain reaction. American Journal of Surgical Pathology 1989; 13: 221–224. [DOI] [PubMed] [Google Scholar]
- 109.Kok TC, et al. No evidence of known types of human papillomavirus in squamous cell cancer of the oesophagus in a low-risk area. Rotterdam Oesophageal Tumour Study Group. European Journal of Cancer 1997; 33: 1865–1868. [DOI] [PubMed] [Google Scholar]
- 110.Koshiol J, et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. International Journal of Cancer 2010; 127: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kulski JK, et al. Survey of histologic specimens of human cancer for human papillomavirus types 6/11/16/18 by filter in situ hybridization. American Journal of Clinical Pathology 1990; 94: 566–570. [DOI] [PubMed] [Google Scholar]
- 112.Lam KY, et al. Presence of human papillomavirus in esophageal squamous cell carcinomas of Hong Kong Chinese and its relationship with p53 gene mutation. Human Pathology 1997; 28: 657–663. [DOI] [PubMed] [Google Scholar]
- 113.Lavergne D, de Villiers EM. Papillomavirus in esophageal papillomas and carcinomas. International Journal of Cancer 1999; 80: 681–684. [DOI] [PubMed] [Google Scholar]
- 114.Lewensohn-Fuchs I, et al. Involvement of aberrant p53 expression and human papillomavirus in carcinoma of the head, neck and esophagus. Anticancer Research 1994; 14: 1281–1285. [PubMed] [Google Scholar]
- 115.Matsha T, et al. Human papillomavirus associated with oesophageal cancer. Journal of Clinical Pathology 2002; 55: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller BA, et al. Human papillomavirus type 16 DNA in esophageal carcinomas from Alaska Natives. International Journal of Cancer 1997; 71: 218–222. [DOI] [PubMed] [Google Scholar]
- 117.Mohseni Mehran SM. Detection of human papilloma virus in esophageal squamous cell carcinoma in Guilan province. Journal of Clinical and Diagnostic Research 2010; 4: 2373–2377. [Google Scholar]
- 118.Morgan RJ, et al. Human papillomavirus and oesophageal squamous cell carcinoma in the UK. European Journal of Surgical Oncology 1997; 23: 513–517. [DOI] [PubMed] [Google Scholar]
- 119.Nakamura T, et al. Expression of p53 protein related to human papillomavirus and DNA ploidy in superficial esophageal carcinoma. Surgery Today 1995; 25: 591–597. [DOI] [PubMed] [Google Scholar]
- 120.Ogura H, et al. Human papillomavirus DNA in squamous cell carcinomas of the respiratory and upper digestive tracts. Japanese Journal of Clinical Oncology 1993; 23: 221–225. [PubMed] [Google Scholar]
- 121.Ono H, et al. Interrelationship between the expression of h-ras p21, C-myc and p53 proteins, and the infection of high-risk human papillomaviruses in Japanese patients with esophageal squamous-cell carcinoma. Oncology Reports 1994; 1: 831–836. [DOI] [PubMed] [Google Scholar]
- 122.Ostrow RS, et al. A survey of human cancers for human papillomavirus DNA by filter hybridization. Cancer 1987; 59: 429–434. [DOI] [PubMed] [Google Scholar]
- 123.Paz IB, et al. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer 1997; 79: 595–604. [DOI] [PubMed] [Google Scholar]
- 124.Poljak M, Cerar A. Human papillomavirus type 16 DNA in oesophageal squamous cell carcinoma. Anticancer Research 1993; 13: 2113–2116. [PubMed] [Google Scholar]
- 125.Poljak M, Cerar A, Seme K. Human papillomavirus infection in esophageal carcinomas: a study of 121 lesions using multiple broad-spectrum polymerase chain reactions and literature review. Human Pathology 1998; 29: 266–271. [DOI] [PubMed] [Google Scholar]
- 126.Rakoczy P, et al. Detection of human papillomavirus type 16 DNA in cervical swabs and formalin-fixed, paraffin-embedded squamous cell carcinomas of non-genital tissues using a synthetic oligodeoxynucleotide probe. Journal of Virological Methods 1989; 25: 325–336. [DOI] [PubMed] [Google Scholar]
- 127.Saegusa M. Absence of human papillomavirus genomic sequences detected by the polymerase chain reaction in oesophageal and gastric carcinomas in Japan. Journal of Clinical Pathology – Molecular Pathology 1997; 50: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shibagaki I, et al. p53 mutation, murine double minute 2 amplification, and human papillomavirus infection are frequently involved but not associated with each other in esophageal squamous cell carcinoma. Clinical Cancer Research 1995; 1: 769–773. [PubMed] [Google Scholar]
- 129.Shimada Y, et al. Genetic alterations in patients with esophageal cancer with short- and long-term survival rates after curative esophagectomy. Annals of Surgery 1997; 226: 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shuyama K, et al. Human papillomavirus in high- and low-risk areas of oesophageal squamous cell carcinoma in China. British Journal of Cancer 2007; 96: 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Si HX, et al. Human papillomavirus infection and loss of heterozygosity in esophageal squamous cell carcinoma. Cancer Letters 2004; 213: 231–239. [DOI] [PubMed] [Google Scholar]
- 132.Si HX, et al. Viral load of HPV in esophageal squamous cell carcinoma. International Journal of Cancer 2003; 103: 496–500. [DOI] [PubMed] [Google Scholar]
- 133.Smits HL, et al. Absence of human papillomavirus DNA from esophageal carcinoma as determined by multiple broad spectrum polymerase chain reactions. Journal of Medical Virology 1995; 46: 213–215. [DOI] [PubMed] [Google Scholar]
- 134.Sobti RC, et al. Telomerase activation and incidence of HPV in human gastrointestinal tumors in North Indian population. Molecular and Cellular Biochemistry 2001; 217: 51–56. [DOI] [PubMed] [Google Scholar]
- 135.Strickler HD, et al. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. European Journal of Cancer Prevention 1998; 7: 305–313. [DOI] [PubMed] [Google Scholar]
- 136.Suzuk L, et al. Detection of human papillomavirus in esophageal squamous cell carcinoma. Cancer 1996; 78: 704–710. [DOI] [PubMed] [Google Scholar]
- 137.Takahashi A, et al. High-risk human papillomavirus infection and overexpression of p53 protein in squamous cell carcinoma of the esophagus from Japan. Diseases of the Esophagus 1998; 11: 162–167. [DOI] [PubMed] [Google Scholar]
- 138.Talamini G, et al. Alcohol, smoking and papillomavirus infection as risk factors for esophageal squamous-cell papilloma and esophageal squamous-cell carcinoma in Italy. International Journal of Cancer 2000; 86: 874–878. [DOI] [PubMed] [Google Scholar]
- 139.Toh Y, et al. Detection of human papillomavirus DNA in esophageal carcinoma in Japan by polymerase chain reaction. Cancer 1992; 70: 2234–2238. [DOI] [PubMed] [Google Scholar]
- 140.Tornesello ML, et al. Detection of mucosal and cutaneous human papillomaviruses in oesophagitis, squamous cell carcinoma and adenocarcinoma of the oesophagus. Journal of Clinical Virology 2009; 45: 28–33. [DOI] [PubMed] [Google Scholar]
- 141.Turner JR, et al. Low prevalence of human papillomavirus infection in esophageal squamous cell carcinomas from North America: analysis by a highly sensitive and specific polymerase chain reaction-based approach. Human Pathology 1997; 28: 174–178. [DOI] [PubMed] [Google Scholar]
- 142.Wang X, et al. Detection of HPV DNA in esophageal cancer specimens from different regions and ethnic groups: a descriptive study. BMC Cancer 2010; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.White RE, et al. Absence of human papillomavirus in esophageal carcinomas from southwestern Kenya. Diseases of the Esophagus 2005; 18: 28–30. [DOI] [PubMed] [Google Scholar]
- 144.Woo YJ, Yoon HK. In situ hybridization study on human papillomavirus DNA expression in benign and malignant squamous lesions of the esophagus. Journal of Korean Medical Science 1996; 11: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng J, et al. Lack of human papillomavirus types 16 and 18 DNA in esophageal squamous carcinoma cell lines. Chinese Medical Journal (English) 1999; 112: 534–537. [PubMed] [Google Scholar]
- 146.Government A. List of Developing Countries as declared by the Minister for Foreign Affairs. AusAID, Canberra, 2012. [Google Scholar]
- 147.Li SY, et al. Meta analysis on etiological relationship between human papillomavirus and esophageal carcinoma [in Chinese]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2009; 23: 85–87. [PubMed] [Google Scholar]
- 148.Wiedorn KH, et al. Comparison of in-situ hybridization, direct and indirect in-situ PCR as well as tyramide signal amplification for the detection of HPV. Histochemistry and Cell Biology 1999; 111: 89–95. [DOI] [PubMed] [Google Scholar]
- 149.Sitas F, et al. InterSCOPE Study: associations between esophageal squamous cell carcinoma and human papillomavirus serological markers. Journal of the National Cancer Institute 2012; 104: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]