SUMMARY
A report on Toxoplasma gondii by the UK Advisory Committee on the Microbiological Safety of Food recommended that more accurate figures on the burden of disease in the UK are needed. We present the first 5 years of data from an enhanced surveillance scheme for toxoplasmosis in England and Wales. Between 2008 and 2012, 1824 cases were reported, with an average of 365 each year. There were 1109 immunocompetent cases, the majority presenting with lymphadenopathy, and 364 immunosuppressed cases, with central nervous system and systemic symptoms most frequently reported. There were also 190 pregnant and 33 congenital cases. Of the pregnant cases, 148 were asymptomatic (probably detected during screening), while 28 suffered a fetal loss or stillbirth. The enhanced surveillance system has led to an improvement in the detection of toxoplasmosis in England and Wales. However, numbers are still likely to be an underestimate, biasing towards the more severe infections.
Key words: Burden of disease, toxoplasmosis, UK
INTRODUCTION
Toxoplasmosis is a zoonotic disease caused by the common protozoan parasite Toxoplasma gondii. While cats are the parasite's definitive host, Toxoplasma can potentially infect all mammalian and avian species. For immunocompetent human hosts, it is estimated that 80–90% of acute Toxoplasma infections are asymptomatic [1]. Clinical presentation in the remaining 10–20% typically includes influenza or glandular fever-like illness, but infection can be severe or life-threatening if acquired during pregnancy or in immunosuppressed individuals. Oocyst contamination of the environment and consumption of undercooked meat are considered to be the common routes of infection but the relative contribution of each is unknown in the UK.
It is estimated that two billion or more people worldwide are infected with T. gondii, although seroprevalence studies show considerable geographical variation both within and between countries. Historical antibody prevalence studies suggest that levels of T. gondii are lower in the UK than in many other European countries, with between 7% and 34% of the UK population infected prior to 2001 [2].
Toxoplasma has been identified as the foodborne pathogen that causes the greatest burden of human disease in The Netherlands [3, 4]. A more recent report from the USA comparing public health burden of foodborne pathogens concluded that, in a combined analysis of QALY loss, economic costs, number of cases and number of deaths, Toxoplasma ranked second only to Salmonella [5]. A report published by the UK's Food Standards Agency (FSA) in 2012 on the risks of T. gondii contamination in the food chain recommended that more accurate figures on infection and burden of disease in the UK population are needed urgently to inform future risk management strategies [6]. This call for improved data on UK toxoplasmosis has been repeated in the Lancet Infection Diseases journal [7, 8].
As toxoplasmosis is not a notifiable disease in England and Wales, information on the disease has historically been collected on an ad hoc basis. In an effort to reduce underreporting and improve our understanding of its clinical presentation, the Health Protection Agency (HPA) (now Public Health England) and the Public Health Wales Toxoplasma Reference Unit (TRU) in Swansea established an enhanced surveillance system for toxoplasmosis in 2008. The system is designed to collect incidence data primarily in vulnerable clinical groups in the UK, where the infection represents the greatest risk to health.
With increasing numbers of immunosuppressed and immunocompromised individuals in the population, it is essential for public health professionals to be aware of this important disease for both at-risk groups and the general public. We present here the findings and analyses from the first 5 years of the enhanced surveillance scheme, describing the epidemiology and clinical presentation of cases of toxoplasmosis identified in England and Wales between 2008 and 2012.
METHODS
Samples and testing
The data presented in this study comprise all samples that were referred to the TRU for specialist diagnosis or reference testing for toxoplasmosis from laboratories in England and Wales between 2008 and 2012. Samples are referred to TRU where routine laboratory testing is not sufficient to achieve a definitive diagnosis or to fully inform clinical management. A significant proportion of such samples come from vulnerable clinical groups. TRU has developed a series of laboratory investigation pathways for the various clinical scenarios where specialist testing is required. Laboratory tests undertaken may include the ‘gold standard’ Sabin–Feldman dye test (DT), enzyme immunoassay (EIA) for the detection of specific IgM antibodies, immunosorbent agglutination assay (ISAGA) for IgA or IgM, IgG avidity measurement, immunoblotting, histological examination of solid tissues, and nucleic acid amplification testing (real-time PCR).
No ethics committee approval was required for this study.
Definition of cases
Each case was systematically categorized by clinical category, clinical presentation, and results/diagnosis (Fig. 1) using the information provided on laboratory request forms (such as demographic information, clinical details, exposure history). Follow-up enquiry by TRU to the requesting clinician was undertaken if the clinical data provided were incomplete.
Fig. 1.
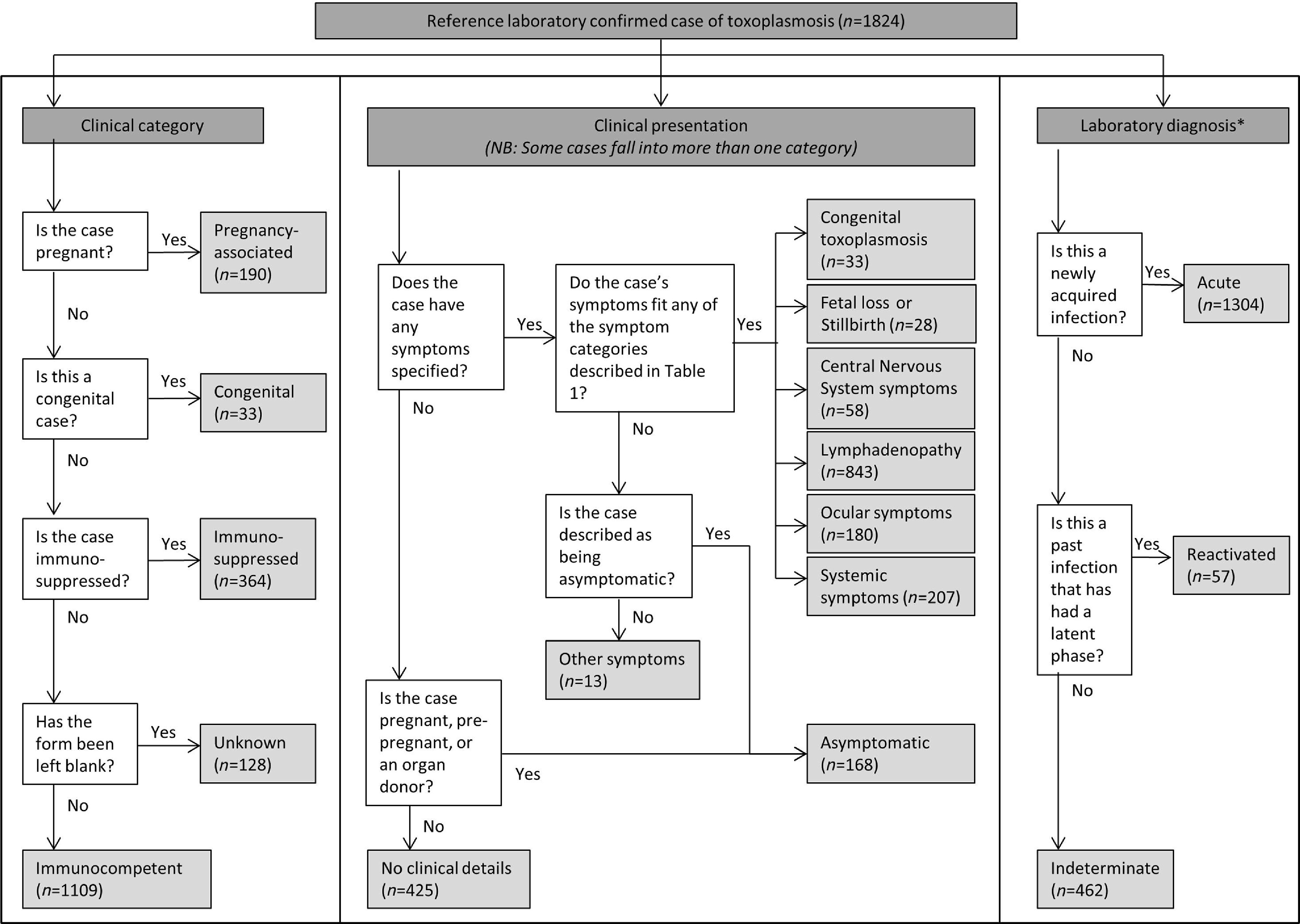
Algorithm depicting the process of case categorization in the enhanced surveillance scheme for toxoplasmosis in England and Wales. * One case did not have an active infection and could not be categorized using the schema presented here.
Clinical category
Cases were categorized into one of four groups based on their clinical status: immunocompetent; immunosuppressed; pregnant and congenital; or unknown. Cases were assumed to be immunocompetent unless otherwise stated, or unless the form was left completely blank (when the case would be classified as ‘unknown’).
Clinical presentation
Cases were categorized based on their symptoms (Table 1). If the case displayed symptoms that did not fall into one of the categories, they were put into the category ‘Other’, e.g. polyhydramnios. If no symptoms were stated – and the patient was either pregnant, pre-pregnant, or an organ donor – cases were assumed to be ‘asymptomatic’. This is because testing of asymptomatic individuals is common within these groups either as part of routine pre-transplant screening or during pregnancy where an exposure risk has been identified. In all other cases where no symptoms were reported, cases were categorized as ‘No clinical details’.
Table 1.
Definitions of the symptom categories for the enhanced surveillance scheme for toxoplasmosis in England and Wales (see also Fig. 1)
| Category | Description |
|---|---|
| Congenital toxoplasmosis | Congenitally acquired cases typically have a common set of symptoms (Sabin's tetrad – hydrocephalus, chorioretinitis, convulsions, intracranial calcifications), including some which can manifest later in life. Cases that acquired infection from their mother are allocated to this symptom category |
| Fetal loss or stillbirth | This symptom category is allocated to the mother of a terminated or lost fetus |
| Central nervous system symptoms | Any symptom or sign associated with the brain, e.g. brain lesion, headaches, ventriculomegaly |
| Lymphadenopathy | Any symptom or sign associated with the lymphatic system, e.g. lymphadenopathy |
| Ocular symptoms | Any symptom or sign associated with the eyes, e.g. chorioretinitis, uveitis |
| Systemic symptoms | Non-specific symptoms or signs, e.g. fever, tiredness, or an illness associated with a specific organ, e.g. bronchiolitis, myocarditis |
Laboratory diagnosis
Categorization was based on interpretation of laboratory test results. Cases with active infection were subdivided into ‘acute’ (newly acquired infection), ‘reactivated’ (infection acquired in the past with an intervening asymptomatic latent phase) or ‘indeterminate’ infections. Cases were put into the latter category when laboratory testing and clinical history could not differentiate acute or reactivated infection.
In addition to the main dataset, a small number of ‘unconfirmed’ cases were recorded where a laboratory specimen was not available for testing but there was a high index of clinical suspicion. There were two classes of ‘unconfirmed’ cases: (i) in the mother where congenital toxoplasmosis was confirmed in the baby but no maternal specimens were provided (where acute infection in the immunocompetent mother was inferred), and (ii) in the fetus where maternal infection in pregnancy was confirmed and the fetus had a clinical presentation of congenital toxoplasmosis, but no specimen was available for testing, e.g. in cases of fetal loss where the parents chose not to have an autopsy or confirmatory testing performed. Unconfirmed cases are not included in the main dataset, but are reported separately at the end of the Results section.
RESULTS
Between 2008 and 2012, 1824 laboratory-confirmed cases of toxoplasmosis were reported to the enhanced surveillance scheme: 405 in 2008, 422 in 2009, 345 in 2010, 341 in 2011, and 311 in 2012. There was a significant decline in numbers diagnosed by TRU over time (P = 0·032), and the total number of samples tested for toxoplasmosis over this period also declined. During 2008, TRU received 13 104 samples for serological testing and 770 samples for PCR analysis, in 2009 it received 12 640 serology samples and 771 PCR samples, in 2010 it received 12 708 serology samples and 724 PCR samples, in 2011 it received 11 783 serology samples and 650 PCR samples, and in 2012 it received 10 425 serology samples and 715 PCR samples.
In total, 967 (53·0%) of the cases were female and 785 (43·0%) were male. In 72 (3·9%), gender was not reported. The majority of cases were reported in the 25–44 years age group (n = 1002, 54·9%) (Fig. 2). There were similar proportions of males and females in each age group, with the exception of the 25–44 years age group where only 39·7% of cases with known gender were male (n = 381). However, when pregnant women were excluded from these data, the sex difference was much reduced and the proportion of males increased to 48·1% of cases (47·5% female, 4·4% unknown). Nonetheless, there remained a significant difference between genders (χ2 = 16·49, P < 0·002).
Fig. 2.
Age and sex distribution of cases (n = 1745, excluding six in utero cases and 73 cases with either unknown age or sex).
Cases were allocated to government office regions [9] based on the source laboratory for each specimen. The 1824 cases in the dataset were unevenly distributed across England and Wales, with 72·6% (n = 1325) of positive specimens coming from just four regions. When rates per 100 000 were calculated (using population data from the mid-point of 2010), the distribution did not change (Fig. 3).
Fig. 3.
Distribution of Toxoplasma cases in England and Wales, with rates per 100 000 population by government office region.
Case submissions were distributed relatively equally across the year by specimen quarter, with a very slight increase in spring and summer [January–March (436, 23·9%), April–June (490, 26·9%), July–September (462, 25·3%), October–December (436, 23·9%)].
There was one report of a death due to toxoplasmosis, in a 1-month-old male infant suffering from congenital infection. Although this was the only death recorded, some of the pregnant cases suffered fetal loss or stillbirth.
The clinical category of cases by year is shown in Figure 4. One hundred and twenty-eight cases (7·0%) were classified as ‘unknown’ clinical category (of which 27 had acute infection and 101 had indeterminate infections). The remaining 1696 cases are described below. The symptoms recorded for each type of case are given; many cases presented with more than one symptom, and are included in the results more than once (see Fig. 1 and Table 2).
Fig. 4.
Clinical category and total number of cases, by year.
Table 2.
Symptoms by clinical category (each case may have more than one symptom)
| Symptoms | Immuno-competent | Immuno-suppressed | Pregnant | Congenital | Unknown | Total |
|---|---|---|---|---|---|---|
| Congenital toxoplasmosis | 0 | 0 | 0 | 33 | 0 | 33 |
| Fetal loss or stillbirth | 0 | 0 | 28 | 0 | 0 | 28 |
| Central nervous system symptoms | 10 | 40 | 0 | 8 | 0 | 58 |
| Lymphadenopathy | 824 | 12 | 7 | 0 | 0 | 843 |
| Ocular symptoms | 163 | 11 | 0 | 6 | 0 | 180 |
| Systemic symptoms | 168 | 31 | 3 | 5 | 0 | 207 |
| Other | 6 | 2 | 5 | 0 | 0 | 13 |
| Asymptomatic | 18 | 2 | 148 | 0 | 0 | 168 |
| No details | 20 | 277 | 0 | 0 | 128 | 425 |
Immunocompetent, non-pregnant cases
There were 1109 immunocompetent (non-pregnant) cases, including nine potential transplant donors. The majority of these patients presented with lymphadenopathy (n = 824, 74·3% of cases), systemic (n = 168, 15·1%) or ocular symptoms (n = 163, 14·7%) (Table 2). Most immunocompetent cases had acute infections (n = 1023). Twenty-one cases had reactivated infections, and 64 had indeterminate infections. In addition, there was one patient (a 31-year-old asymptomatic male transplant donor) who did not have an active infection.
Immunosuppressed, non-pregnant cases
There were 364 immunosuppressed (non-pregnant) cases. There were 273 cases with HIV (including five who had additional other immunosuppressive illnesses), 53 were transplant recipients, and 38 were immunosuppressed for other reasons, e.g. various forms of cancer therapy. Most of the individuals recorded as immunosuppressed had HIV infection, and there was limited clinical information available beyond their HIV status. Where symptoms were reported (n = 87, 23·9%), those involving the central nervous system (n = 40, 46·0%), and systemic symptoms (n = 31, 35·6%) were relatively frequent. Forty-five immunosuppressed cases had acute infection, 33 had reactivated infection, and the remaining 286 were indeterminate.
Pregnancy-associated cases (including congenital)
There were 190 pregnant cases (including 12 who were both pregnant and immunosuppressed with HIV) and 33 congenital cases included in the dataset. From these cases, 29 mother–child pairs could be identified (additional mother–child pairs were formed with unconfirmed cases – see below). Of the 33 congenital cases, seven were defined as in utero infections, 24 occurred in infants aged <1 year, and two were in children aged 1–9 years (one of whom had been a congenital case at birth and first entered the dataset when they developed eye problems in later childhood, and the second was a reactivated infection that was diagnosed when the child was 1 year old).
Over three quarters of pregnant cases were asymptomatic (n = 148, 77·9%), while 28 (14·7%) suffered a fetal loss or stillbirth. The congenital cases were all classified as having congenital toxoplasmosis, but 16 reported additional symptoms. Of the pregnant cases, 179 had acute infection and 11 had indeterminate infection. Thirty of the congenital cases had acute infection, and three had reactivated infections.
Unconfirmed cases
In addition to the 1824 laboratory-confirmed cases, 28 unconfirmed cases were reported by TRU: three pregnant cases and 25 congenital cases. All of these unconfirmed cases formed mother–child pairs with confirmed cases.
DISCUSSION
This study is the first to report on the national epidemiology of toxoplasmosis in England and Wales. The enhanced surveillance system was established to improve our understanding of the burden of toxoplasmosis in the UK, and to provide more detailed information on the presentation of cases. The data suggests that between 300 and 450 cases of toxoplasmosis are confirmed by TRU in England and Wales every year, and there has been a significant decline in the number of cases since the enhanced surveillance scheme was introduced (from a high of 422 in 2009 to 311 in 2012). While it is possible that the early years included a number of patients undergoing repeat testing who had been previously diagnosed (whereas later years included new cases only), this is unlikely to account for the significant difference in case numbers.
There has been a concurrent decrease in the number of specimens submitted to TRU for confirmatory testing, and there are a number of possible explanations for this. Over the last few years many laboratories have acquired new platforms for serological diagnosis offering a wider range of assays which may not need to be referred for confirmatory testing. TRU also has an ongoing programme that aims to educate referring laboratories so that unnecessary referrals are reduced.
Despite the apparent decline in case numbers, the scheme reflects a threefold increase in case detection compared to routine laboratory reports in the UK between 2005 and 2008 [10]. Nonetheless, the numbers detected by the scheme are still likely to be an underestimate of the total burden of toxoplasmosis in England and Wales. Samples are referred to TRU only where primary testing is equivocal or where confirmation will help to exclude other diagnoses that might require significant clinical intervention, e.g. lymphoma. Samples from less seriously ill patients are usually tested in local laboratories only, and therefore not referred to TRU.
The age and sex profiles in this dataset are typical of toxoplasmosis in an industrialized country. Toxoplasmosis often has two age patterns that are dependent on the primary acquisition of the parasite; one showing high rates in children and the second showing a relatively low prevalence in children that increases with age [2]. The latter profile is usually observed in industrialized countries where guidelines are in place to reduce congenital transmission of T. gondii, and is indicated in this dataset. Males are often over-represented in child and adolescent patients, whereas there tends to be an excess of females in adult cases [2], as can be seen in Figure 2.
The high numbers of pregnant women in this dataset may be due to women requesting screening for toxoplasmosis upon becoming pregnant if they have a history of exposure to cats as pets, regardless of whether they are experiencing symptoms. Of the pregnant women who had acute infections, over two thirds were asymptomatic and may not have been routinely detected. This suggests that a significant number of cases of acute Toxoplasma infection in pregnancy may be going undetected since routine testing is not undertaken in the UK.
While most individuals with Toxoplasma infection will report relatively mild symptoms, a small proportion will suffer from severe morbidity. This is especially true of infections in vulnerable groups. Only one death in a confirmed case was reported during the surveillance period; however, the information on outcomes was collected at the time of diagnosis with no follow-up of cases. Toxoplasmosis ranks high in the list of diseases which lead to the death of patients with AIDS, and fetal loss or stillbirth is a common complication of pregnancy-associated cases. It is notable that 28 infections reported in this paper were associated with fetal loss or stillbirth.
Most immunocompetent individuals in our dataset had acute infections, whereas a relatively high proportion of the immunosuppressed individuals had reactivated infection (42·3% excluding indeterminate infections). The presentation of symptoms in immunocompetent individuals, where lymphadenopathy and ocular symptoms dominated, was notably different to that of immunosuppressed individuals in whom central nervous system involvement was the most common presentation. Thus, clinicians should consider the context of an individual's symptoms when considering toxoplasmosis as part of a differential diagnosis.
The worldwide burden of toxoplasmosis shows some significant differences by country, so it is important to describe the presentation of this illness in England and Wales rather than relying upon data from elsewhere. A systematic review of 15 studies in 1996 found annual seroconversion rates of 0·24–1·6% in Europe and 0·2–0·6% in the USA [11]. This variation may be the result of different behaviours, different regional food preferences or differences in the level of viable Toxoplasma oocysts in the environment, possibly related to differences in climate. These factors together with differences in farming practice may also influence the epidemiology of Toxoplasma infection in livestock entering the food chain. England and Wales has traditionally been thought to have a low seroprevalence, with studies from pre-2001 estimating that between 7% and 34% of people in the UK were infected with Toxoplasma [2]. A more recent report by the UK Advisory Committee on the Microbiological Safety of Food (ACMSF) suggests that toxoplasmosis may affect 0·6% of the UK population each year, with half the population showing signs of past infection by age of 50 years [6]. On this basis, the authors of the report estimate that there may be up to 350 000 cases a year in the UK, of which 10–20% are symptomatic.
Data from unlinked anonymous sera collected from throughout the British Isles during 1990–1991 showed levels of between 11% and 40% in UK blood donors, and an east–west gradient in seroprevalence (with higher levels in the west and lower in the east) [6]. This contrasts with the north–south gradient described in our data, where over 70% of positive specimens submitted to TRU came from four regions in the south of England. In general, referrals are more likely to come from specialist centres dealing with clinically vulnerable groups, and regions with proportionately more of these specialist centres are likely to submit a greater number of samples to TRU compared to areas which have fewer of these centres. As such, the dataset described in this paper does not necessarily reflect the geographical distribution of toxoplasmosis in the general population, but may reflect the distribution of more severe infections.
Toxoplasmosis is an important zoonotic infection in European countries, although there is insufficient good quality data on the burden of disease. The European Food Safety Agency (EFSA) has concluded that the disease burden of toxoplasmosis may have been underestimated, and has called for improved and standardized collection of representative data from all member states [12]. In addition, the recent report from ACMSF recognizes that data on the prevalence and burden of disease are incomplete in the UK [6]. These high-level observations underline the importance of our enhanced surveillance system in supporting national risk assessment initiatives. A more precise understanding of the overall burden of infection associated with T. gondii in the UK is necessary to support policy-makers, public health organizations and healthcare providers in ensuring prevention measures are targeted as efficiently and effectively as possible.
ACKNOWLEDGEMENTS
The funding source for the study was the primary authors' institution (Public Health England). No further funding was obtained. The corresponding author declares that she had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors acknowledge the work of Ms. Kirsty Hewitt in setting up the enhanced surveillance scheme in its early days.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Remington J. Toxoplasmosis in the adult. Bulletin of the New York Academy of Medicine 1974; 50: 211–227. [PMC free article] [PubMed] [Google Scholar]
- 2.Hall S, Ryan M, Buxton D. The epidemiology of toxoplasma infection. In: Joynson D, Wreghitt T, eds. Toxoplasmosis: A Comprehensive Clinical Guide. New York: Cambridge University Press, 2001, pp. 58–124. [Google Scholar]
- 3.Havelaar A, et al. Disease burden of foodborne pathogens in the Netherlands, 2009, International Journal of Food Microbiology 2012; 156: 231–238. [DOI] [PubMed] [Google Scholar]
- 4.Kemmeren J, et al. Priority setting of foodborne pathogens: disease burden and costs of selected enteric pathogens. RIVM, 2006. (Report 330080001/2006) (www.rivm.nl/bibliotheek/rapporten/330080001.pdf). Accessed 6 September 2013.
- 5.Batz M, Hoffmann S, Morris G. Jr. Ranking the risks: the 10 pathogen-food combinations with the greatest burden on public health. University of Florida, Emerging Pathogens Institute, 2011. (http://www.epi.ufl.edu/sites/www.epi.ufl.edu/files/RankingTheRisksREPORT.pdf). Accessed 6 September 2013. [Google Scholar]
- 6.ACMSF. Risk profile in relation to toxoplasma in the food chain, 2012. (http://www.food.gov.uk/multimedia/pdfs/committee/acmsfrtaxopasm.pdf). Accessed 6 September 2013.
- 7.Anon. Toxoplasma gondii: an unknown quality. Lancet Infectious Diseases 2012; 12: 737. [DOI] [PubMed] [Google Scholar]
- 8.Kirby T. Calls for more detailed studies on toxoplasmosis. Lancet Infectious Diseases 2012; 12: 912–913. [DOI] [PubMed] [Google Scholar]
- 9.Office for National Statistics. Regions (former GORs) (http://www.ons.gov.uk/ons/guide-method/geography/beginner-s-guide/administrative/england/government-office-regions/index.html). Accessed 6 September 2013.
- 10.Defra. Zoonoses report UK, 2009. 2011. (http://www.defra.gov.uk/publications/files/pb13571-zoonoses2009-110125.pdf). Accessed 6 September 2013.
- 11.Eskild A, et al. Screening for toxoplasmosis in pregnancy: what is the evidence of reducing a health problem? Journal of Medical Screening 1996; 3: 188–194. [DOI] [PubMed] [Google Scholar]
- 12.EFSA. Scientific opinion of the panel on biological hazards, on a request from EFSA on surveillance and monitoring of Toxoplasma in humans, foods and animals. EFSA Journal 2007; 583: 1–64. [Google Scholar]





