SUMMARY
Recently, a number of outbreaks of measles and mumps have occurred within the UK and Europe. Healthcare workers (HCWs) are at risk of contracting and transmitting disease to patients and staff. To examine this risk at the point of entry to healthcare, we assessed the serological results of new HCWs presenting for pre-placement clearance without evidence of measles-mumps-rubella (MMR) immunity between 1 April 2010 and 31 March 2012. Overall rates of serological positivity to MMR across all age groups were 88·2%, 68·8% and 93·9%, respectively. With regard to measles and mumps, there were statistically significant decreases in the percentage of HCWs born after 1980 that had positive serology (P < 0·05). No such differences were seen between healthcare groups. Most seronegative HCWs accepted MMR vaccination. Despite our entry-level findings, the ongoing risk of a MMR outbreak within this cohort of HCWs appears low.
Key words: Infectious disease control, infectious disease epidemiology, MMR vaccination, public health
INTRODUCTION
A number of high-profile outbreaks of measles and mumps have occurred within the UK and Europe in recent times. There remains concern regarding the potential for further outbreaks of disease in vulnerable groups since it is perceived that population vaccination programmes have been ineffective in eliminating this risk.
The first national childhood measles vaccination programme was introduced in 1968, followed by a selective rubella vaccination programme for adolescent girls in 1970. Up until 1988, routine coverage against mumps in the UK was not provided, at which point a single-dose measles-mumps-rubella (MMR) vaccine was introduced for all children. A nationwide ‘catch-up’ campaign was conducted in 1994, in which over eight million children in the UK were vaccinated with measles-rubella (MR) to prevent the predicted measles epidemic at that time [1]. Finally, in 1996 a routine two-dose MMR schedule was introduced into the childhood immunization programme in the UK.
Achieving ‘herd immunity’ to MMR relies upon three key metrics which include vaccination coverage, vaccine efficacy and disease infectivity [2]. In the context of rubella, a wide uptake of highly effective vaccine has led to the virtual disappearance of the condition in the UK within recent years; with the main remaining threat relating to congenital rubella in babies of unvaccinated mothers. In the case of measles, however, poor vaccination coverage has meant only 80% of the population is thought to be protected in some areas of the UK [3]. Given the highly contagious nature of the disease, unprotected individuals tend to be ‘cherry-picked’, which is thought to underlie the recent outbreaks of measles seen in groups such as travelling communities [4]. In the last decade, there has been a rise in mumps cases in the UK seen within the late teenage and young adult age groups [5]. This has been attributed to a cohort effect, in which individuals aged >16 years at that time would not have received a mumps-containing vaccine, as well as the limited efficacy of the mumps vaccine itself. Evidence suggests that the efficacy from a single mumps vaccine in providing immunity is around 78%, and that of the two vaccines around 90% [6].
Healthcare-associated measles and mumps outbreaks remain a public health concern, since non-immune healthcare workers (HCWs) may both contract and transmit MMR to vulnerable patients and staff. Outbreaks of disease in healthcare institutions have occurred previously, and the potential for disease to spread rapidly in these settings has been recognized [7]. Although it is likely that many HCWs possess evidence of immunity to MMR at the time of pre-placement clearance, it is not clear how many of those without such evidence subsequently test seronegative to these conditions, indicating that they are not immune [8]. Such data are necessary in informing the extent of risk posed by this issue, and informing policy and best practice in this area.
The purpose of our study is to quantify this risk by identifying those HCWs that are non-immune to these conditions, as well as identifying variations in this data according to decade of birth and healthcare occupation which may have implications in terms of targeting public health action in this area.
METHODS
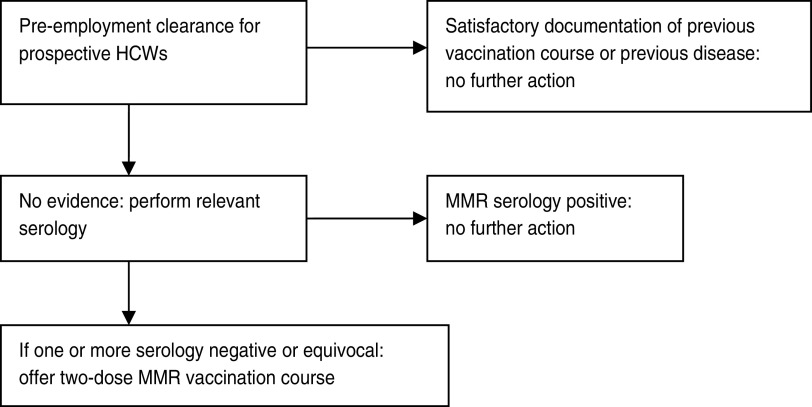
A retrospective analysis of all serological results to MMR from prospective HCWs presenting to Sheffield Occupational Health Service (SOHS) for pre-placement clearance between 1 April 2010 and 31 March 2012 was performed from a computerized database (Cohort, Tempus Software Ltd, UK). At the time of study, HCWs seeking pre-placement clearance were provided with an initial occupational health (OH) appointment in which a pre-placement questionnaire was completed. Documentation of prior MMR vaccination or infection was also confirmed. For those HCWs that were unable to provide satisfactory documentary evidence, serological testing was conducted. In the absence of positive serology, the HCW received two further appointments in which they received a course of MMR vaccine (Fig. 1).
Fig. 1.
Measles-mumps-rubella (MMR) screening policy at Sheffield Occupational Health Service.
For the purpose of further analysis, we assumed that a positive serological result indicated that an individual was likely to be immune to the condition. Results were stratified for further discussion into conventional age groups as set out in the UK Department of Health's Green Book [6], as well as by healthcare occupation. HCWs were classified into six major groups. These included: qualified doctor, qualified nursing including midwifery, allied healthcare professionals, domestic/maintenance/catering, administration/managers, and healthcare student including medical and nursing. Allied HCWs included clinical support workers, phlebotomists, physiotherapists, occupational therapists, dieticians, and podiatrists.
Odds ratios of seronegativity to measles and mumps for HCWs according to decade of birth and occupational status were calculated using a univariate logistic regression model. We believed it was appropriate to choose those HCWs born before 1960 as the age-based reference group from which to calculate odds ratios, since it is widely believed that such individuals are most likely to be immune to measles and mumps through having natural disease. In the absence of evidence from the literature to indicate an appropriate reference group, absolute rates of serological positivity for measles and mumps were calculated for the six occupational categories, and those which had the highest rates acted as reference groups.
All data analysis was conducted using Microsoft Excel. The study was registered as a service evaluation project with the Clinical Effectiveness Unit at Sheffield Teaching Hospitals. We were informed by the Unit that further ethical approval was not required for analysis of this data.
RESULTS
A total of 9697 individuals attended SOHS for pre-placement screening during the study period. Out of these, 3921 (40·4%) HCWs presented to the department without documentary evidence of prior vaccination to at least one of measles, mumps or rubella. Table 1 profiles the HCWs by decade of birth and occupational subgroup.
Table 1.
Study demographics
| Decade of birth | All HCWs | Qualified doctor | Qualified nursing/midwife | Allied healthcare worker | Domestic/ maintenance | Clerical/manager | Healthcare student |
|---|---|---|---|---|---|---|---|
| Up to 1960 | 333 (8·5) | 150 (10·8) | 47 (7·5) | 76 (9·8) | 28 (6·9) | 32 (9·2) | 0 (0) |
| 1960–1969 | 730 (18·6) | 275 (19·9) | 123 (19·6) | 122 (15·7) | 96 (23·5) | 81 (23·2) | 33 (8·8) |
| 1970–1979 | 1042 (26·6) | 400 (28·9) | 155 (24·8) | 226 (29·0) | 112 (27·5) | 89 (25·5) | 60 (16·0) |
| 1980–1989 | 1422 (36·3) | 560 (40·4) | 227 (36·3) | 264 (33·9) | 124 (30·4) | 122 (35·0) | 125 (33·3) |
| After 1990 | 394 (10·0) | 0 (0) | 74 (11·8) | 90 (11·6) | 48 (11·7) | 25 (7·1) | 157 (41·9) |
| All ages | 3921 (100) | 1385 (100) | 626 (100) | 778 (100) | 408 (100) | 349 (100) | 375 (100) |
Values given are n (%).
These 3921 HCWs underwent serological testing only to the conditions for which they did not possess a priori evidence of immunity. Overall rates of serological positivity (used as a proxy for immunity) to measles, mumps and rubella across all age groups was 88·2%, 68·8% and 93·9%, respectively. With regard to measles and mumps, the percentage of HCWs testing serologically positive declined substantially in individuals born after 1980 (Table 2).
Table 2.
Serological test results for HCWs stratified by decade of birth
| Decade of birth | All tests | Measles serology tests | Measles positive | Mumps serology tests | Mumps positive | Rubella serology tests | Rubella positive |
|---|---|---|---|---|---|---|---|
| Up to 1960 | 413 | 268 | 264 (98·5) | 277 | 214 (77·2) | 223 | 204 (91·5) |
| 1960–1969 | 820 | 567 | 549 (96·8) | 588 | 421 (71·6) | 395 | 376 (95·2) |
| 1970–1979 | 1316 | 798 | 736 (92·2) | 831 | 600 (72·3) | 596 | 575 (96·5) |
| 1980–1989 | 2584 | 1009 | 830 (82·3) | 1084 | 729 (67·3) | 809 | 767 (94·8) |
| After 1990 | 2436 | 330 | 242 (73·4) | 329 | 174 (52·9) | 396 | 350 (88·3) |
| All | 7569 | 2972 | 2621 (88·2) | 3109 | 2138 (68·8) | 2419 | 2272 (93·9) |
Values given are n or n (%).
The highest rates of serological positivity for measles were found in the qualified doctor group (754/856, 87·9%), and for mumps in the clerk/managerial subgroup (174/236, 73·7%). Statistical significance was set at P = 0·05. These acted as our reference groups within the univariate logistic regression model.
With regard to age groups, there were statistically significant increases for the odds ratio of testing seronegative to measles from 1970 onwards, with those HCWs born after 1990 being 24 times more likely to test negative than those born before 1960. Similar patterns were seen for mumps for those born after 1980, with HCWs born after 1990 around three times more likely to test negative than those born before 1960 (Table 3).
Table 3.
Odds ratio of seronegativity by decade of birth
| Decade of birth | Measles seronegativity | Mumps seronegativity | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Up to 1960 | 1 | — | 1 | — |
| 1960–1969 | 2·16 (0·73–6·46) | 0·17 | 1·35 (0·97–1·88) | 0·08 |
| 1970–1979 | 5·56 (2·00–15·43) | 0·001 | 1·31 (0·95–1·80) | 0·10 |
| 1980–1989 | 14·23 (5·23–38·71) | <0·0001 | 1·65 (1·22–2·25) | 0·001 |
| After 1990 | 24·00 (8·68–66·36) | <0·0001 | 3·03 (2·12–4·31) | <0·0001 |
OR, Odds ratio; CI, confidence interval.
With regard to healthcare occupation, no statistically significant differences were found between groups with regard to measles testing, but both allied health professionals and qualified doctors approached this value (Table 4).
Table 4.
Odds ratio of seronegativity by occupational subgroup
| Occupational group | Measles seronegativity | Mumps seronegativity | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Qualified doctor | 1 | — | 1·36 (0·99–1·88) | 0·06 |
| Qualified nursing/midwife | 0·94 (0·65–1·36) | 0·74 | 1·2298 (0·86–1·77) | 0·26 |
| Allied health professional | 1·12 (0·84–1·50) | 0·45 | 1·3722 (0·99–1·90) | 0·06 |
| Domestic/maintenance | 1·30 (0·89–1·90) | 0·17 | 1·2458 (0·85–1·82) | 0·26 |
| Clerk/managerial | 1·37 (0·91–2·07) | 0·13 | 1 | — |
| Healthcare student | 1·23 (0·87–1·73) | 0·25 | 1·3051 (0·91–1·86) | 0·1420 |
OR, Odds ratio; CI, confidence interval.
Following pre-placement assessment, 48 individuals that tested seronegative for at least one MMR condition did not receive vaccination. Reasons included refusal and contraindications to products within the MMR vaccine. For these individuals, SOHS provided advice to the manager regarding procedure for suspected MMR cases in staff including symptoms and signs suggestive of disease, and made recommendations to the employing authority regarding exclusion from ‘high-risk’ areas for contracting and transmitting MMR [9]. At SOHS, these areas included maternity units, paediatrics including outreach community work, emergency medicine, critical care, and working with immunocompromised patients.
DISCUSSION
Our findings suggest that although most HCWs present for pre-placement clearance with evidence of immunity to MMR, around 40% do not. Of these 40%, around one-third test seronegative to mumps and 10% test seronegative to measles. At SOHS, the overwhelming majority of these seronegative HCWs accepted MMR vaccination, despite the absence of a mandatory immunization policy in these circumstances.
Very few UK studies have explored the immunological status of HCWs attending pre-placement clearance to MMR, and none with a study population of this size. Studies from Italy and the UK performed around a decade ago identified that immunity to measles in HCWs was relatively high overall [10, 11]. Recent data from a small study in France provide comparable results to those seen here in terms of overall rates of serological immunity to measles, and the susceptibility of individuals aged <30 years [12]. This is of interest, given that the childhood MMR vaccination in both countries, as well as in the rest of Europe, is similar.
It is likely that our results for HCWs are representative of those that would be found within other secondary healthcare institutions. A major concern relates to the falling rates of serological positivity to measles and mumps in those born after 1980. This would suggest that a large number of HCWs in these age groups are unlikely to be immune to these conditions at the time of pre-employment assessment. Furthermore, given the adverse impact upon MMR uptake in the UK in the mid 1990s of unproven claims over vaccine safety, the next few years may yet see even higher proportions of unvaccinated HCWs attending pre-placement screening. Therefore given recent healthcare-associated measles and mumps outbreaks in the UK, we feel that our data further strengthens the argument for improved MMR vaccination coverage. As a response to a recent outbreak in Wales, the UK government has recently initiated a measles vaccination ‘catch-up’ campaign for all 10- to 16-year-olds in the UK. The impact of this programme in reducing future measles outbreaks remains to be seen.
It has been suggested that most individuals born prior to 1970 are likely to be immune to measles and mumps through natural infection [6]. Our findings, at least for measles, would support this statement, although work from Switzerland suggests that it may not be internationally applicable [13]. With regard to mumps, our results indicate that around one-quarter of HCWs in this age group may not be immune. This would support the recommendation that individuals working in high-risk occupations without evidence of documented immunity to mumps (vaccination or previous infection) should receive a course of MMR. One approach to addressing this is to ensure MMR vaccination becomes a condition for pre-placement clearance for HCWs. There are strong ethical arguments around this. One of these relates to the professional responsibility and duty of care for HCWs to ensure their patients do not contract vaccine-preventable conditions from them. Another pertains to the responsibility held by employers in the UK to reduce the personal risk to vulnerable HCWs from work-related infections. It might be assumed that HCWs would consider mandatory MMR vaccination acceptable in light of its free availability, efficacy and excellent safety profile. Some work has been done in this area suggesting that most HCWs would accept such a policy, but there may be a disparity between attitudes to mandatory programmes and actual vaccine uptake rates [14, 15].
Limitations
This study has a number of limitations. It is important to recognize that our study provides an entry-level assessment of serology to MMR in prospective HCWs rather than a true measure of continuing risk. Nevertheless, it is unclear to what extent the small numbers that declined or were unable to accept vaccination present an ongoing hazard to staff and patients despite OH measures to reduce this. Although in our study this is likely to be small, in the absence of a standard policy towards managing HCWs that refuse vaccination in the UK following pre-employment assessment, it is unclear to what degree these findings are comparable elsewhere. Current recommendations in such cases are to consider excluding these HCWs from ‘high-risk’ areas for contracting and transmitting MMR [9], but these definitions are not clearly qualified, nor the processes by which to implement them. Greater clarification can ensure consistency of OH practice in this area, potentially reducing the risk of outbreaks within hospital settings yet further.
The relevance of our results to other settings, particularly beyond Europe, may be restricted. It is likely that they are at least comparable to other OH departments, although it is possible that those entering healthcare may be more likely to have followed health advice around vaccination than others as a prerequisite for employment. Our findings, although more stark, reflect those seen in a recent French study and given the similarity between MMR vaccination programmes across Europe may have significance elsewhere [12]. From an international perspective, however, differing assessment processes, childhood and adult vaccination strategies, as well as healthcare delivery models will influence the immunization rates of HCWs to MMR as well as the likelihood of local outbreaks.
Although we assumed that a positive serological result indicated that a HCW was likely to be immune to the condition, the clinical implications of a positive mumps serological result have been brought into question. In particular, it has been suggested that this may be the least predictive of the three MMR IgG tests in protecting individuals from developing disease [16, 17]. This presents an ongoing dilemma, since the optimal number of vaccination required to protect an individual from contracting mumps is not known, nor is the required serological IgG value to indicate immunity.
We have surmised that all HCWs had a similar risk of exposure to, contracting and transmitting MMR disease to patients and staff alike. Although we recognize it is likely that there will be variation in patient contact time between different HCWs, and that some HCWs will be at greater risk than others of contracting and transmitting MMR within the hospital setting, it is likely that all HCWs in our study would have sufficient interaction with patients and/or hospital staff to justify preventive action for unimmunized individuals.
Further work
Although our results suggest that the ongoing risk of MMR outbreak within healthcare settings from this cohort of HCWs is low, the extent to which our findings are applicable for existing staff is uncertain, particularly if MMR uptake has been variable. This is of particular relevance where MMR catch-up campaigns may be necessary for non-immune HCWs, such as that currently being considered at SOHS. Such data can help deliver targeted and cost-effective programmes in this area. Consideration of worker status (permanent or temporary), as well as shift patterns (night, day, rolling) are related issues in considering the most appropriate approach in delivering such a programme.
A final issue relates to developing monitoring programmes for vaccine uptake both in OH and population settings. While it has been suggested this may be challenging within the secondary-care setting [18], further work may explore the cost-effectiveness of designing, implementing and evaluating a MMR surveillance programme for OH and community settings. Data from such a programme can inform national and local vaccination strategies towards MMR control and prevention, and maximize the impact of interventions when dealing with outbreaks and epidemics of disease.
ACKNOWLEDGEMENTS
The authors thank Dr Alison Rimmer at Sheffield Occupational Health Service who provided permission to conduct the study and Mrs Janet Chapman for assisting with data collection.
This study did not receive any funding.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Vyse AJ, et al. Evolution of surveillance of measles, mumps and rubella in England and Wales: providing the platform for evidence based vaccination policy. Epidemiology Reviews 2002; 24: 125–36. [DOI] [PubMed] [Google Scholar]
- 2.Cox AR, Kirkham H. A case study of a graphical misrepresentation: drawing the wrong conclusions about the measles, mumps and rubella virus vaccine. Drug Safety 2007; 30: 831–836. [DOI] [PubMed] [Google Scholar]
- 3.Watson JC, et al. An evaluation of measles revaccination among school-entry-aged children. Pediatrics 1996; 97: 613–618. [PubMed] [Google Scholar]
- 4.McIntyre P, Leask J. Improving uptake of MMR vaccine. British Medical Journal 2002; 336: 729–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. British Medical Journal 2005; 330: 1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health. Immunisation against Infectious Disease (the Green Book). London: Blackwell Publishing, 2006, pp. 255–276. [Google Scholar]
- 7.Chen SY, et al. Healthcare-associated measles outbreak in the United States after an importation: challenges and economic impact. Journal of Infectious Diseases 2010; 203: 1517–1523. [DOI] [PubMed] [Google Scholar]
- 8.Chen RT, et al. Adverse events following measles-mumps-rubella and measles vaccinations in college students. Vaccine 1991; 9: 297–299. [DOI] [PubMed] [Google Scholar]
- 9.Association of National Health Occupational Physicians. Immunisation of healthcare workers (http://www.anhops.org.uk/guidelines/asp). Accessed 25 September 2013.
- 10.Fedeli U, Zanetti C, Saia B. Susceptibility of healthcare workers to measles, mumps rubella and varicella. Journal of Hospital Infection 2002; 51: 133–135. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler E, Roth C, Wreghitt T. Prevalence of measles susceptibility among health care workers in a UK hospital. Does the UK need to introduce a measles policy for its healthcare workers? Occupational Medicine 2003; 53: 398–402. [DOI] [PubMed] [Google Scholar]
- 12.Freund R, et al. Measles immunity and measles vaccine acceptance among healthcare workers in Paris, France. Journal of Hospital Infection 2013; 27: 38–43. [DOI] [PubMed] [Google Scholar]
- 13.Uçkay I, et al. Age limit does not replace serologic testing for determination of immune status for measles. Infection Control and Hospital Epidemiology 2007; 28: 1117–1120. [DOI] [PubMed] [Google Scholar]
- 14.Seale H, Leask J, Raina MacIntyre C. Do they accept compulsory vaccination? Awareness, attitudes and behaviour of hospital health care workers following a new vaccination directive. Vaccine 2009; 27: 3022–3025. [DOI] [PubMed] [Google Scholar]
- 15.Maltezou HC, et al. Attitudes towards mandatory vaccination and vaccination coverage against vaccine-preventable disease among health-care workers in tertiary-care hospitals. Journal of Infection 2012; 64: 319–324. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Immunizations of healthcare personnel: recommendations of the Advisory Committee on Immunization Practices 2011 (http://www.cdc.gov/mmwr/pdf/rr/rr6007.pdf). Accessed 21 January 2013.
- 17.Health Protection Agency. MMR immunisation (http://www.hpa.org.uk/Topics/InfectiousDiseases). Accessed 24 January 2013.
- 18.Pezzoli L, et al. Can we know the immunisation status of healthcare workers? Results of a feasibility study in hospital trusts, England, 2008. Epidemiology and Infection 2010; 138: 45–52. [DOI] [PubMed] [Google Scholar]