SUMMARY
Although co-infection by multiple groups of pathogens is the norm rather than the exception in nature, most research on the effects of pathogens on their hosts has been largely based on a single or few pathogen species. Nevertheless, the health impact of co-occurring infections is evident, and it is important that scientists should consider pathogen communities rather than single relevant pathogen species when assessing the impact of multiple infections. In this work we illustrate the consequences of neglecting different pathogen taxa (viruses, protozoa, helminths, arthropods) in the explanatory power of a set of Partial Least Squares Regression (PLS-R) models used for exploring the impact of co-infections on the body condition of 57 adult feral cats; 71·5% cats were co-infected by ⩾3 groups of pathogens. The best two PLS-R models provided a first component based on the combination of helminths, protozoa and viruses, explaining 29·15% of body-condition variability. Statistical models, partially considering the pathogen community, lost between 24% and 94% of their explanatory power for explaining the cost of multiple infections. We believe that in the future, researchers assessing the impact of diseases on host life-history traits should take into account a broad representation of the pathogen community, especially during early assessment of the impact of diseases on host health.
Key words: Feral cats, multiple infections, partial least squares regression, poliparasitism
INTRODUCTION
Understanding the causes and consequences of co-infection remains one of the major challenges in disease biology. Although co-infection is the rule rather than the exception [1], the consequences of multiple infections have been traditionally ignored not only by animal and human health scientists but also by wildlife ecologists. Nevertheless, there is an increasing interest to move from the ‘one-disease-one-parasite’ perspective to a more holistic view of hosts as ecosystems of parasites [2], partially motivated by the health impact of co-occurring infections [3]. In fact, in such complex ‘host–parasite ecosystems’ a variety of both direct and indirect interactions between parasites and their hosts [4, 5] must be taken into account. For example, acquired immunity to one pathogen species may have negative effects on a second species, but also can produce immunosuppression increasing infection susceptibility [6]. On the other hand, direct competition for resources are also common [7]. Thus, both the host's immune response (top-down regulations [4]) and competition between parasites (bottom-up regulations) have to be considered in order to explain the observed variability in susceptibility to a new infection [8], infectiousness [6] and even pathogen shedding [9] in an infected host.
One of the most relevant consequences of co-infection is its effect on disease severity. The cost of infection relies on both the number of species involved in the infection (see [10] for the combined effects of two pathogens, but [11] for a broad community), and on pathogen burden [12]. However, certain combinations could have a protective effect [13], making it extremely difficult to assess the cost of infection by considering only a part of the whole host–pathogen community [14]. Nonetheless, most published information on the consequences of co-infection in wildlife [15] and humans [16] have been largely based on the combined effects of only two species, and thus whether the outcomes of such studies are conditioned by the number and type of pathogen species considered is yet to be determined.
In this work, we provide and example on the consequences of considering only a part of the complete pathogen community when assessing the cost of infection in adult feral cats (Felis silvestris catus). Cats are particularly suitable for our purposes since they do not store fat reserves in anticipation of food shortage (i.e. income breeders). An evaluation of the nutrient requirements of domestic cats has led to the conclusion that cats are metabolically attuned to a carnivorous diet [17] and hence a high protein diet limits the likelihood of obesity and deviations from a balanced body condition often results in disease [18]. Therefore, poor body condition in cats has often been linked to infections caused by viruses [19], helminths [20] and mixed infections [21].
More specifically, the aim of this study was to evaluate the consequences of overlooking a part of the pathogen community in the explanatory power (EP) of statistical models designed for evaluating body-condition losses due to co-infection.
METHODS
Sampling procedure
In autumn 2008, 57 adult feral cats were live-trapped in Mallorca Island (Spain) with baited traps during a pest control campaign carried out to protect the endemic avifauna. Most of a cat's diet in the study area relies on rodent predation [22], hence we assumed that highly parasitized cats would show limited opportunities to hunt and hence the opportunity to compensate the cost of infection.
Once captured, cats were anaesthetized with a combination of ketamine (Imalgène®, Merial, France) plus xylazine (Rompun®, Bayer, Spain). Blood samples were collected from the cephalic vein and whole blood preserved in EDTA and sera were stored at −20°C until assayed. Subsequently, cats were humanely euthanatized with sodium thiopental (B. Braun Medical S.A., Spain). Only cats showing permanent teeth (aged >6 months) were used in this study. At the laboratory cats were necropsied in detail. Later, different species belonging to viruses, protozoa, helminths and arthropods were detected using specific laboratory methodologies that are summarized in Table 1. On the other hand, kidney fat reserves were used as a proxy for body condition in cats. Kidney fat reserves have already been used as a proxy for body reserves in other felids (e.g. European lynx, Lynx lynx [23]) and their statistical properties are well know [24].
Table 1.
Detection method, and associated pathology for the specific pathogen community for exploring the effects of multiple infections on body-condition losses in adult feral cats from Majorca Island, Spain
| Agent | Type of assay | Detects | Kit or reference | Symptoms and lesions | Source |
|---|---|---|---|---|---|
| Virus | |||||
| FeLV | c-ELISA | C | Ingezim FeLV (Ingenasa) | Can cause a variety of diseases, including neoplasias (malignant lymphoma, leukaemia, myelofibrosis, fibrosarcoma, and others), anaemia, pancytopenia, immune suppression, and death | [40] |
| FPV | c-ELISA | C | Ingezim CPV (Ingenasa) | Aetiological agent of feline panleukopaenia. Can cause enteritis and diarrhoea, lymphopenia, neutropenia, thrombocytopenia and anaemia, fetal death and cerebral ataxia in kittens | [40] |
| FIV | c-ELISA | AI | Ingezim FIV (Ingenasa) | Non-specific clinical signs (fever, dermatitis, stomatitis, neurological and ocular disease, diarrhoea, renal insufficiency). Immunosuppression. Usually leads to death | [40] |
| Protozoa | |||||
| Toxoplasma gondii | MAT | AI | [36] | In adult cats: anorexia, fever, weight loss, neurological and ocular signs, hepatitis, respiratory tract disease, vomiting, diarrhoea, among others. Death can occur | [40] |
| Leishmania infantum | PCR | AI | [37] | Cutaneous ulcerative or nodular lesions. Often asymptomatic | [40] |
| Helminths | |||||
| Ancylostoma tubaeforme | Direct retrieval at necropsy | AI | [38] | Can cause haemorrhage from multiple lacerations to the intestine leading to anaemia | [41] |
| Toxocara cati | Diarrhoea, potbelly, poor coat | [42] | |||
| Oslerus rostratus | No studies available on the signs of infection with this parasite. Oslerus osleri can cause chronic tracheobronchitis, respiratory distress, and cough in dogs | [42] | |||
| Cestodes* | Anal discomfort, itching. Competition for resources | [42] | |||
| Arthropods | |||||
| Ticks† | Direct retrieval | AI | [39] | Heavy infestations can cause restlessness and exsanguination, leading to anaemia and sometimes to death; saliva can cause toxicosis and allergies. Vectors of infectious diseases | [43] |
| Fleas‡ | Heavy infestations cause intense pruritus, papulocrustous. dermatitis or patchy alopecia. Vectors of infectious diseases | [43] | |||
FIV, Feline immunodeficiency virus; FeLV, feline leukaemia virus; FPV, feline parvovirus; c-ELISA, competitive enzyme-linked immunosorbent assay; MAT, modified agglutination test; AI, active infection; C, contact.
Joyeuxiella pasqualei, Diplopylidium acanthotetra, Dipylidium carracidoi, Taenia taeniaformis.
Mostly Rhipicephalus sanguineus
Mostly Ctenophalides felis. For FIV, the presence of antibodies indicates active infection; for Toxoplasma gondii cats showing high MAT titres (<1:200) were considered recently infected.
Statistical modelling
The effects of co-infection with viruses, protozoa, helminths and arthropods on cats' body condition were assessed by a Partial Least Squares Regression (PLS-R) approach. This statistical tool is an extension of multiple regression analysis. In the PLS-R, associations between explanatory and response variables are established with latent factors inferred through a mathematical model from variables measured directly. These latent factors are defined as linear combinations between predictor and response variables that maximize the explained variance in the dependent variables. During this iterative method the original multidimensionality is reduced to a lower number of orthogonal factors to detect the structure in the relationships between predictor variables and between these latent factors and the response variables. The extracted factors account for successively lower proportions of original variance [25].
PLS-R is probably the least restrictive of the multivariate techniques [26]. This flexibility allows it to be used when there are fewer observations than predictor variables, in case of multicollinearity and in case of studies of covariance in both explanatory and predictor variable groups [27]. A comprehensive reading on the suitability of PLS-R in the fields of ecology and evolution can be found in Carrascal et al. [28].
In our case, body condition (i.e. kidney fat) was the single explanatory variable while selected pathogen group combinations from the cats' pathogen community served as the predictor factor. Each pathogen group was defined by several species (Table 1). Furthermore, pathogen richness (e.g. the number of pathogen species actually observed per host) was also calculated and included as a part of the X component representing co-infection. Intensity of helminth infection was calculated according to Bush et al. [29].
Finally the EP for each of the candidate models, defined as the relative proportion of observed body-condition variability explained by the best PLS-R model, was also calculated. PLS-R was estimated with ‘plspm’ version 0.3·7 [30] of the Statistical package of R, version 3.0·1 [31].
RESULTS AND DISCUSSION
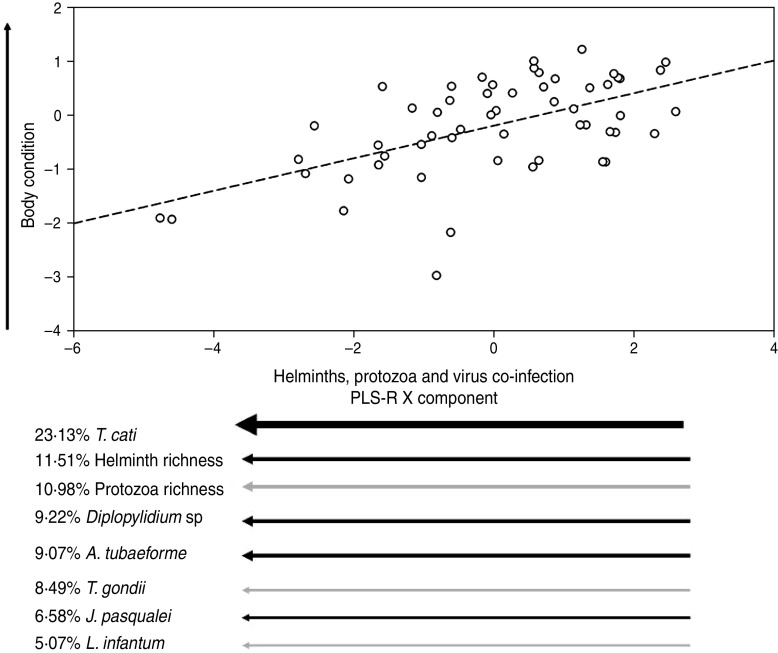
Only one cat was exclusively infected by a single group of parasites (helminths), confirming that multiple infections are the rule rather than the exception in wildlife [32]. In fact, co-infections by only two parasite groups were rare (between 1·7% by arthropods and viruses, or 8·7% by helminths and protozoa), being 71·5% of individuals co-infected by ⩾3 parasite types (e.g. 31·5% by arthropods, helminths, protozoa and viruses, 19·0% by arthropods, helminths, and viruses, 12·3% by arthropods, helminths, and protozoa, and 8·7% by helminths, protozoa and viruses). The best two PLS-R models (see Table 2) provided a first component based on the combination of helminths, protozoa and viruses explaining 29·15% of body-condition variability of cats. Hence we assumed that this model had the maximum explanatory power (EP = 1). Such models suggested that cats in better condition were parasitized by few helminth and protozoa species (low species richness), i.e. lower Diplopylidium acanthotetra, Ancylostoma tubaeforme and Joyeuxiella pasqualei burdens, and were not infected by Toxoplasma gondii or with Leishmania infantum (Table 3, Fig. 1).
Table 2.
Explanatory power (EP) of pathogen combinations belonging to different taxonomic groups for explaining the variations of fat reserves in free-roaming cats from Majorca Island, Spain
| Co-infection | R2X (%) | Eigenvalue | R2Y (%) | Q2 | Sign | EP |
|---|---|---|---|---|---|---|
| Helminths + protozoa + viruses | 20·68 | 2·67 | 29·15 | 0·17 | S | 1·00 |
| Arthropods + helminths + protozoa + viruses | 18·18 | 2·82 | 28·87 | 0·15 | S | 0·99 |
| Helminths + protozoa | 26·17 | 2·68 | 27·42 | 0·16 | S | 0·94 |
| Arthropods + helminths + protozoa | 22·14 | 2·86 | 26·91 | 0·16 | S | 0·92 |
| Helminths + viruses | 23·16 | 2·39 | 23·29 | 0·14 | S | 0·80 |
| Arthropods + helminths + viruses | 20·13 | 2·58 | 22·96 | 0·11 | S | 0·79 |
| Helminths | 30·79 | 2·33 | 22·03 | 0·14 | S | 0·76 |
| Arthropods + helminths | 25·42 | 2·57 | 21·34 | 0·11 | S | 0·73 |
| Protozoa + viruses | 34·46 | 1·99 | 13·37 | 0·07 | S | 0·46 |
| Arthropods + protozoa | 37·26 | 2·23 | 11·47 | 0·05 | S | 0·39 |
| Protozoa | 73·39 | 2·20 | 10·07 | 0·06 | S | 0·35 |
| Arthropods + viruses | 26·20 | 1·45 | 5·42 | −0·08 | n.s. | 0·19 |
| Viruses | 55·08 | 1·53 | 2·89 | −0·01 | n.s. | 0·10 |
| Arthropods | 67·57 | 1·89 | 1·83 | −0·02 | n.s. | 0·06 |
Models were ranked with respect to the percentage of observed body-condition variability (R2Y) explained by a specific co-infection. The R2X value indicates the percentage of variability of the X component explained by a specific co-infection of parasites belonging to one or different taxonomic groups.
Q2 index (i.e. leave-one-out cross-validation); S indicates statistically significant Partial Least Squares Regression (PLS-R) model (α = 0·05); n.s. non-significant. EP indicates the percentage of explained variance with respect to the PLS-R model (i.e. helminths + protozoa + viruses) explaining the greatest proportion of observed body-condition variability (i.e. 29·15%).
Table 3.
Predictor weights of the most parsimonious Partial Least Squares Regression (PLS-R) model explaining the effects of co-infection by several viruses, protozoa, helminth and arthropod species on body condition in 57 feral cats from Majorca Island, Spain
| Pathogen group | Predictor variables | Weights | % Variance explained |
|---|---|---|---|
| Helminths (nematodes) | Toxocara cati | −0·483 | 23·35 |
| Protozoa | Protozoa richness | −0·359 | 12·88 |
| Helminths (cestodes) | Diplopylidium acanthotetra | −0·325 | 10·58 |
| Helminths | Helminth richness | −0·325 | 10·53 |
| Helminths (nematodes) | Ancylostoma tubaeforme | −0·323 | 10·42 |
| Protozoa | Toxoplasma gondii | −0·317 | 10·04 |
| Helminths (cestodes) | Joyeuxiella pasqualei | −0·272 | 7·42 |
| Protozoa | Leishmania infantum | −0·235 | 5·50 |
| Viruses | Feline leukaemia virus | 0·200 | 4·01 |
| Helminths (cestodes) | Dipylidium carracidoi | −0·144 | 2·08 |
| Viruses | Virus richness | 0·126 | 1·58 |
| Helminths (nematodes) | Oslerus rostratus | −0·098 | 0·97 |
| Helminths (cestodes) | Taenia taeniformis | −0·074 | 0·55 |
Predictor weights represent the contribution of each pathogen infection to the PLS-R x-axis. Predictor weights explaining more than 5% of the total variance in each response variable are shown in bold type.
Fig. 1.
Relationship between co-infecting helminths (black arrows) and protozoa (grey arrows) on a PLS-R component describing the body condition of adult feral cats. This plot represents the best PLS-R model shown in Table 2. Arrow direction indicates either an increase or a decrease of the component value, and arrow thickness directly indicates the weight of the component. Viruses explained <5% of the PLS-R X component and were therefore excluded from the plot.
It is interesting to note that helminths alone accounted for 76% of the EP (Table 2). This parasite group is normally acquired when cats consume an infected host or by kittens during nursing from an infected mother [20]. The early infected animals are more likely to be clinically affected than cats infected at the adult stage, mainly because their immunological system is not fully developed. In fact, helminth-infected cats (especially young animals) can display diarrhoea, vomiting, permanent failure to grow and probably a reduced ability to store body reserves. However, while the influence of the whole helminth on body condition of cats is clear, Toxocara cati appears to be the most relevant species driving normal fat storage (Table 3, Fig. 1). In fact, T. cati is the cause of general failure to thrive in kittens harbouring moderate burdens, producing during its migration severe lesions in internal organs of adults [33]. Cats parasitized by several parasite species harboured lower Toxocara loads, probably suggesting that few animals were able to deal with the effect of multiple infections in the case of Toxocara infection.
On the other hand, both helminth and protozoa richness were included within the set of variables with substantial support underlining the role of protozoa infection (Toxoplasma and Leishmania) for normal body-condition storage. In addition, the exclusion of certain pathogen groups had a clear impact on the EP of models for explaining body-condition losses. In fact those including only one pathogen group lost between 24% and 94% of EP, depending on the pathogen group. For example, those models including helminths showed a slight reduction (<10%) in terms of EP with respect to the best model. Finally, we were surprised to observe the low impact of viral infections on body condition of cats. This could be explained by the fact that feline leukaemia virus and feline immunodeficiency virus infections can kill cats at the kitten or adult stage, and thus those infected animals we sampled may have shown some degree of resistance to these viral infections and hence a normal ability for fat storage.
We have shown here that neglecting some taxa of the host–parasite community diminishes the EP of models for exploring the cost of infection. The fact that both helminth and protozoa richness were included within the set of variables with substantial support, suggests the importance of considering as many pathogen species as possible in early models for studying the cost of infection. Helminths appeared to be the main group involved in explaining such variations in body condition, having been included in the models with the best EP. The high prevalence of this group of parasites and the fact that cats usually become infected at an early age [20], suggests a relevant role for helminths in shaping the composition and impact of the remaining pathogen community. Evidence of this has been described for a broad range of host models [15] including humans [3], but still largely ignored in large-scale studies of co-infection [8].
Our results support this idea and highlight a serious gap in the research directed to assess not only the impact of single infections but also that of co-infections on both animal and human health. From the community ecology point of view, outcomes of co-infection are difficult to generalize but particularly so when a part of the parasite community is neglected [4]. Synergistic or antagonistic interactions between all types of pathogen combinations are, in fact, possible in natural conditions [34], and thus further research should take into account a broad representation of the whole pathogen community, especially those species interacting with the host from the early stages of development. This new view, based on considering pathogen communities rather than single relevant pathogens, will allow exploration of the interaction between such ‘host–parasite ecosystems’ and their natural environments [35] providing perspectives for management and control of infectious diseases.
DECLARATION OF INTEREST
None.
ACKNOWLEDGEMENTS
The authors thank the Fundació Natura Parc for collaboration during fieldwork, Professor L. L. Vizcaino (UM) for laboratory analyses and Dr F. J. Márquez (UJAEN) for arthropod identification. This work was partially funded by Conselleria de Medi Ambient, Govern de les Illes Balears (contract no. 5893/2008). E. Serrano was supported by the Beatriu de Pinós programme and J. Millán by the Ramón y Cajal programme from the MICINN, Spain. Field work complied with current Spanish law.
REFERENCES
- 1.Brooker S. Estimating the global distribution on disease burden of intestinal nematode infections: adding up the numbers – a review. International Journal for Parasitology 2010; 40: 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez JM, et al. Parasites and conservation biology: the ‘Ibex-ecosystem’. Biodiversity and Conservation 2006; 15: 2033–2047. [Google Scholar]
- 3.Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology 2008; 135: 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends in Ecology and Evolution 2007; 22: 133–139. [DOI] [PubMed] [Google Scholar]
- 5.Hatcher MJ, Dunn AM. Parasites in Ecological Communities, from Interactions to Ecosystems, 1st edn. Cambridge, UK: Cambridge University Press, 2011. [Google Scholar]
- 6.Telfer P, et al. Parasite interactions in natural populations: insights from longitudinal data. Parasitology 2008; 135: 767–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lello J, et al. Competition and mutualism among the gut helminths of a mammalian host. Nature 2004; 428, 840–844. [DOI] [PubMed] [Google Scholar]
- 8.Telfer S, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science 2010; 330: 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lass S, et al. Generating super-shedders: co-infection increases bacterial load and egg production of a gastrointestinal helminth. Journal of the Royal Society Interface. Published online: 19 December 2012. doi: 10.1098/rsif.2012.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CMD, et al. Immunological interactions between 2 common pathogens, Th1-Inducing protozoan Toxoplama gondii and the Th2-inducing helmint Fasciola hepatica. PLoS One 2009; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petney TN, Andrews RH. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. International Journal for Parasitology 1998; 28: 377–393. [DOI] [PubMed] [Google Scholar]
- 12.Marinho CRF, et al. Influence of acute-phase parasite load on pathology, parasitism, and activation of the immune system at the late chronic phase of Chagas' disease. Infection and Immunity 1999; 67: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nkuo-Akenji TK, et al. Malaria and helminth co-infection in children living in a malaria endemic setting of mount Cameroon and predictors of anemia. Journal of Parasitology 2006; 96: 1191–1195. [DOI] [PubMed] [Google Scholar]
- 14.Boel M, et al. Complex interactions between soil-transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PLoS Neglected and Tropical Diseases 2010; 4: e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezenwa V, Jolles A. From host immunity to pathogen invasion: the effects of helminth coinfection on the dynamics of microparasites. Integrative and Comparative Biology 2011; 4: 540–551. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths EC, et al. The nature and consequences of coinfection in humans. Journal of Infection 2013; 63: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald ML, Rogers QR, Morris JG. Nutrition of the domestic cat, a mammalian carnivore. Annual Review of Nutrition 1984; 4: 521–562. [DOI] [PubMed] [Google Scholar]
- 18.Becvarova I. Focus on nutrition: canine and feline obesity: frequently asked questions and their answers. Compedium 2011; 33: 1–5. [PubMed] [Google Scholar]
- 19.Gilot-Fromont E, et al. Prevalence and pathogenicity of retroviruses in wildcats in France. Veterinary Record 2000; 146: 317–319. [DOI] [PubMed] [Google Scholar]
- 20.Millán J, Casanova JC. High prevalence of helminth parasite in feral cats in Majorca Island (Spain). Parasitology Research 2009; 106: 183–188. [DOI] [PubMed] [Google Scholar]
- 21.Sobrinho LSV, et al. Coinfection of Leishmania chagasi with Toxoplasma gondii, feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) in cats from an endemic area of zoonotic visceral leishmaniasis. Veterinary Parasitology 2012; 187: 302–306. [DOI] [PubMed] [Google Scholar]
- 22.Millán J. Feeding habits of feral cats Felis sylvestris catus in the countryside of Majorca Island. Wildlife Biology in Practice 2010; 6: 32–38. [Google Scholar]
- 23.Pulliainen E, Lindgres E, Tunkkari PS. Influence of food availability and reproductive status on the diet and body condition of the European lynx in Finland. Acta Theriologica 1995; 40: 181–196. [Google Scholar]
- 24.Serrano E, et al. A half a century of measuring ungulate body condition using indices: is it time for a change? European Journal of Wildlife Research 2008; 54: 675–680. [Google Scholar]
- 25.Abbi H. Partial least square regression, PLS-R. In: Salkind N, ed. Encyclopedia of Measurement and Statistics. California: Thousand Oaks, 2007. [Google Scholar]
- 26.Haenlein M, Kaplan AM. A beginner's guide to Partial Least Squares analysis. Understanding Statistics 2004; 3: 283–297. [Google Scholar]
- 27.Geladi P, Kowalski B. Partial Least Squares Regression: a tutorial. Analytica Chimica Acta 1986; 185: 1–17. [Google Scholar]
- 28.Carrascal LM, Galván I, Gordo O. Patial least squares regression as an alternative to current regression methods used in ecology. Oikos 2009; 118: 681–690. [Google Scholar]
- 29.Bush AO, et al. Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology 1997; 83: 575–583. [PubMed] [Google Scholar]
- 30.Sánchez G, Trinchera LR. plspm: Partial Least Squares data analysis methods. R package version 0 3 7 (http://cranr-projectorg/web/packages/plspm/indexhtml). Accessed 15 April 2013.
- 31.R Development Core Team. 3·0·1. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (http://wwwR-projectorg). Accessed 1 May 2013.
- 32.Bordes F, Morand S. The impact of multiple infections on wild animal hosts: a review. Infection Ecology and Epidemiology 2011; 1: 7346. doi: 10.3402/iee.v1i0.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowman DD, et al. Feline clinical parasitology. Iowa: Iowa State University Press, 2002. [Google Scholar]
- 34.Cox FE. Concomitant infections, parasites and immune responses. Parasitology 2001; 122 (Suppl.): 23–38. [DOI] [PubMed] [Google Scholar]
- 35.Serrano E, et al. The use of null models and partial least squares approach path modelling (PLS-PM) for investigating risk factors influencing post-weaning mortality in indoor pig farms. Epidemiology and Infection. Published online: 3 June 2013 doi: 10.1017/S0950268813001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubey JP, Desmonts G. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Veterinary Journal 1987; 19: 337–339. [DOI] [PubMed] [Google Scholar]
- 37.Millán J, et al. An investigation into alternative reservoirs of canine leishmaniasis on the endemic island of Mallorca (Spain). Transbound Emerging Diseases 2011; 58: 352–357. [DOI] [PubMed] [Google Scholar]
- 38.Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals, 7th edn, 809 pp. Philadelphia: Lea & Febiger, 1982. [Google Scholar]
- 39.Millán J, et al. Ectoparasites of the endangered Iberian lynx and sympatric wild and domestic carnivores in Spain. Medical and Veterinary Entomology 2007; 21: 248–254. [DOI] [PubMed] [Google Scholar]
- 40.Greene CE. Infectious Diseases of Dog and Cat. Missouri, USA: Saunders-Elsevier, 1987. [Google Scholar]
- 41.Kalkofen UP. Hookworms of dogs and cats. Veterinary Clinics of North America: Small Animal Practice 1987; 17: 1341–1354. [DOI] [PubMed] [Google Scholar]
- 42.Taylor MA, Coop RL, Wall RL. Veterinary Parasitology, 3rd edn. Oxford: Blackwell, 2007. [Google Scholar]
- 43.Durden LA, Mullen GR. Medical and Veterinary Entomology. New York: Academic Press, 2002. [Google Scholar]