SUMMARY
We used data from the Genitourinary Medicine Clinic Activity Dataset (GUMCAD) over a 3-year period (2009–2011) to investigate the distribution and risk factors of Trichomonas vaginalis infection in England. Socio-demographic and clinical risk factors associated with a diagnosis of T. vaginalis were explored using multivariable logistic regression. Rates of T. vaginalis infection were highest in London and the West Midlands. For men and women, T. vaginalis infection was significantly associated with: older age compared to those aged 20–24 years, non-white ethnicity (in particular black Caribbean and black ‘other’ ethnic groups), and birth in the Caribbean vs. birth in the UK. Current gonorrhoea or chlamydia infection was associated with a diagnosis of T. vaginalis in women. Further research is required to assess the public health impact and cost-effectiveness of introducing targeted screening for women at high risk of infection in areas of higher prevalence.
Key words: Epidemiology, Trichomonas, public health, sexually transmitted infections (STIs)
INTRODUCTION
Trichomoniasis is a sexually transmitted infection (STI) caused by the flagellated protozoan Trichomonas vaginalis [1]. In 2008, the World Health Organization estimated that 276 million new cases occurred worldwide, exceeding comparable estimates for both Chlamydia trachomatis and Neisseria gonorrhoeae [2].
In women, T. vaginalis infection of the genital tract can present with vaginal discharge, which is classically frothy in nature and yellow-green in colour, unusual odour, vulvar itching and signs of vulvitis [3–5]. Some women may also present with lower abdominal pain and dysuria [6]. Asymptomatic infections are common and occur in up to half of all female cases [7]. Less is known about T. vaginalis infections in men; the majority do not experience any clinical manifestations although some may present with non-gonococcal urethritis or, rarely, with balanitis [4, 5].
As the symptoms of trichomoniasis tend to be mild or absent, the importance of the infection is often overlooked [8]. However, there is evidence that infection with T. vaginalis may be associated with reproductive health complications, including pelvic inflammatory disease and adverse pregnancy outcomes [3, 9, 10]. Importantly, T. vaginalis infection may also facilitate HIV transmission [11, 12]. The biological mechanism for the latter is poorly understood but probably involves inflammatory processes which may amplify viral shedding from the genital tract of HIV-seropositive individuals or, alternatively, lead to the recruitment of high levels of CD4+ and other HIV target cells in HIV-seronegative individuals [13]. T. vaginalis infection can also promote changes in the normal vaginal flora and increase susceptibility to bacterial vaginosis, both of which are important co-factors for HIV transmission [14].
Current UK guidelines [British Association for Sexual Health and HIV (BASHH)] do not recommend routine screening for T. vaginalis infection in asymptomatic individuals [15]. Thus, not only is the prevalence of T. vaginalis infection probably underestimated, undiagnosed asymptomatic infections could help sustain transmission, potentially leading to poor reproductive health outcomes in affected populations [16].
Little is known about the recent epidemiology and public health impact of T. vaginalis infection in England. We used comprehensive, patient-level data collected from all genitourinary medicine (GUM) clinics in England [17], to investigate the distribution and risk factors of T. vaginalis infection and assessed whether the potential burden of infection could warrant a review of existing screening guidelines.
METHODS
Data source
We extracted data from the Genitourinary Medicine Clinic Activity Dataset (GUMCAD) which is the national STI surveillance and reporting system in England. GUMCAD collects anonymized patient-level data on all diagnoses from, and services provided by, GUM clinics in England and includes extensive clinical and socio-demographic data associated with each patient attendance [17].
In England, GUM clinics are commissioned as free, open-access services. STI testing and treatment is therefore available to a broad population base. All patients are offered basic screening for chlamydia, gonorrhoea, syphilis and HIV regardless of symptoms. Further testing (e.g. T. vaginalis infection, herpes simplex virus and non-STI conditions) is performed on patients who have STI-related symptoms. All clinical data are coded in the GUMCAD dataset using Sexual Health and HIV Activity Property Type (SHHAPT) codes, as defined by BASHH.
All GUM clinics in England are required to submit GUMCAD data. Two hundred and six GUM clinics submitted data in 2009 and 2010, and 209 GUM clinics submitted data in 2011. We included data on all attendances by individuals resident in England (3 221 854 attendances by 1 432 526 men and 3 363 563 attendances by 1 552 799 women).
STI data on all T. vaginalis diagnoses in England since records began (1995) were also used to generate trend lines by gender. Data from 1995 to 2008 were sourced from the KC60 GUM clinic returns [18], and data from 2009 to 2011 were sourced from GUMCAD returns.
Data management
All epidemiological analyses undertaken using GUMCAD data were restricted to first episodes (patients diagnosed with T. vaginalis infection) or first visits (all other patients). Individual patient records within each GUM clinic were linked by a local unique patient identifier which enabled removal of repeat attendances. A repeat diagnosis of T. vaginalis infection was defined as having a second occurrence at least 42 days after the first episode. The 42-day interval reflects the typical STI-specific episode length used to analyse GUMCAD data.
We used the 2010 English Index of Multiple Deprivation (IMD) score to provide an overall measure of deprivation at the small area level. The IMD score for each Lower Layer Super Output Area (LSOA) in England is calculated based on a broad range of issues which are experienced by people living within that geographical area [19]. In our study, the IMD score for all areas in England was categorized into five similar-sized groups and then mapped to the individuals in our dataset by LSOA of residence.
Linking patient records also allowed us to identify diagnoses of other acute STIs or vaginal conditions including candidiasis and bacterial vaginosis. Of those diagnosed with T. vaginalis, current infection with another acute STI (chlamydia, gonorrhoea, syphilis, first-episode warts, or first-episode herpes) or a vaginal condition (candidiasis or bacterial vaginosis) was defined as having any infection within 42 days either side of their T. vaginalis diagnosis. These patients were also defined as being newly diagnosed with HIV if they had a new diagnosis recorded within 42 days either side of their T. vaginalis diagnosis. For all other patients, diagnoses of STIs and vaginal conditions were limited to a period of 42 days after the date of their first visit.
Data analysis
The socio-demographic and clinical characteristics of patients diagnosed with first-episode T. vaginalis were explored. Univariable analyses and multivariable logistic regression were used to investigate risk factors associated with presentation with T. vaginalis infection in English residents attending GUM clinics, with all patients who had not been diagnosed with T. vaginalis during 2009–2011 as the comparison group. Only data on first episodes (cases) or first visits (comparison group) were included in the analyses. Attendances of men reporting a homosexual orientation were excluded, as a contemporary discussion with local GUM clinic staff had shown that diagnoses of T. vaginalis were incorrectly coded in this patient group. Furthermore, male-to-male transmission of T. vaginalis is known to be a rare occurrence [20]. Risk factors associated with a diagnosis of T. vaginalis were tested for statistical significance using the χ2 test. Variables of interest included: age group, ethnic group, world region of birth, IMD score, Strategic Health Authority (SHA) of residence and diagnoses of other acute STIs or vaginal conditions. Those variables with a P value of <0·1 were included in the multivariable logistic regression model. Records with missing data on any of the variables were excluded from the regression modelling. Separate models were fitted for men and women. The presence of the first episode of T. vaginalis was taken as the outcome variable. All statistical analyses were performed using Stata version 12 (StataCorp, USA).
RESULTS
Trends in T. vaginalis diagnoses
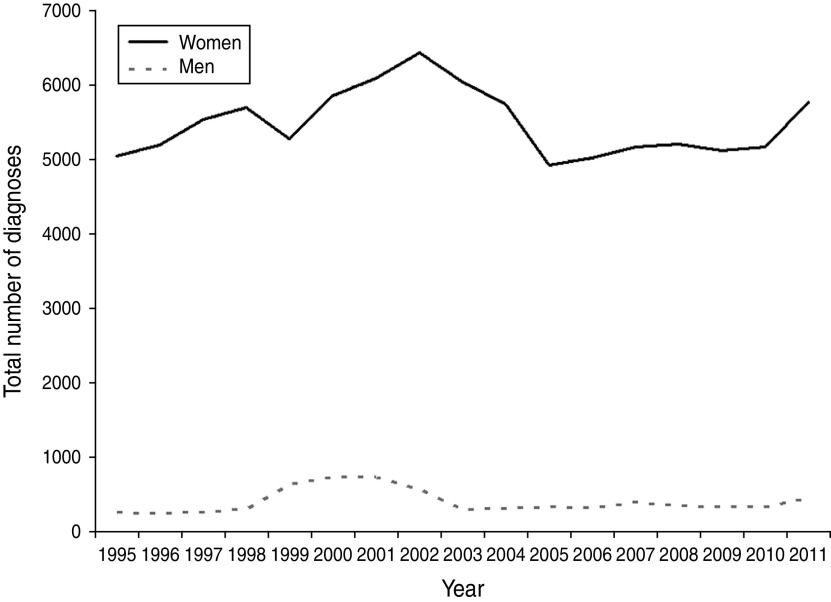
There was a gradual increase in T. vaginalis diagnoses in England during the 1990s with cases peaking at 708 in 2001 for men and at 6430 in 2002 for women (Fig. 1). Diagnosis numbers then rapidly dropped to their previous levels. A slow increase was then observed in both men and women up to 2009, when this trend showed signs of stabilizing. In 2011, however, there was a 13% increase in all cases seen. In total, 6216 new cases were diagnosed in GUM clinics in England, of which 93% occurred in women.
Fig. 1.
Diagnoses of Trichomonas vaginalis at genitourinary medicine (GUM) clinics in England, 1995–2011. STI data in England are sourced from GUM clinic KC60 returns (1995–2008) and Genitourinary Medicine Clinic Activity Dataset (GUMCAD) returns (2009–2011), Public Health England.
T. vaginalis in English residents
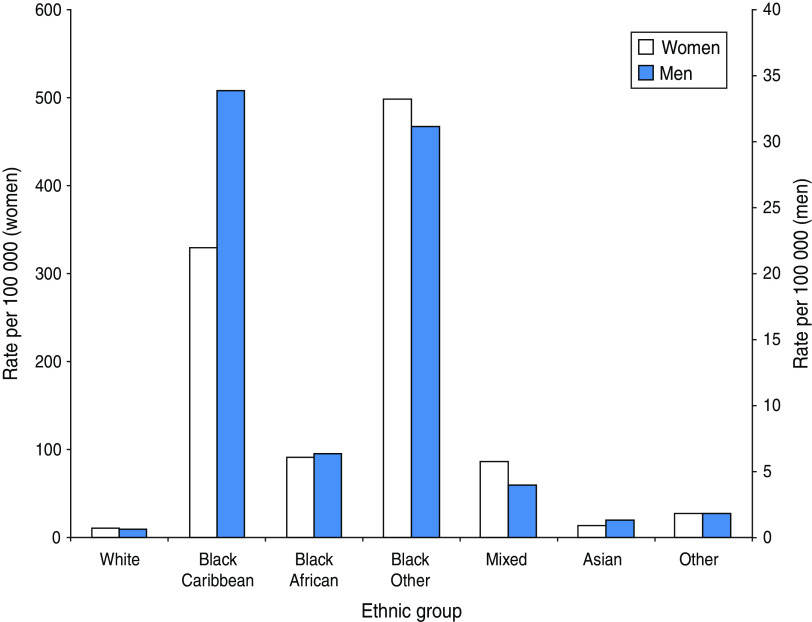
During 2009–2011, there were 16 794 episodes of T. vaginalis diagnosed in 15 366 patients. Just under half (48%) were white and 20% were black Caribbean. The characteristics of patients by both ethnic group and region of birth varied by gender; a higher percentage of men reported black Caribbean ethnicity or birth in the Caribbean. Rates of infection were highest in patients of black Caribbean or black ‘other’ ethnicity (which consists mainly of individuals who identify themselves as black British) and lowest in whites (Fig. 2). Nearly a quarter (24%) of patients were aged 20–24 years, 43% lived in London and 15% in the West Midlands. Population rates were also highest in Local Authorities in these areas (Fig. 3). About half (46%) of all patients were living in the most deprived areas of England.
Fig. 2.
[colour online]. Trichomonas vaginalis diagnosis rates, per 100 000 population, by ethnic group, 2009–2011.
Fig. 3.
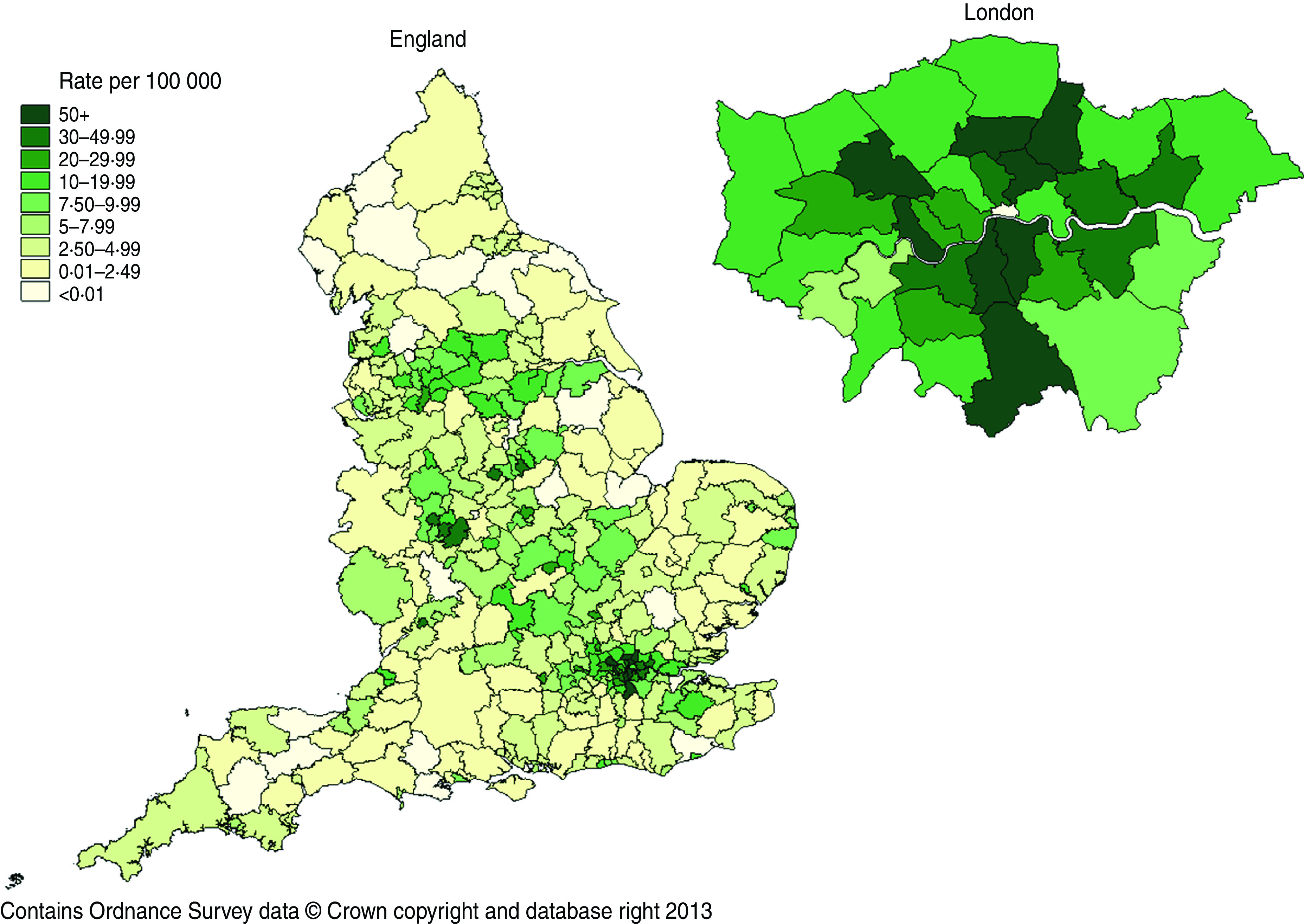
Trichomonas vaginalis diagnosis rates, per 100 000 population, by English Local Authority of residence, 2011. Rates calculated using the Office for National Statistics mid-2010 Population Estimates for England.
Risk factors for T. vaginalis
In the multivariable analyses, 11 929 women diagnosed with T. vaginalis were compared with 1 240 114 GUM clinic patients. Acute infection with syphilis (P = 0·186) and new HIV diagnosis (P = 0·458) were not significant by univariable analysis and were excluded from the final model. All other variables were included and remained significantly associated with a diagnosis of T. vaginalis (Table 1). Similarly, 836 men diagnosed with T. vaginalis infection were compared with 1 036 056 GUM clinic patients. Acute infection with chlamydia (P = 0·859), herpes (P = 0·172) and syphilis (P = 0·224), and new HIV diagnosis (P = 0·896) were excluded from the final model. Independent risk factors associated with a diagnosis of T. vaginalis in men are presented in Table 2.
Table 1.
Risk factors associated with presentation with Trichomonas vaginalis infection in English residents attending GUM clinics 2009–2011: women (n = 1 252 043)*
| Variable | Number (% T. vaginalis) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted P value |
|---|---|---|---|---|
| Age group (yr) | <0·001 | |||
| <15 | 40 (0·3) | 0·55 (0·40–0·75) | 0·47 (0·34–0·64) | |
| 15–19 | 1906 (16·0) | 0·98 (0·93–1·04) | 0·94 (0·88–0·99) | |
| 20–24 | 2927 (24·5) | 1·00 | 1·00 | |
| 25–29 | 2085 (17·5) | 1·07 (1·01–1·13) | 1·05 (0·99–1·11) | |
| 30–34 | 1298 (10·9) | 1·15 (1·08–1·23) | 1·12 (1·05–1·20) | |
| 35–39 | 1067 (8·9) | 1·48 (1·38–1·59) | 1·48 (1·37–1·59) | |
| 40–44 | 1073 (9·0) | 2·04 (1·90–2·19) | 1·97 (1·83–2·12) | |
| 45–49 | 858 (7·2) | 2·46 (2·28–2·66) | 2·38 (2·20–2·58) | |
| 50–54 | 444 (3·7) | 2·51 (2·27–2·78) | 2·62 (2·36–2·90) | |
| 55–59 | 135 (1·1) | 1·63 (1·37–1·94) | 1·93 (1·62–2·30) | |
| ⩾60 | 96 (0·8) | 1·28 (1·04–1·57) | 1·57 (1·28–1·93) | |
| Ethnic group | <0·001 | |||
| White | 6204 (52·0) | 1·00 | 1·00 | |
| Mixed | 964 (8·1) | 3·82 (3·56–4·09) | 3·01 (2·80–3·23) | |
| Black Caribbean | 2362 (19·8) | 8·56 (8·16–8·99) | 4·23 (3·98–4·50) | |
| Black African | 858 (7·2) | 2·46 (2·29–2·64) | 2·70 (2·45–2·99) | |
| Black other | 725 (6·1) | 6·79 (6·28–7·34) | 4·13 (3·80–4·49) | |
| Asian | 548 (4·6) | 1·94 (1·78–2·12) | 1·83 (1·66–2·00) | |
| Other | 268 (2·3) | 1·42 (1·26–1·61) | 1·44 (1·27–1·64) | |
| Region of birth | <0·001 | |||
| UK | 9414 (78·9) | 1·00 | 1·00 | |
| Europe (non-UK) | 563 (4·7) | 0·67 (0·61–0·72) | 0·66 (0·60–0·72) | |
| Caribbean | 862 (7·2) | 6·53 (6·08–7·02) | 1·27 (1·16–1·38) | |
| Sub-Saharan Africa | 677 (5·7) | 1·18 (1·09–1·28) | 0·42 (0·38–0·46) | |
| Other | 413 (3·5) | 0·64 (0·59–0·71) | 0·44 (0·40–0·49) | |
| IMD score | <0·001 | |||
| 1 (most deprived) | 5794 (48·6) | 1·00 | 1·00 | |
| 2 | 3287 (27·6) | 0·60 (0·57–0·62) | 0·67 (0·64–0·70) | |
| 3 | 1507 (12·6) | 0·35 (0·33–0·37) | 0·45 (0·43–0·48) | |
| 4 | 838 (7·2) | 0·23 (0·22–0·25) | 0·34 (0·32–0·37) | |
| 5 (least deprived) | 503 (4·2) | 0·16 (0·15–0·17) | 0·25 (0·23–0·28) | |
| Strategic Health Authority | <0·001 | |||
| London | 5326 (44·7) | 1·00 | 1·00 | |
| North East | 136 (1·1) | 0·15 (0·13–0·18) | 0·26 (0·22–0·31) | |
| North West | 1048 (8·8) | 0·44 (0·41–0·47) | 0·64 (0·60–0·69) | |
| Yorkshire and the Humber | 1064 (8·9) | 0·64 (0·60–0·69) | 0·97 (0·90–1·04) | |
| East Midlands | 785 (6·6) | 0·66 (0·61–0·71) | 1·06 (0·98–1·15) | |
| West Midlands | 1497 (12·6) | 1·02 (0·96–1·08) | 1·15 (1·09–1·23) | |
| East of England | 806 (6·8) | 0·49 (0·45–0·53) | 0·96 (0·89–1·04) | |
| South East | 400 (3·4) | 0·25 (0·22–0·27) | 0·54 (0·49–0·60) | |
| South Central | 384 (3·2) | 0·28 (0·25–0·31) | 0·61 (0·54–0·67) | |
| South West | 483 (4·1) | 0·37 (0·33–0·40) | 0·73 (0·66–0·81) | |
| Acute STI infection | ||||
| Chlamydia | 1259 (10·6) | 1·57 (1·48–1·67) | 1·58 (1·49–1·68) | <0·001 |
| Gonorrhoea | 456 (3·8) | 5·65 (5·13–6·22) | 3·66 (3·30–4·05) | <0·001 |
| Herpes† | 250 (2·1) | 0·78 (0·69–0·88) | 0·86 (0·76–0·98) | 0·020 |
| Warts‡ | 311 (2·6) | 0·46 (0·41–0·51) | 0·62 (0·55–0·69) | <0·001 |
| Other vaginal condition | ||||
| Bacterial vaginosis | 2054 (17·2) | 1·65 (1·57–1·73) | 1·14 (1·07–1·21) | <0·001 |
| Candidiasis | 1283 (10·8) | 1·22 (1·15–1·29) | 1·13 (1·07–1·18) | <0·001 |
OR, Odds ratio; CI, confidence interval; IMD, Index of Multiple Deprivation.
Analysis based on first episode of T. vaginalis or first patient visit. Mixed ethnic group includes white and black Caribbean, white and black African, white and Asian and any other mixed background. Unadjusted and adjusted odds ratios calculated using logistic regression.
First episode of herpes.
First episode of warts.
Table 2.
Risk factors associated with presentation with Trichomonas vaginalis infection in English residents attending GUM clinics 2009–2011: men (n = 1 036 892)*
| Variable | Number (% T. vaginalis) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted P value |
|---|---|---|---|---|
| Age group (yr) | <0·001 | |||
| 15–19 | 38 (4·6) | 0·69 (0·48–0·98) | 0·63 (0·44–0·90) | |
| 20–24 | 138 (16·5) | 1·00 | 1·00 | |
| 25–29 | 164 (19·6) | 1·46 (1·16–1·83) | 1·36 (1·08–1·70) | |
| 30–34 | 118 (14·1) | 1·70 (1·33–2·17) | 1·47 (1·14–1·89) | |
| 35–39 | 97 (11·6) | 2·10 (1·62–2·73) | 1·78 (1·37–2·31) | |
| 40–44 | 107 (12·8) | 3·08 (2·39–3·96) | 2·48 (1·72–3·45) | |
| 45–49 | 83 (9·9) | 3·33 (2·54–4·38) | 2·68 (2·04–3·53) | |
| 50–54 | 43 (5·1) | 2·94 (2·09–4·15) | 2·44 (1·72–3·45) | |
| 55–59 | 16 (1·9) | 1·89 (1·12–3·16) | 1·74 (1·03–2·93) | |
| ⩾60 | 32 (3·8) | 3·04 (2·07–4·47) | 2·70 (1·83–4·01) | |
| Ethnic group | <0·001 | |||
| White | 358 (42·8) | 1·00 | 1·00 | |
| Mixed | 40 (4·8) | 2·92 (2·11–4·05) | 2·75 (1·96–3·85) | |
| Black Caribbean | 248 (29·7) | 14·0 (11·9–16·5) | 8·00 (6·48–9·87) | |
| Black African | 70 (8·4) | 3·32 (2·57–4·30) | 2·76 (1·91–4·00) | |
| Black Other | 52 (6·2) | 7·70 (5·75–10·3) | 5·75 (4·22–7·83) | |
| Asian | 52 (6·2) | 2·20 (1·65–2·95) | 1·91 (1·38–2·65) | |
| Other | 16 (1·9) | 1·68 (1·02–2·77) | 1·50 (0·88–2·53) | |
| Region of birth | 0·001 | |||
| UK | 573 (68·5) | 1·00 | 1·00 | |
| Europe (non-UK) | 30 (3·6) | 0·82 (0·57–1·18) | 0·89 (0·61–1·30) | |
| Caribbean | 115 (13·8) | 12·1 (9·91–14·8) | 1·63 (1·28–2·09) | |
| Sub-Saharan Africa | 69 (8·3) | 1·96 (1·52–2·51) | 0·78 (0·54–1·11) | |
| Other | 49 (5·9) | 1·11 (0·83–1·48) | 0·77 (0·55–1·07) | |
| IMD score | <0·001 | |||
| 1 (most deprived) | 385 (46·1) | 1·00 | 1·00 | |
| 2 | 213 (25·5) | 0·60 (0·51–0·71) | 0·73 (0·62–0·87) | |
| 3 | 106 (12·7) | 0·37 (0·30–0·46) | 0·57 (0·45–0·71) | |
| 4 | 78 (9·3) | 0·32 (0·25–0·41) | 0·59 (0·46–0·77) | |
| 5 (least deprived) | 54 (6·5) | 0·25 (0·18–0·33) | 0·50 (0·37–0·68) | |
| Strategic Health Authority | <0·001 | |||
| London | 361 (43·2) | 1·00 | 1·00 | |
| North East | 8 (1·0) | 0·12 (0·06–0·25) | 0·32 (0·16–0·65) | |
| North West | 97 (11·6) | 0·55 (0·44–0·69) | 1·09 (0·86–1·38) | |
| Yorkshire and the Humber | 65 (7·8) | 0·50 (0·38–0·65) | 0·97 (0·74–1·28) | |
| East Midlands | 62 (7·4) | 0·67 (0·51–0·88) | 1·34 (1·01–1·76) | |
| West Midlands | 107 (12·8) | 0·99 (0·80–1·23) | 1·30 (1·04–1·62) | |
| East of England | 29 (3·5) | 0·24 (0·17–0·35) | 0·52 (0·35–0·77) | |
| South East | 38 (4·6) | 0·34 (0·24–0·47) | 0·85 (0·60–1·20) | |
| South Central | 38 (4·6) | 0·41 (0·29–0·57) | 0·91 (0·64–1·29) | |
| South West | 31 (3·7) | 0·32 (0·22–0·46) | 0·74 (0·61–1·08) | |
| Acute STI infection | ||||
| Gonorrhoea | 16 (1·9) | 1·51 (0·92–2·48) | 0·97 (0·59–1·60) | 0·918 |
| Warts† | 12 (1·4) | 0·18 (0·10–0·31) | 0·26 (0·15–0·46) | <0·001 |
OR, Odds ratio; CI, confidence interval; IMD, Index of Multiple Deprivation.
Analysis based on first episode of T. vaginalis or first patient visit. Mixed ethnic group includes white and black Caribbean, white and black African, white and Asian and any other mixed background. Unadjusted and adjusted odds ratios calculated using logistic regression.
First episode of warts.
For both men and women, the odds of T. vaginalis infection were higher in older age groups compared to those aged 20–24 years. Those of black Caribbean [women: adjusted odds ratio (aOR) 4·23, 95% CI 3·98–4·50; men: aOR 8·00, 95% CI 6·48–9·87] and black ‘other’ (women: aOR 4·13, 95% CI 3·80–4·49; men: aOR 5·75, 95% CI 4·22–7·83) ethnicity were significantly more likely to be diagnosed with T. vaginalis than those who were white. The adjusted OR for T. vaginalis infection for those born in the Caribbean was 1·27 (95% CI 1·16–1·38) for women and 1·63 (95% CI 1·28–2·09) for men compared to those born in the UK. The odds of T. vaginalis infection were significantly higher in women diagnosed with gonorrhoea (aOR 3·66, 95% CI 3·30–4·05) and chlamydia (aOR 1·58, 95% CI 1·49–1·68) but not in men diagnosed with these infections.
DISCUSSION
Using national surveillance data, we have described the epidemiology of T. vaginalis infection in English residents attending GUM clinics in England during 2009–2011.
Women were on average 15 times more likely to be diagnosed with T. vaginalis compared to men. This gender imbalance was expected as, first, it has been documented that the prevalence of T. vaginalis is generally higher in women [8], and second, it is normal practice in GUM clinics to test women, but not men, with genital discharge for T. vaginalis infection.
Although the number of T. vaginalis diagnoses peaked in women aged 20–24 years, our analysis revealed that the odds of infection increased significantly with age, with those aged 40–59 years at greatest risk. Other studies have also reported an association between T. vaginalis infection and older age [21, 22]. The reasons for this association, which is in contrast to that of other curable STIs, are still a matter of speculation; however, the occurrence of asymptomatic and/or persistent infection may play an important role [23]. There is also evidence that T. vaginalis infection can be incidentally detected in older women through routine cervical screening [24]. However, the sensitivity of this technique as a diagnostic tool for T. vaginalis detection is low [25].
The odds of being diagnosed with T. vaginalis infection were greatest in individuals of black ethnicity, in particular those of black Caribbean and black ‘other’ origin, as well as in those born in the Caribbean. Black ethnicity has previously been identified as a risk factor for T. vaginalis infection and could be explained by a combination of social and cultural factors which influence sexual mixing and individual behaviour [26]. Concurrency of sexual relationships and the number of sexual partners may differ by ethnic group [27]. Lack of condom use, unwillingness to access healthcare and high rates of douching may also contribute to the increased rate of infection in these populations [28]. A similar association with black ethnicity has been observed for bacterial STIs such as gonorrhoea and chlamydia, and for bacterial vaginosis [27, 29], suggesting underlying risks are similar. The very high ORs associated with ethnic group observed in the univariable analyses fell considerably in the adjusted analysis, probably because of confounding effect of region of birth, IMD score and SHA.
Concurrent infection with T. vaginalis is commonly reported; especially co-infection with chlamydia or gonorrhoea [3, 21, 30], and we found the same association in the women in our study population. It is possible that women who have either of these infections are more likely to present at GUM clinics with symptoms such as vaginal discharge, dyspareunia or lower abdominal pain, in comparison to those who only have T. vaginalis infection. As such, testing positive for T. vaginalis could be related to having another STI diagnosed. However, only a small proportion of our study population had a concurrent chlamydial or gonococcal infection. Although we have underestimated the frequency of T. vaginalis infection, we do not believe that our results are biased. Co-infection with either gonorrhoea or chlamydia has been associated with T. vaginalis infection in young women in separate studies [26, 30]. This adds to the suggestion that there may be similarities in behaviour and/or other socio-demographic risk factors between these populations. Acute STI co-infection was not associated with a diagnosis of T. vaginalis infection in men.
We found no association between a diagnosis of T. vaginalis infection and a new diagnosis of HIV. However, as T. vaginalis infection has been implicated in the acquisition and onward transmission of HIV, future analyses could explore the incidence of HIV following a diagnosis of T. vaginalis. Other studies have found an increased rate of HIV seroconversion in patients with a previous diagnosis of T. vaginalis [31, 32], which could have important implications for HIV transmission in at-risk populations in England.
There are a number of limitations to our study. The influence of sexual behaviour could not be evaluated as these data are not collected within GUMCAD. It is likely that most of the patients in our study were diagnosed with T. vaginalis infection after presenting to a clinic with typical signs and symptoms. Although this information is not available in GUMCAD, this would reflect current UK guidelines which recommend testing in symptomatic patients only. As such, our comparison group may have included patients who were infected but undiagnosed, thereby inflating the differences observed. Testing for T. vaginalis is also dependent upon individual GUM clinic policies and practices. Few clinics may screen for T. vaginalis in asymptomatic patients and prevalence may be elevated as a result of these local practices [33]. Additionally, the type of test used could influence incidence estimates. Mahto et al. found that wet-film microscopy was the most common diagnostic approach used by GUM clinics in England. However, rates of infection were higher in areas where an alternative or additional confirmatory test (culture or acridine orange staining) was used [34]. Nucleic acid amplification-based tests (NAATs), including the FDA-approved APTIMA® T. vaginalis assay are now available for use and offer a means of detecting T. vaginalis with higher sensitivity in both men and women, including asymptomatic women [24, 35]. Several clinics have assessed the use of these in their unique clinical settings and discovered increased detection rates compared to normal clinical practice (culture and/or wet-film microscopy as per UK guidelines) [36–38]. Data on test type are not available within GUMCAD which limits our ability to assess the impact of this within our study. There is no suitable SHHAPT code which would have allowed us to identify patients who received a negative test result for T. vaginalis. Thus, trend lines presented in Figure 1 may be a function of the number of symptomatic patients presenting at clinics, rather than a real representation of changes in T. vaginalis rates over time. Our findings show that T. vaginalis infection is strongly associated with black ethnicity and deprivation. It is important to note that black minority populations are more likely to be from deprived areas so are less likely to access private healthcare in relation to those of white ethnicity. However, the impact of this on our results is likely to be minimal since private healthcare is less well accessed in England [39].
At present, testing for T. vaginalis infection is restricted to individuals presenting with symptoms, which has important implications for onward transmission, particularly since a large proportion of infected individuals are asymptomatic. For those T. vaginalis cases that are detected, it is important to achieve successful outcomes for partner referral to ensure that re-infection is avoided. In England, all services are expected to initiate partner notification. This can take place either through patient referral (where the patient notifies their sexual partners of the need to attend a clinic for testing and treatment) or provider referral (where the service provider contacts sexual partners). Onward pathways of referral should be in place when a service is unable to fully undertake partner notification [40]. For T. vaginalis, epidemiological treatment should be given to any sexual contacts within the 4 weeks prior to presentation [41].
Screening for T. vaginalis has previously been limited by insufficient diagnostic techniques. Both wet-film microscopy and culture lack sensitivity, particularly in men. However, the introduction of more sensitive NAATs has the ability to improve diagnosis. Testing can also be incorporated without additional clinic time since the same specimen can also be used to test for chlamydia and gonorrhoea [42]. The clinical performance of the APTIMA assay has been validated in both asymptomatic and symptomatic women [24, 35]. Further research is required to evaluate the cost-effectiveness and public health benefits of introducing targeted T. vaginalis screening in high-risk women in moderate to high prevalence settings, such as GUM clinics in London and the West Midlands, where prevalence of infection is highest.
Our study has provided a comprehensive and unique insight into the epidemiology of T. vaginalis infection in English residents attending GUM clinics by characterizing at-risk populations and identifying areas of higher prevalence. This information could inform public health interventions to improve sexual health and help raise awareness of this often neglected STI in policy-makers, clinicians and the general public.
ACKNOWLEDGEMENTS
This work was undertaken by Public Health England as part of routine public health surveillance.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Petrin D, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clinical Microbiology Reviews 1998; 11: 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Department of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections 2008. Geneva: WHO, 2012. [Google Scholar]
- 3.Swygard H, et al. Trichomoniasis: clinical manifestations, diagnosis and management. Sexually Transmitted Infections 2004; 80: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krieger JN. Consider diagnosis and treatment of trichomoniasis in men. Sexually Transmitted Diseases 2000; 27: 241–242. [DOI] [PubMed] [Google Scholar]
- 5.Wolner-Hanssen P, et al. Clinical manifestations of vaginal trichomoniasis. Journal of the American Medical Association 1989; 261: 571–576. [DOI] [PubMed] [Google Scholar]
- 6.Schwebke JR, Burgess D. Trichomoniasis. Clinical Microbiology Reviews 2004; 17: 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis DA. Trichomoniasis. Medicine 2010; 38: 291–293. [Google Scholar]
- 8.Van der Pol B. Trichomonas vaginalis infection: the most prevalent nonviral sexually transmitted infection receives the least public health attention. Clinical Infectious Diseases 2007; 44: 23–25. [DOI] [PubMed] [Google Scholar]
- 9.Moodley P, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clinical Infectious Diseases 2002; 34: 519–522. [DOI] [PubMed] [Google Scholar]
- 10.Cotch MF, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sexually Transmitted Diseases 1997; 24: 353–360. [DOI] [PubMed] [Google Scholar]
- 11.McClelland RS, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. Journal of Infectious Diseases 2007; 195: 698–702. [DOI] [PubMed] [Google Scholar]
- 12.Laga M, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet 1994; 344: 246–248. [DOI] [PubMed] [Google Scholar]
- 13.Sorvillo F, Kerndt P. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet 1998; 351: 213–214. [DOI] [PubMed] [Google Scholar]
- 14.Moodley P, Connolly C, Sturm AW. Interrelationships among human immunodeficiency virus type 1 infection, bacterial vaginosis, trichomoniasis, and the presence of yeasts. Journal of Infectious Diseases 2002; 185: 69–73. [DOI] [PubMed] [Google Scholar]
- 15.Sherrard J. United Kingdom National Guideline on the Management of Trichomonas vaginalis. London: British Association for Sexual Health and HIV, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Roth AM, et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sexually Transmitted Diseases 2011; 38: 398–400. [DOI] [PubMed] [Google Scholar]
- 17.Savage EJ, et al. Rapid increase in gonorrhoea and syphilis diagnoses in England in 2011. Eurosurveillance 2012; 17. [PubMed] [Google Scholar]
- 18.Hughes G, Simms I, Leong G. Data from UK genitourinary medicine clinics, 2006: a mixed picture. Sexually Transmitted Infections 2007; 83: 433–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLennan D, et al. The English Indices of Deprivation 2010. London: Communities and Local Government, 2011. [Google Scholar]
- 20.Kelley CF, et al. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sexually Transmitted Diseases 2012; 39: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller WC, et al. The prevalence of trichomoniasis in young adults in the United States. Sexually Transmitted Diseases 2005; 32: 593–598. [DOI] [PubMed] [Google Scholar]
- 22.Joyner JL, et al. Comparative prevalence of infection with Trichomonas vaginalis among men attending a sexually transmitted diseases clinic. Sexually Transmitted Diseases 2000; 27: 236–240. [DOI] [PubMed] [Google Scholar]
- 23.Johnston VJ, Mabey DC. Global epidemiology and control of Trichomonas vaginalis. Current Opinion in Infectious Diseases 2008; 21: 56–64. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann LH, et al. Trichomonas vaginalis genital infections: progress and challenges. Clinical Infectious Diseases 2011; 53 (Suppl. 3): 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lara-Torre E, Pinkerton JS. Accuracy of detection of Trichomonas vaginalis organisms on a liquid-based papanicolaou smear. American Journal of Obstetrics & Gynecology 2003; 188: 354–356. [DOI] [PubMed] [Google Scholar]
- 26.Helms DJ, et al. Risk factors for prevalent and incident Trichomonas vaginalis among women attending three sexually transmitted disease clinics. Sexually Transmitted Diseases 2008; 35: 484–488. [DOI] [PubMed] [Google Scholar]
- 27.Hughes G, et al. Comparison of risk factors for four sexually transmitted infections: results from a study of attenders at three genitourinary medicine clinics in England. Sexually Transmitted Infections 2000; 76: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorvillo F, et al. Trichomonas vaginalis, HIV, and African-Americans. Emerging Infectious Diseases 2001; 7: 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhury B, et al. Identification of individuals with gonorrhoea within sexual networks: a population-based study. Lancet 2006; 368: 139–146. [DOI] [PubMed] [Google Scholar]
- 30.Krashin JW, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sexually Transmitted Diseases 2010; 37: 440–444. [DOI] [PubMed] [Google Scholar]
- 31.Mavedzenge SN, et al. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sexually Transmitted Diseases 2010; 37: 460–466. [DOI] [PubMed] [Google Scholar]
- 32.Van der Pol B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. Journal of Infectious Diseases 2008; 197: 548–554. [DOI] [PubMed] [Google Scholar]
- 33.Woodland H, Rogstad KE. Trichomonas vaginalis in a Sheffield genitourinary medicine department. International Journal of STD & AIDS 2005; 16: 491–493. [DOI] [PubMed] [Google Scholar]
- 34.Mahto M, et al. Finding cases of Trichomonas vaginalis infection in England. International Journal of STD & AIDS 2011; 22: 471–473. [DOI] [PubMed] [Google Scholar]
- 35.Schwebke JR, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. Journal of Clinical Microbiology 2011; 49: 4106–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng A, Ross J. Nuclei acid amplification tests (NAAT) for Trichomonas vaginalis: should they change who we screen for infection [Abstract]. Sexually Transmitted Infections 2012; 88 (Suppl. 1): A29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawton M, Schembri G, Kingston M. Testing for Trichomonas vaginalis (TV) by transcription mediated amplification (TMA). An evaluation in a large city clinic [Abstract]. Sexually Transmitted Infections 2013; 88 (Suppl. 1): A32. [Google Scholar]
- 38.Watts A, et al. Clinical evaluation of diagnostic methods for the detection of Trichomonas vaginalis from high vaginal specimens [Abstract]. International Journal of STD & AIDS 2013; 24 (Suppl. 1): 19. [Google Scholar]
- 39.Boyle S. United Kingdom (England): health system review. Health Systems in Transition 2011; 13: 1–483. [PubMed] [Google Scholar]
- 40.British Association for Sexual Health and HIV & The Medical Foundation for AIDS and Sexual Health. Standards for the management of sexually transmitted infections (STIs). London: British Association for Sexual Health and HIV & The Medical Foundation for AIDS and Sexual Health; 2010. [Google Scholar]
- 41.McClean H, et al. BASHH statement on partner notification for sexually transmissible infections. London: British Association for Sexual Health and HIV, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sexually Transmitted Infections 2013; 89: 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]