SUMMARY
In July 2011, a cluster of Yersinia enterocolitica infections was detected in southwestern Pennsylvania, USA. We investigated the outbreak's source and scope in order to prevent further transmission. Twenty-two persons were diagnosed with yersiniosis; 16 of whom reported consuming pasteurized dairy products from dairy A. Pasteurized milk and food samples were collected from this dairy. Y. enterocolitica was isolated from two products. Isolates from both food samples and available clinical isolates from nine dairy A consumers were indistinguishable by pulsed-field gel electrophoresis. Environmental and microbiological investigations were performed at dairy A and pasteurization deficiencies were noted. Because consumption of pasteurized milk is common and outbreaks have the potential to become large, public health interventions such as consumer advisories or closure of the dairy must be implemented quickly to prevent additional cases if epidemiological or laboratory evidence implicates pasteurized milk as the outbreak source.
Key words: Foodborne infections, outbreaks, Yersinia enterocolitica
INTRODUCTION
Enteropathogenic Yersinia enterocolitica, a foodborne pathogen, causes an estimated 116 716 human infections annually in the USA [1]. Yersiniosis can manifest as diarrhoea, abdominal pain, fever, acute mesenteric lymphadenitis mimicking appendicitis, and systemic infection. Severe and even fatal disease can occur. Diagnosis is determined by using stool or blood culture, although a limited number of laboratories routinely screen human clinical specimens for Yersinia species [2].
The epidemiology of Y. enterocolitica is complex and poorly understood because outbreaks are infrequent and investigation of sporadic infections rarely identifies an apparent exposure source. Raw or undercooked pork consumption is considered a primary mode of sporadic transmission, although other food items have been implicated in outbreaks, including pasteurized milk, tofu, and chitterlings [3–10]. Consumption of unpasteurized milk and untreated ground water are also considered risk factors for infection [11, 12].
Since the late 1980s, serogroup O:3 has been the predominant strain associated with human infections in the USA, although other serogroups including O:8 and O:9 are well established human pathogens [2–6]. Healthy pigs are frequently colonized with serogroups that cause human illness; pathogenic serogroups have also been isolated from cows, sheep, goats, dogs, and cats [13–16]. Pathogenic Y. enterocolitica strains have been isolated from unpasteurized milk and cow faeces, although the prevalence in cows is typically lower than in pigs and the association between veterinary isolates and human disease is unclear [17, 18].
Y. enterocolitica is a ubiquitous microorganism; however, most isolates recovered from asymptomatic carriers, food, and the environment are not pathogenic [19]. Isolates are rarely recovered from food or environmental samples because a limited number of Y. enterocolitica organisms might be present, compared to other microflora in food and environmental samples [19].
Yersiniosis is not mandatorily reportable in Pennsylvania, but case reports are sporadically received by health departments. On 22 July 2011, a physician notified the Allegheny County Health Department (ACHD) of a patient with Y. enterocolitica infection. An examination of the Pennsylvania Department of Health's database of routinely submitted case reports identified four additional yersiniosis patients in Allegheny and adjacent Beaver County with onset of illness in June–July 2011. During 2008–2010, a total of 11 Y. enterocolitica cases had been reported in these two counties. Because of the recent cases, an investigation was launched to identify a potential common source, assess scope of illnesses, and prevent additional infections.
METHODS
Initial investigation
Using a standardized questionnaire, interviewers initially queried patients about traditional Y. enterocolitica risk factors including exposure to raw pork, chitterlings, and unpasteurized milk. During these interviews, one patient was identified as a consumer of pasteurized milk that was home-delivered from a farm-based southwestern Pennsylvania dairy (dairy A). After further questioning, the other four additional patients also reported consumption of dairy A pasteurized milk. Thereafter, the investigation focused on dairy A, which on 27 July voluntarily stopped production after joint notification of the illnesses by the Pennsylvania Department of Health (PADOH) and the Pennsylvania Department of Agriculture (PDA). Dairy A also notified all home delivery customers and retail stores about the illnesses and recommended that they discard any remaining milk.
Case ascertainment
We classified a case as culture-confirmed Y. enterocolitica infection during 1 January 2011–31 August 2011, occurring in a resident of any of the four southwestern Pennsylvania counties (Allegheny, Beaver, Butler, Lawrence) served by dairy A. To identify cases, we reviewed state surveillance data, used shopper card information from a retail store to contact purchasers of dairy A milk, issued a press release, notified clinicians, and contacted hospital-based and commercial laboratories in southwestern Pennsylvania to identify clinical isolates of Y. enterocolitica. All identified patients, or their guardians, or next of kin were interviewed about potential exposures, including consuming dairy A products.
Environmental investigation
Samples of dairy A product were collected from a retail store and from the homes of dairy A consumers where available. Dairy A was certified by PDA to pasteurize on site and sell non-grade A cow's milk (milk for pasteurization that is not certified under the Interstate Milk Shippers Program) within Pennsylvania from a herd of about 150–200 cows. The dairy produced skim, 1%, 2%, whole, chocolate- and strawberry-flavoured milk, cream, buttermilk, and ice cream, and distributed ∼4500 US gallons of milk (10 000 containers) weekly to ∼645 households and 40 retail outlets and restaurants in southwestern Pennsylvania; ∼85% of the milk was distributed in returnable glass bottles, which were washed and sanitized by the dairy's mechanical bottle washer. Milk was also sold in plastic jugs. Milk flavourings and ice cream mix were added before pasteurization. Ice cream was packaged in 56-oz paperboard containers.
Milk sanitarians from PDA inspected dairy A and reviewed pasteurization and equipment cleaning logs. The dairy owners and employees were asked about illness in themselves and in family members. On 4 August, microbiological samples from the pasteurizer, ice cream machine, drainage collectors, milk bottle washer, bottle filler, crate washer, unwashed crates, door handles, water delivery truck, chocolate powdered milk flavouring, and sweetwater (cold water passed through piping to cool pasteurized milk) tank were obtained during a site visit by ACHD, PADOH, and PDA. During the visit, unpasteurized milk and water samples from the pasteurization plant (a mixture of chlorinated municipal and well water), cow herd water, standing water from two floor drains, municipal water from a water delivery truck, and sweetwater were also obtained. Environmental samples were collected in Cary–Blair transport media, refrigerated, and delivered to the state public health laboratory in <24 h.
On 1 September, dead-end ultrafiltration was used to filter 100-litre chlorine quenched water samples from each of the following dairy A locations: pasteurization plant, cow herd water, sweetwater, and municipal (truck) water [20]; 2-litre grab samples were also collected from the same locations. Filters and water samples were shipped in <24 h to the Centers for Disease Control and Prevention (CDC) for extraction and testing. In October 2011, a third party, separate from the health department investigation, collected manure samples from the dairy herd for Y. enterocolitica testing.
Laboratory investigation
All available Y. enterocolitica patients' isolates were shipped from clinical laboratories to PADOH or ACHD public health laboratory for confirmation.
Laboratories at PADOH, ACHD, PDA, and CDC tested environmental or dairy A products for Y. enterocolitica by using variations of cold temperature enrichment methods that are typically used to provide Y. enterocolitica with a competitive advantage (Table 1). Dairy A product samples sent to the ACHD laboratory were also tested by using a novel room temperature enrichment method developed specifically for this investigation based upon aspects of published research methods [21–25] and theory. In this method, dairy A product was added to 225 ml peptone sorbitol bile broth and refrigerated at 5°C for 1 day. On day 2, the broth was alkalinized by using 1 ml of 0·25% potassium hydroxide (KOH) and incubated at room temperature (25°C) for 2 days. MacConkey and cefsulodin-irgasan-novobiocin agar plates were inoculated with the room temperature broth on days 3 and 4, and incubated at ± 25°C for 1–2 days.
Table 1.
Yersinia enterocolitica isolation methods used by local, state, and federal laboratories during an investigation of Y. enterocolitica infections associated with improperly pasteurized milk products – southwest Pennsylvania, March–August, 2011
| Laboratory | Samples | Enrichment | Incubation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Broth or Buffer | Temperature | Time (days) | Potassium hydroxide | Inoculation day | Agar plates | Temperature | Time (days) | ||
| ACHD | |||||||||
| Method 1 | Dairy A products | PSBB | 5°C | 21 | 0·5% to agar plates | 10, 21 | MacConkey, CIN | ±25°C | 1–2 |
| Method 2* | Dairy A products | PSBB | 5°C, 25°C | 1, 2 | 0·25% to broth after 1 day cold enrichment | 3, 4 | MacConkey, CIN | ±25°C | 1–2 |
| PDA | |||||||||
| Method 1 (FDA BAM) [27] | Dairy A products and environmental | PSBB | 10°C | 10 | 0·5% to agar plates† | 10 | MacConkey, CIN | 30°C | 1–2 |
| PADOH | |||||||||
| Method 1 (FDA BAM) | Dairy A products and environmental | PSBB | 10°C | 10 | 0·5% to agar plates† | 10 | MacConkey, CIN | 30°C | 1–2 |
| Method 2 | Dairy A products and environmental | PSBB | 4°C | 21 | 0·5% to agar plates† | 7, 14, 21 | MacConkey, CIN | 30°C | 1–2 |
| CDC | |||||||||
| Method 1 | Environmental | n.a. | n.a. | n.a. | n.a. | Direct plating | MacConkey, CIN | 25°C, 30°C | 2 |
| Method 2 | Environmental | PBS | 4°C | 21 | Broth treated with 0·25% for 2 min, then 0·5% for 15 s before plating | 21 | MacConkey, CIN | 25°C, 30°C | 2 |
| Method 3 | Environmental | n.a. | 25°C | 2 | Broth treated with 0·25% for 2 min, then 0·5% for 15 s before plating | 1, 2 | MacConkey, CIN | 25°C, 30°C | 2 |
ACHD, Allegheny County Health Department; PSBB, peptone sorbitol bile broth; CIN, cefsulodin-irgasan-novobiocin agar; PDA, Pennsylvania Department of Agriculture; FDA, Food and Drug Administration; BAM, Bacterial Analytical Manual [27]; PADOH, Pennsylvania Department of Health; CDC, Centers for Disease Control and Prevention; PBS, phosphate buffered saline; n.a., not applicable.
In this method, dairy A product was added to 225 ml peptone sorbitol bile broth and refrigerated at 5°C for 1 day. On day 2, the broth was alkalinized by using 1 ml of 0·25% potassium hydroxide (KOH) and incubated at room temperature (25°C) for 2 days. MacConkey and cefsulodin-Irgasan-novobiocin agar plates were inoculated with the room temperature broth on days 3 and 4, and incubated at ± 25°C for 1–2 days.
1:10 dilution of enrichment without potassium hydroxide also plated on agar per BAM guidelines.
Available Y. enterocolitica isolates were subtyped by pulsed-field gel electrophoresis (PFGE) and restricted with NotI and ApaI at PADOH. Y. enterocolitica isolates were biotyped and serogrouped at the National Yersinia Reference Laboratory (CDC, Atlanta, GA) [26–28] and also at the WHO Collaborating Center for Yersinia (Pasteur Institute, Paris) for confirmation of unusual results (L. Martin and E. Carniel, unpublished data).
Cohort study
To determine outbreak extent and identify particular dairy A products or other exposures associated with illness, we conducted a cohort study of dairy A home delivery customers by using a list provided by dairy A. Information was obtained regarding illness, food consumption, types and quantity of dairy A product consumption, and animal exposures during 2011 for all household members. A suspected case of yersiniosis was defined as a household member receiving dairy A products during 2011 and experiencing ⩾2 of the following: abdominal cramping, diarrhoea (>3 loose stools during a 24-h period), or fever (measured temperature >38·05°C) since 1 January 2011.
On the basis of review by the PADOH Institutional Review Board and the CDC Scientific Education and Professional Development Program Office Human Subjects' Protection Coordinator, this investigation was considered a public health response and therefore non-research.
Statistical analysis
Relative risks (RRs) and 95% confidence intervals (CIs) were calculated by using generalized estimating equations and Poisson regression with multilevel modelling to account for household clustering. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., USA).
RESULTS
Patients' characteristics
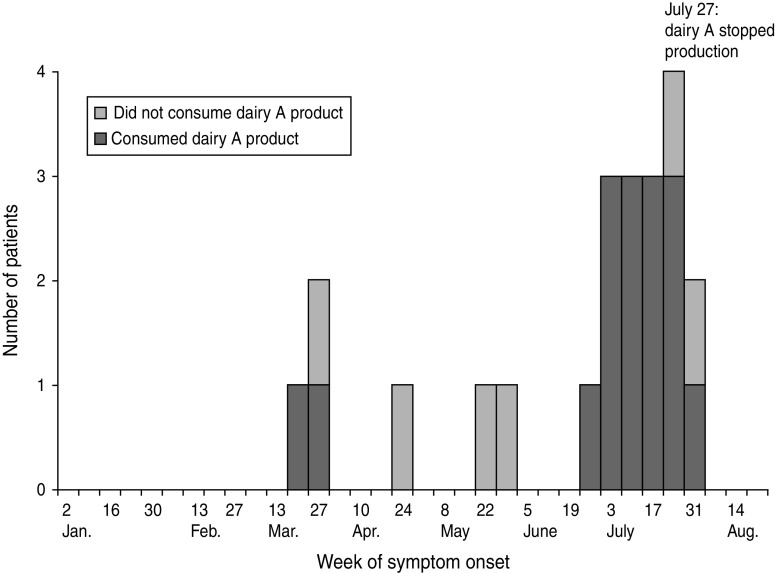
A total of 22 patients with culture-confirmed yersiniosis were identified; 16 (73%) had consumed dairy A products. Of the 16 patients with dairy A exposure, symptom onset occurred during 24 March–5 August 2011 (Fig. 1). Median age was 27 years (range 1–76 years); 63% were female (Table 2). Clinical information was available for 14 patients and included diarrhoea (71%), fever (50%), abdominal cramping (43%), nausea (21%), sore throat (14%), and rash (14%). Seven patients (44%) were hospitalized; three (19%) required intensive care; one (6%) had abdominal surgery to rule out lymphoma and one (6%) patient died. Y. enterocolitica was isolated from the stool of 14 dairy A-associated patients and the blood of two other patients. All 16 reported having consumed dairy A's glass-bottled pasteurized milk; three patients also had eaten dairy A ice cream. The patients had consumed multiple types of dairy A milk and ice cream. The six patients who did not consume dairy A products had no recognized common exposures.
Fig. 1.
Patients with culture-confirmed Yersinia enterocolitica infection (n = 22), by week of onset, southwest Pennsylvania, March–August, 2011.
Table 2.
Characteristics of patients with culture-confirmed Yersinia enterocolitica who reported exposure to dairy A products (n = 16), southwest Pennsylvania, January–August 2011
| Characteristics | No. (%) |
|---|---|
| Age, median (range) | 27 (1–76) years |
| Female | 10 (63) |
| Time from symptom onset to diagnosis, mean (range) | 14·5 (3–38) days |
| Hospitalized | 7 (44) |
| Intensive care unit | 3 (19) |
| Died | 1 (6) |
| Ate dairy A ice cream | 3 (19) |
| Consumed dairy A milk from glass bottles | 16 (100) |
| Consumed 2% milk only | 7 (44) |
| Consumed skim milk only | 1 (6) |
| Consumed chocolate milk only | 1 (6) |
| Consumed multiple types of milk | 4 (25) |
| Consumed 2% milk and ice cream | 3 (19) |
| Received home delivery | 8 (50) |
| Purchased from dairy A onsite store | 4 (25) |
| Purchased from retail store | 4 (25) |
Patients' specimens
Antimicrobial susceptibility testing was performed on isolates of 6/7 hospitalized patients; five isolates demonstrated resistance to ampicillin/sulbactam, ampicillin, and cefazolin and one isolate demonstrated intermediate sensitivity to ampicillin (ampicillin/sulbactam and cefazolin susceptibility testing was not performed). Y. enterocolitica isolates from nine patients who consumed dairy A products were indistinguishable by PFGE (no bands of difference) and were confirmed as bioserotype 1B/O:8, a highly pathogenic bioserotype of Y. enterocolitica. Available isolates from the two patients who did not consume dairy A products with Y. enterocolitica infection had PFGE patterns and biotype/serogroup combinations that differed from each other and from the outbreak strain. Isolates were not available for the remaining four patients who did not consume dairy A products.
Environmental investigation
Temperature and time logs indicated that standards for adequate pasteurization (72°C for 15 s) [29] were met, but other deficiencies were noted including worn and broken pasteurizer gaskets. A sanitizer spray pump used to clean the pasteurization equipment was underpowered. Water temperatures used during cleaning were too hot (>93°C). Milk soils (residue) were observed in the pasteurization equipment and multiple items were inadequately cleaned. An employee reported that the mechanical bottle washer's iodine spray pump had been underpowered for an undetermined period, probably resulting in inadequately cleaned glass bottles. Additionally, iodine concentrations and caustic soak tank concentrations were measured incorrectly (dairy A had been using pH strips rather than per cent causticity). Milk soils were observed in the rubber filler gaskets and stainless nozzles of the bottle filler.
Water sources for the herd and pasteurization plant varied. When well-water levels were sufficient, well water was pumped into an underground concrete reservoir. During dry periods, the dairy obtained municipal water by using its own tanker trunk. This water was gravity-fed into the reservoir where it mixed with well water. The water was then pumped into the pasteurization plant where it was used for multiple purposes including pasteurizer cleaning and to supply the bottle washer. During 2011, the dairy began using municipal water in mid-June. The inside of the water tanker truck was not cleaned between its last use in November 2010 and first use in June 2011. Drinking water for the cow herd was obtained from a cistern containing a mixture of rain water and municipal water (when necessary).
New calves were born onsite and housed in a separate barn. The milking barn was adjacent to the processing plant. One milk processing room adjoined both the bottle-washing room and a cooling room. Used glass bottles and crates were stored in the same room as the bottle washer.
The farmhand who tended cows did not process milk or ice cream, but did deliver milk. This farmhand reported a 2-week history of watery, non-bloody diarrhoea without abdominal cramping during late July. He reportedly did not consume dairy A milk, but did drink the dairy's well water. A stool specimen obtained on 8 August did not yield Y. enterocolitica or any other enteric pathogen. No other dairy personnel reported illness in themselves or their family members during June–July 2011; asymptomatic dairy personnel were not tested. Dogs and cats but not pigs were observed on dairy premises. No pig farms were located in the vicinity of dairy A, and employees denied delivering milk to any pig farms.
Food and environmental specimens
Standard plate counts, coliform counts, somatic cell counts, and phosphatase (indicative of inadequate pasteurization) in routine regulatory samples of dairy A milk collected during January–June 2011 met PDA requirements. Ice cream testing during February–June 2011 detected intermittently elevated coliforms (>10 coliforms/ml).
Y. enterocolitica was not cultured from any of the 31 environmental or product samples tested at PADOH, the 16 environmental or 18 product samples tested at PDA, or the eight environmental samples tested at CDC. All manure samples collect from the dairy herd in October 2011 were reported as negative for Y. enterocolitica. None of the 19 food samples tested at the ACHD laboratory yielded Y. enterocolitica by using cold enrichment; however, two samples (unopened ice cream from a patient's home and homemade yogurt made by using dairy A milk from an asymptomatic customer) tested by using the room temperature enrichment method yielded Y. enterocolitica. Both food isolates were confirmed as bioserotype 1B/O:8 and were indistinguishable by PFGE (no bands of difference) from dairy A-associated patients' isolates.
Cohort study
Of 645 dairy A home delivery households, interviews were completed for 356 (55%). The 356 participating households included 992 persons. Dairy A products were consumed by 849 (86%) household members. Twenty (2%) respondents reported direct contact with pigs. Symptoms consistent with the case definition for suspected yersiniosis were reported by 64 (6%) household members (Table 3). Ten (16%) respondents had stool specimens tested before interview; one yielded Y. enterocolitica and this person's illness was included as a confirmed case.
Table 3.
Symptom prevalence in cohort study participants who reported a Yersinia-like illness, southwest Pennsylvania, January–August 2011
| Symptom | No. | % |
|---|---|---|
| Diarrhoea | 64 | 100 |
| Abdominal cramping or pain | 62 | 97 |
| Fatigue | 35 | 55 |
| Headache | 24 | 38 |
| Fever | 23 | 36 |
| Chills | 19 | 30 |
| Sore throat | 16 | 25 |
| Joint pain | 13 | 20 |
| Vomiting | 12 | 19 |
| Swollen lymph nodes | 11 | 17 |
| Bloody diarrhoea | 6 | 9 |
| Rash | 3 | 5 |
Drinking any milk was associated with illness (RR 4·0, 95% CI 0·5–28·6) (Table 4), although not significantly. Those consuming ⩾3 cups of milk/day (10%) were more likely to be ill, compared to those who drank <1 cup/day (5%, RR 2·0, 95% CI 1·0–4·1). Drinking only glass-bottled milk, compared to drinking only milk distributed in plastic jugs was not significantly associated with illness (RR 1·8, 95% CI 0·2–12·8). No specific milk type was associated with illness, nor were traditional Y. enterocolitica risk factors.
Table 4.
Risk factors for Yersinia-like illness in dairy a cohort study survey respondents, southwest Pennsylvania, January–August 2011
| Exposure | Exposed | Unexposed | RR | 95% CI | ||
|---|---|---|---|---|---|---|
| Ill | AR (%) | Ill | AR (%) | |||
| Drank any dairy A milk (ref = never drinks milk) | 61/910 | 7 | 1/59 | 2 | 4·0 | 0·5–28·6 |
| Amount of milk consumed per day* | ||||||
| <1 glass per day (reference) | 18/340 | 5 | Reference | |||
| 1–2 glasses per day | 30/476 | 6 | 1·2 | 0·6–2·3 | ||
| ⩾3 glasses per day | 14/134 | 10 | 2·0 | 1·0–4·1 | ||
| Ate any dairy A ice cream (ref = no ice cream) | 8/168 | 5 | 56/824 | 7 | 0·7 | 0·3–1·4 |
| Drank dairy A milk from glass bottles only (ref = drink only plastic) | 54/787 | 7 | 1/26 | 4 | 1·8 | 0·2–12·8 |
| Drank dairy A milk varieties | ||||||
| Any fat free milk | 26/288 | 9 | 38/704 | 5 | 1·7 | 0·9–3·0 |
| Any 1% milk | 13/135 | 10 | 51/857 | 6 | 1·6 | 0·8–3·5 |
| Any 2% milk | 28/429 | 7 | 36/527 | 6 | 1·0 | 0·6–1·8 |
| Any whole milk | 18/213 | 8 | 46/779 | 6 | 1·4 | 0·8–2·7 |
| Any chocolate-flavoured milk | 25/370 | 7 | 39/622 | 6 | 1·1 | 0·6–2·0 |
| Any strawberry-flavoured milk | 3/64 | 5 | 61/928 | 7 | 0·7 | 0·2–3·0 |
| Any buttermilk | 5/43 | 12 | 59/949 | 6 | 1·9 | 0·8–4·4 |
| Any cream | 2/34 | 6 | 62/960 | 6 | 0·9 | 0·3–3·7 |
| Only fat free milk | 13/150 | 9 | 51/842 | 6 | 1·4 | 0·7–2·8 |
| Only 1% milk | 2/45 | 4 | 62/947 | 7 | 0·7 | 0·2–2·7 |
| Only 2% milk | 8/168 | 5 | 56/768 | 7 | 0·7 | 0·3–1·8 |
| Only whole milk | 3/75 | 4 | 61/917 | 7 | 0·6 | 0·1–2·5 |
| Only chocolate-flavoured milk | 0/15 | 0 | 64/977 | 7 | n.a. | n.a. |
| Only strawberry-flavoured milk | 0/0 | n.a. | 64/992 | 6 | n.a. | n.a. |
| Only buttermilk | 0/0 | n.a. | 64/992 | 6 | n.a. | n.a. |
| Only cream | 0/2 | 0 | 64/990 | 6 | n.a. | n.a. |
| Drank raw milk | 0/5 | 0 | 64/987 | 6 | n.a. | n.a. |
| Contact with pigs | 3/20 | 15 | 61/972 | 6 | 2·4 | 0·6–8·8 |
| Contact with cows | 4/42 | 10 | 60/950 | 6 | 1·5 | 0·6–4·0 |
| Ate raw or undercooked pork | 0/5 | 0 | 64/987 | 6 | n.a. | n.a. |
| Chitterlings | 0/1 | 0 | 64/991 | 6 | n.a. | n.a. |
| Well water | 10/136 | 7 | 54/855 | 6 | 1·2 | 0·5–2·7 |
AR, Attack rate; RR, relative risk; CI, confidence interval; n.a., not applicable.
χ2 test for trend = 3·4, P = 0·07.
DISCUSSION
This outbreak of yersiniosis was associated with consumption of pasteurized milk products from a dairy in southwestern Pennsylvania. The dairy was a family-run, farm-based operation that performed on-site pasteurization and distributed the majority of its products through home delivery. The dairy was inspected annually by PDA. Multiple lines of epidemiological evidence implicated the dairy, including 16 culture-confirmed illnesses in consumers of products from the dairy, high relative risk and dose–response observed in the dairy's home delivery customers, and abrupt decline in illnesses when the dairy ceased production. Isolation of Y. enterocolitica from unopened ice cream and from yogurt made with the dairy's milk that were indistinguishable by PFGE from patients' isolates confirmed the dairy as the outbreak source, although the specific mechanism for contamination was not identified.
The extent of the outbreak was probably greater than the 16 culture-confirmed cases. Yersiniosis is not reportable in Pennsylvania; many clinicians are unfamiliar with this infection, and a limited number of laboratories routinely culture clinical isolates for Yersinia species. Although the total number of illnesses cannot be estimated, the 6% rate of Yersinia-like illness in the dairy's interviewed home delivery customers indicates additional illnesses had occurred. The closure of dairy A after the likely source was identified probably prevented additional cases.
Yersiniosis outbreaks in the USA are rare. However, this is the fifth reported outbreak of yersiniosis associated with pasteurized milk since 1976 [6–10]. The most recently reported milk outbreak [6] in New England also involved bioserotype 1B/O:8 and occurred in a similar setting to our investigation. That outbreak occurred at a farm-based dairy that pasteurized glass-bottled milk on site. A specific cause or mechanism for product contamination was not determined, and similar types of deficiencies in pasteurization and bottle-washing were identified. This indicates that transmission opportunities for Y. enterocolitica in farm-based dairy operations are possible.
Although Y. enterocolitica was isolated from dairy products left over in patients' households, we were unable to isolate Y. enterocolitica from environmental samples. Pathogenic strains of Y. enterocolitica are often difficult to isolate from the environment, possibly because of the fastidious enrichment requirements of the organism, combined with the limited number of pathogenic strains present in correlation to a substantial burden of background flora [19]. In each of the four previously published outbreaks regarding Y. enterocolitica associated with pasteurized milk, multiple environmental samples were collected and few yielded Y. enterocolitica, and similar to our investigation, the exact mechanism of contamination was difficult to determine [6–8, 10]. The long incubation period for Y. enterocolitica (3–7 days) is another reason why conditions at the dairy at the time of inspection might not reflect the conditions at the time of the outbreak. Our investigation was further hindered by the fact that dairy A began extensive cleaning of the pasteurization plant and dairy processing and packaging equipment as soon as they were notified of illnesses; samples were not obtained until cleaning was completed. The dairy subsequently took additional corrective actions including replacement of worn pasteurizer gaskets, installation of a new crate washer in a room separated from sanitized bottles, and use of titration to measure the bottle washer's causticity and iodine concentration.
Post-pasteurization contamination was postulated in all outbreaks of Y. enterocolitica associated with pasteurized milk; potential mechanisms included flavouring ingredients added post-pasteurization [7] and contamination of a bottle-filling valve, milk bottles, or crates [7–10]. Although pigs were not identified on the dairy and swabs from milk crates tested negative, our cohort study identified several home-delivery customers who reported direct contact with pigs; therefore, cross-contamination from workers who handled crates or bottles potentially contaminated by other persons who cared for pigs cannot be ruled out.
Because all 16 patients in our investigation had consumed glass-bottled milk, we focused our initial investigation on the mechanical bottle washer. However, after the unopened container of ice cream, which would not have been affected by the bottle washer, yielded Y. enterocolitica, additional contamination hypotheses were considered.
The results of our investigation and cohort study indicate that no single product or milk type could explain illness in the majority of persons with either confirmed or suspected yersiniosis. Therefore, the likely contamination source either from pasteurization or post-pasteurization deficiencies resulted from a mechanism common to production pathways for all dairy A products such as dairy personnel. Although the single ill farmhand tested negative for Y. enterocolitica, asymptomatic dairy personnel were not tested and cannot be excluded as possible sources of contamination.
Because dairy A added all ingredients (including flavourings and ice-cream mix ingredients) before pasteurization, the chocolate powder tested negative for Y. enterocolitica, and chocolate milk and ice cream were reportedly consumed by few of the patients, these were unlikely to be the source of contamination. Dairy A pasteurization temperatures were adequate, although the equipment was old and worn. No apparent post-pasteurization mechanism for cross-contamination with raw milk was identified, and the absence of phosphatase in tested samples makes the presence of raw milk in finished products unlikely. Contamination from sweetwater was possible. Thoroughly cleaned milk systems are critical in preventing product contamination; however, during this outbreak, pasteurization equipment was inadequately cleaned. Water temperatures exceeding 77°C, which occurred in this investigation, can result in denaturation of milk proteins, allowing milk residue to adhere to surfaces [30]. Multiple areas of milk soiling were observed, providing a potential mechanism for milk contamination. The dairy only started using municipal water in June. Because onset for the earliest cases occurred in March, contamination of the tanker truck or municipal water could not explain all infections. If the bacterial load in pre-pasteurized bulk tank milk is high, some Yersinia might survive pasteurization [31]. Although the single sample of unpasteurized bulk tank milk tested during our investigation did not yield Y. enterocolitica, we cannot rule out the possibility that intermittent faecal shedding prevented detection or that the test was falsely negative given the challenges of isolating Y. enterocolitica from food. Although specific methods varied, all laboratories in this investigation used cold enrichment, the standard isolation method used in the USA [32]. Cold temperatures, while providing a competitive advantage for Y. enterocolitica, also slow its growth; as a result, long enrichment times, typically 10–21 days are required. Although the International Organization for Standardization method for the detection of Y. enterocolitica recommends room temperature enrichment [25], the use of room temperature enrichment after 1 day of cold enrichment and KOH treatment to inhibit growth of background flora was novel and shortened incubation time. Isolation of Y. enterocolitica from yogurt and ice cream through this novel method confirmed the epidemiological association; furthermore, isolation from ice cream led to a broadened environmental investigation and a second press release. Validation studies of this method are necessary to determine its potential benefit during future Y. enterocolitica outbreaks.
Our investigation had limitations, including that (1) collection of environmental samples occurred weeks after the initial case was identified and after thorough equipment cleaning; (2) cow manure samples were not obtained as part of our investigation; and (3) isolates were not available for PFGE from patients with onset during spring to determine the association of these illnesses to later ones.
Nonetheless, this outbreak serves as a reminder of Y. enterocolitica's importance as a foodborne pathogen in North America, severe consequences of infection, and difficulties identifying and investigating outbreaks caused by this organism. In fact, bioserotype 2/O:8 was originally suspected in this outbreak since lipase activity was not detected; however, further work indicated that lipase activity was weak and delayed. The high pathogenicity island (HPI) specific for Y. enterocolitica 1B was detected by polymerase chain reaction of the HPI fyuA gene, asserting that this outbreak was caused by a strain of 1B/O:8 [33–35; L. Martin and E. Carniel, unpublished data]. In the future, molecular methods will be useful in discriminating and identifying strains where inconsistencies in phenotype exist.
Because of the potential for Y. enterocolitica to cause serious illness, hospitalization, and death, clinicians should consider yersiniosis in patients with unexplained febrile diarrhoea and abdominal pain. Although most dairy-associated outbreaks in the USA are linked to raw (unpasteurized) milk [36], this outbreak also highlights that improperly pasteurized milk can also serve as a vehicle for foodborne illness. The ability of Yersinia species to survive and multiply at refrigeration temperatures makes milk a particularly good vehicle for human infections if the pasteurization process is insufficient or if post-pasteurization contamination occurs. The importance of thorough cleaning and maintenance of pasteurization plant equipment, as well as cross-contamination and control should be routinely emphasized to dairy owners [36, 37]. Pasteurization is widely regarded as the most effective method for decreasing the number of pathogenic organisms in milk; outbreaks associated with pasteurized milk are rare [36]. However, because consumption of pasteurized milk is common and outbreaks have the potential to become widespread, public health interventions (e.g. consumer advisories or dairy closures) should be implemented immediately to prevent additional illnesses if epidemiological or laboratory evidence implicates pasteurized milk as the exposure source.
ACKNOWLEDGEMENTS
We thank the many Pennsylvania Department of Health and Allegheny County Health Department staff members who participated with data collection and data entry; Dr Virginia Dato and Dr Atmaram Nambiar (Pennsylvania Department of Health), and Megan Casey and Sharon Silvestri (Allegheny County Health Department) for assistance with the epidemiological investigation; Paul Hoge and David Trotter (Pennsylvania Department of Agriculture), for participation in the environmental investigation; Vincent Hill (Centers for Disease Control and Prevention), for assisting with dead-end ultrafiltration; Cheryl Bopp, Cheryl Tarr (Centers for Disease Control and Prevention), Sharon Shoop and Michael Hydock (Pennsylvania Department of Agriculture), Barry Perry and James Tait (Pennsylvania Department of Health), and Hideo Morimoto (Allegheny County Health Department) for laboratory support; Hannah Gould (Centers for Disease Control and Prevention) for assistance with manuscript review; local hospital infection preventionists for assistance with medical information; and the World Health Organization Collaborating Center for Yersinia (Pasteur Institute, Paris) for assisting in confirmation of the bioserotype.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Scallan E, et al. Foodborne illness acquired in the United States – major pathogens. Emerging Infectious Diseases 2011; 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostroff S. Yersinia as an emerging infection: epidemiologic aspects of yersiniosis. Contributions to Microbiology and Immunology 1995; 13: 5–10. [PubMed] [Google Scholar]
- 3.Tacket CO, et al. An outbreak of Yersinia enterocolitica infections caused by contaminated tofu (soybean curd). American Journal of Epidemiology 1985; 121: 705–711. [DOI] [PubMed] [Google Scholar]
- 4.Tauxe RV, et al. Yersinia enterocolitica infections and pork: the missing link. Lancet 1987; 1: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 5.Lee LA, et al. Yersinia enterocolitica O:3 infections in infants and children, associated with the household preparation of chitterlings. New England Journal of Medicine 1990; 322: 984–987. [DOI] [PubMed] [Google Scholar]
- 6.Ackers ML, et al. An outbreak of Yersinia enterocolitica O:8 infections associated with pasteurized milk. Journal of Infectious Diseases 2000; 181: 1834–1837. [DOI] [PubMed] [Google Scholar]
- 7.Black RE, et al. Epidemic Yersinia enterocolitica infection due to contaminated chocolate milk. New England Journal of Medicine 1978; 298: 76–79. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood MH, Hooper WL. Excretion of Yersinia spp. associated with consumption of pasteurized milk. Epidemiology and Infection 1990; 104: 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood MH, Hooper WL, Rodhouse JC. The source of Yersinia spp. in pasteurized milk: an investigation at a dairy. Epidemiology and Infection 1990; 104: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacket CO, et al. A multistate outbreak of infections caused by Yersinia enterocolitica transmitted by pasteurized milk. Journal of the American Medical Association 1984; 251: 483–486. [PubMed] [Google Scholar]
- 11.Ostroff SM, et al. Sources of sporadic Yersinia enterocolitica infections in Norway: a prospective case-control study. Epidemiology and Infection 1994; 112: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver SP, Jayarao BM, Almeida RA. Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathogens and Disease 2005; 2: 115–129. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima H, et al. Differentiation of Yersinia enterocolitica serotype O:5,27 strains by phenotypic and molecular techniques. Journal of Clinical Microbiology 1993; 31: 1672–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fearnley C, et al. Application of fluorescent amplified fragment length polymorphism for comparison of human and animal isolates of Yersinia enterocolitica. Applied and Environmental Microbiology 2005; 71: 4960–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman A, et al. Yersinia enterocolitica: Epidemiological studies and outbreaks. Journal of Pathogens 2011; 2011: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhaduri S, Wesley IV, Bush EJ. Prevalence of pathogenic Yersinia enterocolitica strains in pigs in the United States. Applied and Environmental Microbiology 2005; 71: 7117–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNally A, et al. Comparison of the biotypes of Yersinia enterocolitica isolated from pigs, cattle and sheep at slaughter and from humans with yersiniosis in Great Britain during 1999–2000. Letters in Applied Microbiology 2004; 39: 103–108. [DOI] [PubMed] [Google Scholar]
- 18.Jayarao BM, et al. A survey of foodborne pathogens in bulk tank milk and raw milk consumption among farm families in Pennsylvania. Journal of Dairy Science 2006; 89: 2451–2458. [DOI] [PubMed] [Google Scholar]
- 19.Fredriksson-Ahomaa M, Korkeala H. Low occurrence of pathogenic Yersinia enterocolitica in clinical, food, and environmental samples: a methodological problem. Clinical Microbiology Reviews 2003; 16: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CM, Hill VR. Dead-end hollow-fiber ultrafiltration for recovery of diverse microbes from water. Applied and Environmental Microbiology 2009; 75: 5284–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer E. Isolation of Yersinia enterocolitica from foods. International Journal of Food Microbiology 1992; 17: 75–84. [DOI] [PubMed] [Google Scholar]
- 22.Doyle MP, Hugdahl MB. Improved procedure for recovery of Yersinia enterocolitica from meats. Applied and Environmental Microbiology 1983; 45: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Damme I, Habib I, De Zutter L. Yersinia enterocolitica in slaughter pig tonsils: enumeration and detection by enrichment versus direct plating culture. Food Microbiology 2010; 27: 158–161. [DOI] [PubMed] [Google Scholar]
- 24.Schiemann DA, Olson SA. Antagonism by gram-negative bacteria to growth of Yersinia enterocolitica in mixed cultures. Applied and Environmental Microbiology 1984; 48: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Organization for Standardization. Microbiology of food and animal feedings stuffs–Horizontal method for the detection of presumptive pathogenic Yersinia enterocolitica (ISO10273:2003). Geneva, Switzerland: British Standard International Organization for Standardization, 2003. [Google Scholar]
- 26.Wauters G. Antigens of Yersinia enterocolitica. In: Bottone E, ed. Yersinia enterocolitica. Boca Raton: CRC Press, 1981, pp. 41–53. [Google Scholar]
- 27.Wauters G, Kandolo K, Janssens M. Revised biogrouping scheme of Yersinia enterocolitica. Contributions to Microbiology and Immunology 1987; 9: 14–21. [PubMed] [Google Scholar]
- 28.Wauters G, et al. Somatic and flagellar antigens of Yersinia enterocolitica and related species. Contributions to Microbiology and Immunology 1991; 12: 239–243. [PubMed] [Google Scholar]
- 29.Food and Drug Administration. Grade A pasteurized milk ordinance, 2011 revision. Silver Spring, MD, USA: United States Department of Health and Human Services, Public Health Service, 2011. [Google Scholar]
- 30.Jones G. Cleaning and sanitizing milking equipment. Virginia Cooperative Extension, 2009, vol. 404-400.
- 31.Schiemann DA, Toma S. Isolation of Yersinia enterocolitica from raw milk. Applied and Environmental Microbiology 1978; 35: 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weagant SD, Feng P, Stanfield JT. Yersinia enterocolitica and Yersinia pseudotuberculosis, chapter 8. In: Bacteriological Analytical Manual, 8th edn, revision A. Silver Spring, MD, USA: United States Department of Health and Human Services, Food and Drug Administration, 1998. Revised August 2007. [Google Scholar]
- 33.Heesemann J, et al. Virulence of Yersinia enterocolitica is closely associated with siderophore production, expression of an iron-repressible outer membrane of polypeptide of 65,000 Da and pesticin sensitivity. Molecular Microbiology 1993; 8: 397–408. [DOI] [PubMed] [Google Scholar]
- 34.Fàbrega A, Vila J. Yersinia enterocolitica: pathogenesis, virulence and antimicrobial resistance. Enfermedades Infecciosas y Microbiología Clínica 2012; 30: 24–32. [DOI] [PubMed] [Google Scholar]
- 35.Carniel E, Guilvout I, Prentice M. Characterization of a large chromosomal ‘high-pathogenicity island’ in biotype 1B Yersinia enterocolitica. Journal of Bacteriology 1996; 178: 6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langer AJ, et al. Nonpasteurized dairy products, disease outbreaks, and state laws – United States, 1993–2006. Emerging Infectious Diseases 2012; 18: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Outbreak of Listeria monocytogenes infections associated with pasteurized milk from a local dairy – Massachusetts, 2007. Morbidity and Mortality Weekly Report 2008; 57: 1097–1101. [PubMed] [Google Scholar]