SUMMARY
We used a national survey of 7578 randomly selected respondents in 2008–2009 to identify the period prevalence of acute respiratory infection (ARI) by season and state, and to estimate the incidence of ARI in the Australian community. A case was defined as any episode of cold or flu with at least one of the following symptoms: fever, chills, sore throat, running nose, or cough in the past 4 weeks. Frequency data were weighted to the Australian population. The response rate to the survey was 49%, and 19·9% (1505/7578) of respondents reported an ARI in the previous 4 weeks, which extrapolated to 68·9 million cases [95% confidence interval (CI) 65·1–72·7] of ARI in Australia annually. The incidence was 3·2 (95% CI 3·0–3·4) cases of ARI/person per year, and was highest in young children and lowest in older people. ARI imposes a significant burden on Australian society.
Key words: Acute respiratory infection (ARI), Australia, epidemiology, incidence, survey
INTRODUCTION
Acute respiratory infections (ARI) are very common illnesses that usually have a minor and temporary impact. However, they can also have severe consequences, accounting for about 5·8 million deaths globally in 2010 [1]. They are a significant causes of morbidity and mortality in developing countries, primarily in children aged <5 years [2]. In industrialized countries, the sheer number of people infected in a given year means that the non-health impacts, such as lost productivity, social disruption, and use of healthcare services, are significant [1, 3].
Respiratory infections usually follow a seasonal pattern in temperate regions of the world; highest during autumn and winter. The onset, duration and severity of ARI depend on many factors such as the characteristics of pathogens causing infection, population susceptibility and environmental factors [4]. Knowledge of the magnitude of the disease burden and how the disease occurs by time, place and person provides a baseline for future monitoring and understanding the epidemiology of this common illness.
Australian studies of respiratory infections report that the incidence of respiratory illness varies between 2·2 and 5·6 cases/person per year [5–7]. The variations in the methods and definitions used in these studies make comparisons problematic. Australian studies have been conducted in selected communities or hospital settings and may not represent incidence of ARI in the general population.
In Australia, it is estimated that respiratory tract infections account for 6–7 million visits to general practitioners (GPs) each year [8]. The combined death rate for pneumonia and influenza places respiratory infections as the sixth leading cause of death in Australia – accounting for 2715 deaths (2% of all deaths), and 61 014 hospital admissions in 2006–2007 [9].
The aim of this study was to estimate the incidence and describe the epidemiology of respiratory episodes from a national cross-sectional survey to better understand the health burden imposed by ARI in Australia.
METHODS
As part of a national study assessing changes in the prevalence of infectious diseases in Australia, a computer-assisted telephone-based survey was administered to collect data regarding respiratory symptoms and associated complaints over a 1-year period during 2008–2009. We randomly selected the household and recruited the person who had the last birthday in that household into the study. The full methodology applied in this study was a repeat of an earlier cross-sectional survey that has been described previously [10]. The questionnaire covered information on gastrointestinal and respiratory symptoms, specifically cold or flu and associated fever or chills. Potential exposures were explored and participants were asked about the duration of illness, healthcare-seeking behaviour, medication usage as well as the effect of the illness on their work. This paper presents and analyses information collected regarding respiratory symptoms.
Case definition for analysis
A case of ARI was defined as any episode of cold or flu, with at least one of the following symptoms: fever, chills, sore throat, runny nose, or cough in the past 4 weeks. Respondents who reported respiratory symptoms were asked the date their symptoms began.
The number and duration of respiratory symptoms were evaluated, and a new episode was defined as following 1 week or more without any of cold or flu, runny nose, sore throat, fever or cough. Duration was calculated from the first to the last day of respiratory symptoms. The proportion of participants with vomiting or diarrhoea associated with respiratory symptoms was also determined. Seasons were defined as follows: spring (September–November), summer (December–February), autumn (March–May), winter (June–August).
Analysis
The primary aim of the analysis was to estimate the annual number of ARI cases in the Australian community in a single year. The period prevalence in the last 4 weeks was estimated by state, season and demographic factors. As the sample was stratified by state, we incorporated post-stratification in analysis to adjust for known differences between the survey sample and the target population by weighting to the 2008 resident population for age, sex and state from the Australian Bureau of Statistics (www.abs.gov.au). We used survival analysis to estimate the mean duration of symptoms in people with ongoing illness at interview. Analysis was performed with Stata statistical package, version 12.1 [11]. The ‘svyset’ command in Stata was used to weight the data by age, sex and state during analysis.
Ethical considerations
During interview, verbal consent was obtained from all participants and from parents or guardians on behalf of children. Questions about children aged <15 years were answered by their carer. Adolescents aged 15–17 years answered questions themselves following verbal consent from their guardian. The study was approved by the ethics committees of the Australian Government Department of Health and Ageing, the Australian National University and the Cancer Council in New South Wales.
RESULTS
Overall, 49% (7578/15 465) of contacted households participated in the survey. Of the 7578 study participants, 498 (6·6%) participants were aged ⩽9 years, 497 (6·6%) were aged 10–19 years, and 6583 (87·8%) were aged ⩾20 years; 161 (2·1%) were Aboriginal and Torres Strait Islander respondents (see Appendix).
Period prevalence
Overall, 19·9% (1505/7578) met the case definition for ARI, of which 34·8% were associated with fever or chills and 69·5% with cough. There were 19·2% (1449/7566) of respondents that reported one episode of respiratory symptoms during the last month, followed by 1·3% (95/7566) of respondents with two episodes, and 0·1% (7/7566) who experienced ⩾3 episodes. The mean duration of reported acute respiratory symptoms by each age group ranged from 8·3 days [95% confidence interval (CI) 6·5–10·1] in the 10–19 years age group to 15·4 days (95% CI 13·6–17·2) in respondents ⩾65 years. Respondents reported that respiratory symptoms lasted an average of 12·6 days (95% CI 11·9–13·3). Following post-stratification of survey results to the Australian population, the resulting weighted 4-week period prevalence of ARI was 24·8% (95% CI 23·4–26·2).
Males reported fewer ARI episodes than females (Table 1). The weighted period prevalence of ARI was highest in the youngest age group (0–4 years) at 46·3%, while people aged ⩾65 years had the lowest prevalence at 9·7%. Young children were also most likely to experience a greater number of episodes. The period prevalence of ARI in the previous 4 weeks among Indigenous respondents was 39·2%.
Table 1.
Period prevalence of acute respiratory infections by age, gender and Indigenous status, 2008–2009, Australia
| Characteristic | Period prevalence (%) | 95% CI |
|---|---|---|
| Sex | ||
| Male | 23·1 | 21·1–25·2 |
| Female | 26·4 | 24·5–28·3 |
| Age (years) | ||
| 0–4 | 46·3 | 38·9–53·7 |
| 5–9 | 41·0 | 33·8–48·1 |
| 10–19 | 35·8 | 30·9–40·8 |
| 20–29 | 29·4 | 24·5–34·2 |
| 30–39 | 24·2 | 20·7–27·7 |
| 40–49 | 19·9 | 16·9–22·8 |
| 50–59 | 15·4 | 13·1–17·7 |
| 60–64 | 14·5 | 11·4–17·7 |
| ⩾65 | 9·7 | 8·3–11·1 |
| Indigenous status | ||
| Indigenous | 39·2 | 27·1–51·3 |
| Non-Indigenous | 24·5 | 23·1–25·9 |
Data weighted for state, age and sex.
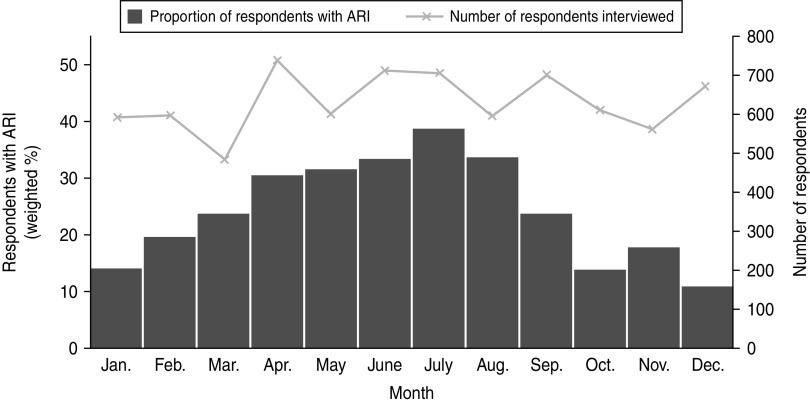
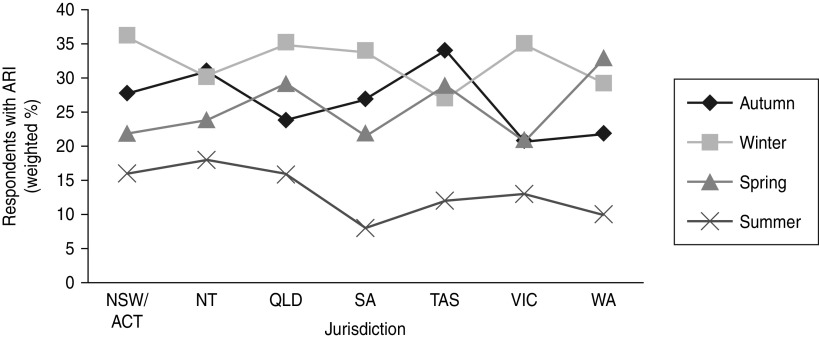
Overall a greater number of ARI episodes were reported in winter compared to summer (Fig. 1). The period prevalence of ARI was 34·3% (95% CI 31·4–37·2) in winter. Compared to winter, a lower prevalence of ARI was observed in spring (24·6%, 95% CI 21·8–27·4) and autumn (24·9%, 95% CI 22·0–27·8). The lowest prevalence was observed in summer (14·1%, 95% CI 11·6–16·5).The seasonal peak varied by state and territory, with the Northern Territory weighted period prevalence being 31·4% (95% CI 24·1–38·6) in autumn to New South Wales at 35·6% (95% CI 31·1–40·1) in winter (Fig. 2).
Fig. 1.
Summary of respondents interviewed and weighted proportion of survey respondents experiencing acute respiratory infection (ARI) in the previous 4 weeks by month of interview, 2008–2009, Australia.
Fig. 2.
The weighted 4-week period prevalence of acute respiratory infections (ARI) by state or territory and season, 2008–2009, Australia. NSW/ACT, New South Wales/Australian Capital Territory; NT, Northern Territory; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
There were 7·4% (111/1505) of respondents with an ARI that reported concomitant gastrointestinal symptoms. Concurrent diarrhoea and vomiting were present during 4·5% and 3·9% of ARI episodes, respectively. Children aged <5 years suffered most from concomitant gastrointestinal symptoms during respiratory episode with a proportion of 41·1%.
ARI incidence
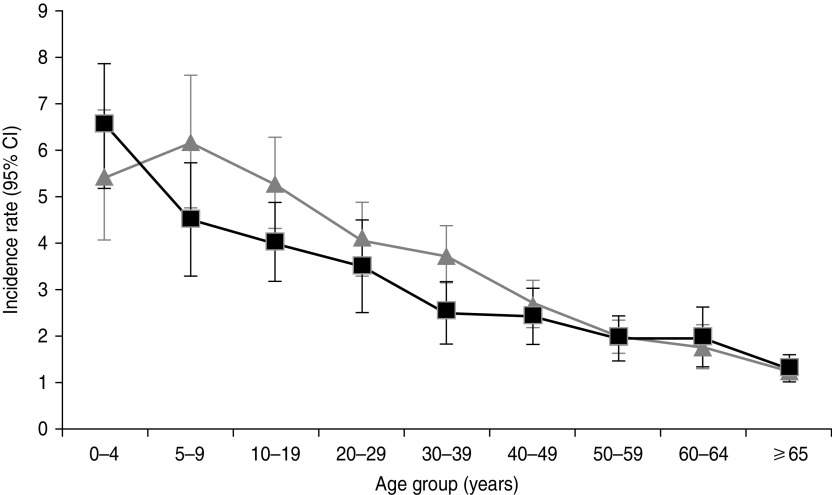
Extrapolating data to the Australian population, there were 68·9 million cases (95% CI 65·1–72·7 million) of ARI in Australia in one year, equating to an incidence of 3·2 (95% CI 3·0–3·4) cases of ARI per person annually. In comparing the estimated incidence of ARI by state or territory, Queensland (3·4, 95% CI 3·0–3·8 episodes/person per year) and New South Wales/Australian Capital Territory (3·4, 95% CI 3·1–3·7 episodes/person per year) had the highest incidence rates. Victoria had the lowest ARI incidence with an estimated rate of 2·9 (95% CI 2·5–3·3) episodes/person per year (Table 2). Overall the estimated community incidence for females was 3·4 (95% CI 3·2–3·7) ARI cases/person per year, compared to 3·0 (95% CI 2·7–3·2) cases/person per year for males. When incidences in different age groups were examined against gender, males aged 0–4 years showed the highest incidence of 6·5 (95% CI 5·2–7·9) cases/person per year, followed by females aged 5–9 years with an incidence of 6·2 (95% CI 4·8–7·6) cases/person per year. It was also observed that ARI incidence decreased with increasing age (Fig. 3).
Table 2.
Incidence of acute respiratory infections by state and territory, 2008–2009, Australia
| State/territory | Incidence rate (per person per years) | 95% CI |
|---|---|---|
| New South Wales/Australian Capital Territory | 3·4 | 3·1–3·7 |
| Northern Territory | 3·4 | 2·9–3·8 |
| Queensland | 3·4 | 3·00–3·8 |
| South Australia | 3·1 | 2·7–3·6 |
| Tasmania | 3·3 | 2·9–3·8 |
| Victoria | 2·9 | 2·5–3·4 |
| Western Australia | 3·1 | 2·6–3·6 |
Data weighted for state, age and sex.
Fig. 3.
Incidence of acute respiratory infection/person per year, by age and sex (weighted to the Australian population), 2008–2009. Error bars represent 95% confidence intervals (CIs) around point estimates. –■–, Males; –▲–, females.
DISCUSSION
This national survey shows that the incidence of ARI in Australia is very high, with an estimated 68·9 million cases in a single year. Australia has an annual incidence rate of 3·2 cases/person per year, similar to other developed countries [12], but higher than results from some studies, such as those conducted in China [13]. However, the differences in study design, demographics of target population, definition and classification of ARI, the period required between episodes, and the methodology employed make any direct comparisons between other studies and our study problematic. For example, some studies were prospective longitudinal designs while cross-sectional telephone interviews were used in our study. Whether a recall method causes an overestimate due to ‘telescoping’ events into a shorter time-frame is unknown [14]. Differences in data collection methods have an impact on the final estimation. The definition of ARI should be similar when comparing across countries, as even an apparently small change in the definition can cause a large change in the ARI incidence. Although Queensland had the highest period prevalence of ARI in Australia, there was no significant variation in different jurisdictions. The Northern Territory, which has a population comprising almost 30% Indigenous persons, had a similar peak prevalence of ARI as the other states, which are about 2% Indigenous. This could be because that the small numbers of Indigenous respondents in most states makes interpretation of the impact on Indigenous people difficult. As the survey was conducted by telephone, remote Indigenous communities would not have been included. Although the period prevalence of ARI in the Northern Territory was similar compared to other states, this jurisdiction showed peaks of respiratory infection in both autumn and winter and exhibited less variability between seasons. One explanation could be that as a tropical area, the weather condition in Northern Territory differed to other temperate zones of Australia, which may benefit the transmission of certain pathogens. According to the Northern Territory Tropical Influenza Surveillance Scheme, in 2008 the rate of influenza-like illness (ILI) presentations in Northern Territory was higher in May and June compared to earlier in the year, and then increased consistently until mid-August [15, 16]. The higher notification rate of ILI during autumn and winter seasons in the Northern Territory area is consistent with the appearance of two peaks of ARI in our study.
Seasonally, a greater number of episodes per week were observed in winter compared to summer months, which reflects what is known from syndromic surveillance for ILI [15, 17]. Notably, mechanisms of seasonality are thought to vary for the different pathogens; some have increased carriage in birds in certain seasons, while some have enhanced replication in cold and humid condition [18–20]. Physical mechanisms involving the pathogens and susceptibility of the host immune system may be responsible for the transmission of infectious diseases. Breathing cold and dry air can slow mucociliary clearance of the nasal passage, which has been used to explain the often observed rise in ARI following a cold-air event [21–23].
Our study showed that children aged <5 years had the highest incidence of ARI compared to other age groups, which is similar to findings in other studies [2, 5]. This rate decreased progressively in each age group, and people aged ⩾65 years had the lowest incidence of ARI. Greater number of recurrences and shorter duration of episodes were also observed in the youngest age group. Women also had a higher risk of acquiring ARI. It is likely that the behaviour of young children may increase their exposure to pathogens via person-to-person contact. Compared to males and older people, women with ARI had a higher probability of having a young child with ARI in the house (data not shown), suggesting that the transmission between a child and their carer is important. The role of sex hormones in the regulation of immune system may also contribute to the differences in ARI incidence [24]. The elderly have the lowest risk of contracting ARI, which has been reported from previous observational studies [25]. This is possibly due to living in isolation and minimal contact with children. Additionally, a certain degree of prior natural immunity to influenza has been recognized in the Australian population, particularly in those aged ⩾60 years [26]. This might be another explanation for the lower incidence of ARI in older age groups.
Identification of groups of people vulnerable to ARI is useful to guide interventions to minimize transmission and public health resource allocation. Young children and their carers are clearly at higher risk. It is important to promote hygiene in childcare centres and in the home, and identify simple measures that may prevent transmission [27, 28]. Older people living in the community do not appear at risk of increased episodes of ARI, but may suffer more severe consequences of infection [13, 29]. We did not include influenza and pneumococcal immunization status in our questionnaire and were unable to compare the incidence of participants with vaccination to those without. Given these pathogens are only two of the many that cause acute respiratory illness and vaccines only provide coverage to certain strains, we believe that this will have minimal impact on our estimates of incidence. Our results showed that 7·4% of respiratory episodes were associated with gastrointestinal symptoms and children aged <5 years were most likely to have concurrent gastrointestinal symptoms during respiratory episodes. An association between respiratory and gastrointestinal symptoms has been reported previously [14, 30, 31]. Certain pathogens, such as enterovirus, known to cause respiratory symptoms are frequently associated with gastrointestinal symptoms in children, and it is often impossible to differentiate illnesses that are primarily respiratory infections vs. those that have respiratory symptoms secondary to a gastrointestinal illness. Microbiological confirmation of respiratory illness was not possible in this study. Consequently, we were unable to comment on the incidence of specific respiratory pathogens as a cause of illness in Australia. Instead, we used a sensitive symptomatic definition for an acute respiratory episode. Thus, this should provide a close estimation of the epidemiology of ARI in the community.
The strength of our data relates to its nationwide study coverage with large numbers of participants from each of the jurisdictions. The participation rate for the survey was reasonable, although elderly females comprised the largest proportion of the respondents. The impact of the skewed age and sex distribution of study participants was taken into account through weighting data to the Australian population to adjust for known difference between the sample and target population. However, we did not collect data on the non-respondents, and therefore, were not able to assess other aspects of participation bias. Additionally, our study was based on self-reported symptoms and we did not attempt to validate these reports.
In summary, we used national data to provide an overarching perspective of ARI in Australia. The overall incidence of ARI in the Australian community, and incidence by state confirmed that ARI imposes a significant burden across Australia. Factors associated with ARI and these findings can assist in targeting further investigation and, ultimately, strategic interventions to reduce the incidence in certain groups. It is important that future research further investigates the transmission pathways between children and their carers in the home and how to best interrupt infection. Additional study about transmission modes of pathogens causing ARI in risk groups may also be important, as it is likely that sources of infection vary. These findings can assist in targeting interventions to reduce the incidence of ARI in high-risk populations.
ACKNOWLEDGEMENTS
This study was funded by the Australian Government Department of Health & Ageing.
APPENDIX
Demographic characteristics of respondents by age, gender and state, 2008–2009, Australia
| Demographic characteristics | No. of respondents (%) |
|---|---|
| Gender | |
| Male | 3024 (39·9) |
| Female | 4554 (60·1) |
| Age (years) | |
| 0–4 | 249 (3·3) |
| 5–9 | 249 (3·3) |
| 10–19 | 497 (6·6) |
| 20–29 | 507 (6·7) |
| 30–39 | 802 (10·6) |
| 40–49 | 1064 (14·0) |
| 50–59 | 1374 (18·1) |
| 60–64 | 755 (10·0) |
| ⩾65 | 2081 (27·5) |
| State/territory | |
| New South Wales/Australian Capital Territory | 2367 (31·2) |
| Northern Territory | 869 (11·5) |
| Queensland | 869 (11·5) |
| South Australia | 870 (11·5) |
| Tasmania | 868 (11·5) |
| Victoria | 867 (11·4) |
| Western Australia | 868 (11·5) |
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Rafael L, Mohsen N, Kyle F. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew J, Patwari A, Gupta P. Acute respiratory infection and pneumonia in India: a systematic review of literature for advocacy and action: UNICEF-PHFI Series on Newborn and Child Health, India. India Pediatrics 2011; 48: 191–218. [DOI] [PubMed] [Google Scholar]
- 3.Berman S. Acute respiratory infections: the forgotten pandemic. Communique from the International Conference on Acute Respiratory Infections, held in Canberra, Australia, 7–10 July 1997. Clinical Infectious Diseases 1999; 28: 188.10357581 [Google Scholar]
- 4.Vega T, Lozano JE, Meerhoff T. Influenza surveillance in Europe: establishing epidemic thresholds by the moving epidemic method (http://www.ncbi.nlm.nih.gov/pubmed/22897919). Accessed 4 April 2013. [DOI] [PMC free article] [PubMed]
- 5.Lambert SB, et al. Respiratory illness during winter: a cohort study of urban children from temperate Australia. Journal of Paediatrics and Child Health 2005; 41: 125–129. [DOI] [PubMed] [Google Scholar]
- 6.Leder K, et al. A community-based study of respiratory episodes in Melbourne, Australia. Australian and New Zealand Journal of Public Health 2003; 27: 6. [DOI] [PubMed] [Google Scholar]
- 7.Najnin N, et al. Community based study to compare the incidence and health services utilization pyramid for gastrointestinal, respiratory and dermal symptoms. BMC Health Services Research 2012; 23: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards B, Jenkins C. Respiratory infectious disease burden in Australia (http://www.thoracic.org.au/documents/papers/2007_RID_Case_Statement.pdf). Accessed 4 April 2013.
- 9.Anon. Asthma, chronic obstructive pulmonary disease and other respiratory diseases in Australia, in Asthma series, 2010. Cat. no. ACM 20. Canberra: AIHW.
- 10.Hall GV, Kirk MD, Ashbolt R. Frequency of infectious gastrointestinal illness in Australia, 2002: regional, seasonal and demographic variation. Epidemiology and Infection 2006; 134: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang HJ, Min J, Wang B. Analysis on influencing factors of acute respiratory infection in family. Chinese Journal of Public Health 2006; 22: 302–303. [Google Scholar]
- 12.Taussing LM, Wright AL. The Tucson children's respiratory study. I. design and implementation of a prospective study of acute respiratory illness in children. American Journal of Epidemiology 1989; 129: 1219–31. [DOI] [PubMed] [Google Scholar]
- 13.Wang JB, Wang QQ, Bi ZQ. Acute respiratory infections diasese research and surveillance. Chinese Journal of Epidemiology 1994; 15: 141–144.7834688 [Google Scholar]
- 14.Thomas MK, Perez E, Majowicz SE. Burden of acute gastrointestinal illness in the metropolitan region, Chile. Epidemiology Infection 2011; 139: 560–71. [DOI] [PubMed] [Google Scholar]
- 15.Kaczmarek M, Owen R, Barr IG. Annual report of the national influenza surveillance scheme, 2008. Clinical Infectious Diseases 2010; 34: 8–22. [PubMed] [Google Scholar]
- 16.Flint S, Su JY, Scott L. The early experience of pandemic (H1N1) 2009 influenza in the Northern Territory, Australia. Northern Territory Disease Control Bulletin 2009; 16: 1–8. [Google Scholar]
- 17.Anon. Australian Influenza surveillance report. No. 2, 2012. Australian Government: Department of Health and Ageing.
- 18.Mäkinen TM, Juvonen R, Jokelainen J. Cold temperature and low humidity are associated with increased occurrence of respiratory tract infections. Respiratory Medicine 2009; 103: 456–462. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert M, Silingenbergh J, Xiao X. Climate change and avian influenza. Review of Science Technology 2008; 27: 459–466. [PMC free article] [PubMed] [Google Scholar]
- 20.Weber T, Stilianakis N. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. Journal of Infection 2008; 57: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assaad FA, Reid D. Some factors influencing mortality from influenza. Bulletin of the World Health Organization 1997; 45: 113–117. [PMC free article] [PubMed] [Google Scholar]
- 22.Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngologica 2002; 122: 183–191. [DOI] [PubMed] [Google Scholar]
- 23.Fuhrmann C. The effects of weather and climate on the seasonality of influenza: what we know and what we need to know. Geography Compass 2010; 4: 718–730. [Google Scholar]
- 24.Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respiratory Medicine 2007; 101: 1845–1863. [DOI] [PubMed] [Google Scholar]
- 25.Zambon MC, et al. Contribution of influenza and respiratory synctytial virus to community cases of influenza-like illness: an observational study. Lancet 2001; 358: 1410–16. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg ME, Lai MH, Hartel GF. Response after one dose of a monovalent influenza A (H1N1) 2009 vaccine – preliminary report. New England Journal of Medicine 2009; 361: 2405–2413. [DOI] [PubMed] [Google Scholar]
- 27.Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet 2003; 3: 547–556. [DOI] [PubMed] [Google Scholar]
- 28.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet 2003; 3: 275–281. [DOI] [PubMed] [Google Scholar]
- 29.Zhang CG. Epidemiological survey on 174 cases of mild acute respiratory infection. Disease Surveillance 2005; 20: 5–7. [Google Scholar]
- 30.Lina B, Valette M, Foray S. Surveillance of community-acquired viral infections due to respiratory viruses in Rhone-Alpes (France) during winter 1994 to 1995. Journal of Clinical Microbiology 1996; 34: 3007–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang ZY, Chen X, Yang XQ. Analysis of enterovirus in hospitalized children with acute respiratory tract infections in Chongqing. Journal of Pediatric Density 2008; 26: 473–476. [Google Scholar]