SUMMARY
Two community-based density case-control studies were performed to assess risk factors for cholera transmission during inter-peak periods of the ongoing epidemic in two Haitian urban settings, Gonaives and Carrefour. The strongest associations were: close contact with cholera patients (sharing latrines, visiting cholera patients, helping someone with diarrhoea), eating food from street vendors and washing dishes with untreated water. Protective factors were: drinking chlorinated water, receiving prevention messages via television, church or training sessions, and high household socioeconomic level. These findings suggest that, in addition to contaminated water, factors related to direct and indirect inter-human contact play an important role in cholera transmission during inter-peak periods. In order to reduce cholera transmission in Haiti intensive preventive measures such as hygiene promotion and awareness campaigns should be implemented during inter-peak lulls, when prevention activities are typically scaled back.
Key words: Cholera, risk factors, endemic, epidemic, Haiti, prevention, transmission, Vibrio cholerae
INTRODUCTION
Since October 2010 Haiti has been experiencing a cholera epidemic for the first time in over 100 years [1]. As of March 2013, the epidemic has resulted in more than 650 000 cases and 7441 deaths [2]. Immunological naivety of the population to the cholera agent and the contamination of river waters explain most of the high attack rate [3]. Several epidemic peaks have occurred, all during the rainy seasons. The first peak (October–December 2010) was explosive, with very rapid transmission throughout the country; the second peak (May–July 2011) was lower than the first in some places, and higher in others. Since then, peaks have occurred twice a year corresponding to the rainy season. Between peaks a low but persistent number of cholera cases are reported.
Waterborne transmission was clearly identified as the main transmission route during the peak periods [4–6]; however, other factors may increase in importance during the inter-peak periods. Here we present findings from two studies which investigated the risk factors associated with clinical cholera cases that occur during the lull in transmission, factors that therefore may contribute to the maintenance of cholera transmission in urban settings.
METHODS
Study design and settings
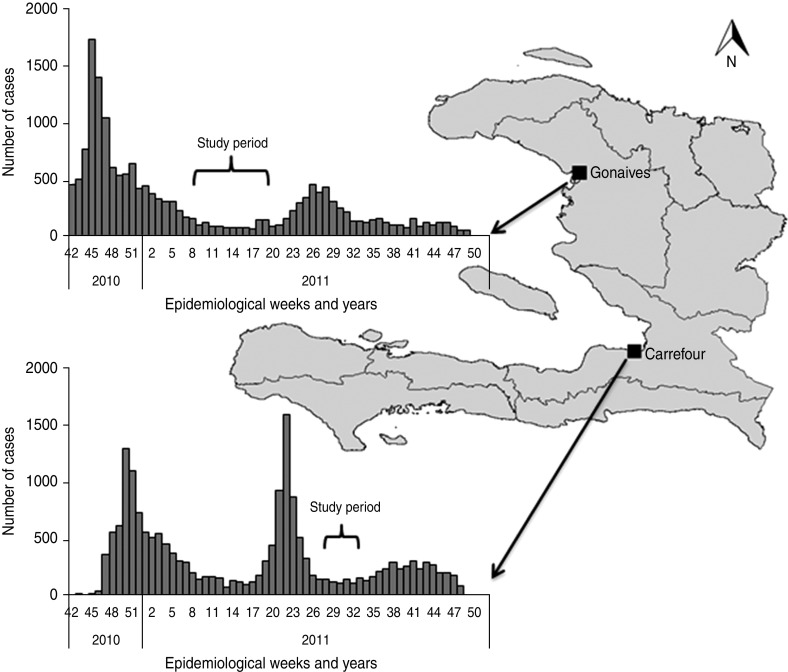
The two studies were community-based density case-control surveys, with cases and controls matched by age and gender. The first study was conducted in Gonaives, a city of 230 000 inhabitants [7] and capital of the Artibonite department, from 23 March 2011 to 30 May 2011. The second study was conducted in Carrefour (a suburb of the capital Port-au-Prince; 430 000 inhabitants) [7] from 22 July 2011 to 22 August 2011 (Fig. 1). At the time of the study, Carrefour still sheltered ∼40 000 displaced people in camps as a consequence of the January 2010 earthquake [8]. Data were collected from individuals as well as from household observations.
Fig. 1.
Location of the towns of Gonaives and Carrefour and periods of participants' interviews in relation to the epidemic curves of the communes, where the towns are located, Haiti, 2011.
Gonaives was chosen because it was among the first and most affected towns; Carrefour was chosen to explore additional risk factors related to the post-earthquake conditions of the survivors and because of the high incidence reported in previous epidemic waves.
Case and control definitions
A case was defined as a person (1) living in Gonaives or Carrefour since the beginning of the cholera outbreak in October 2010; (2) aged >5 years; (3) presenting with symptoms of acute watery diarrhoea; and (4) with a cholera diagnosis confirmed by a rapid test (Crystal VC® Rapid Dipstick test, Span Diagnostics, India) for Vibrio cholerae O1 or O139. In Gonaives, cases were included from the cholera treatment centre (CTC) managed by Médecins Sans Frontières (MSF). In Carrefour, cases were included from two CTCs: one managed by MSF and another by Save the Children. Participation in the study was proposed to all eligible patients upon admission to the CTC. Written consent was sought after patients tested positive by Crystal VC test and before inclusion in the study.
Two controls of the same sex and age group were selected for each case. Age groups were 5–9, 10–14, 15–19, 20–29, 30–39, 40–49 and ⩾50 years. A control was defined as a person (1) living in Gonaives or Carrefour since the beginning of the epidemic in October 2010; (2) who had not experienced acute watery diarrhoea since that time; and (3) reported that they would have sought treatment at the CTC if they had developed acute watery diarrhoea.
Controls were selected using spatial random sampling [9]. Two polygons were first drawn to define the urban areas of Gonaives and Carrefour. Points were then drawn randomly within the polygons and superposed onto Google Earth® maps. Points coinciding with a house were retained; two points were randomly attributed to each case as locations to find controls. Investigators located the corresponding houses using GPS devices and verified the presence of a household member eligible for participation as a control. If none was eligible, investigators continued to the nearest house, and so on, until they found a willing control.
Sample size was determined based on the hypothesis that the presence of free chlorine in drinking water stored at home would result in a 2·5-fold decrease in the risk of transmitting cholera. This hypothesis was tested with an alpha risk of 5%, a statistical power of 80% and an estimated loss of 10%, resulting in a sample of 90 cases and 180 controls for each study.
Data collection and management
Trained investigators conducted face-to-face interviews with all cases and controls aged ⩾16 years; for participants aged <16 years, interviews were conducted with the child's guardian. A locally tailored questionnaire was written in French and translated into Creole, and then back-translated for verification.
Patients who agreed to participate were interviewed either on the day of admission to the CTC or the following day, depending on the severity of their clinical condition. On the day of a case's interview, investigators visited his/her household to assess the hygiene conditions of the latrine (presence of hand washing soap at latrine; overall latrine condition) and to conduct chemical and biological tests of the household's drinking water. The interview and the household assessment of controls were carried out on the same day as, or the day following, the matched case interview.
During the interview, data was collected on the following variables of potential relevance to cholera transmission: origin and quality of food and water, hygiene and sanitation habits, contact with cholera-infected patients, knowledge of transmission and prevention measures, and socioeconomic status.
Evaluating quality of drinking water
The level of free chlorine in households' drinking water was measured with a HANNA HI 701 Checker® HC spectrophotometer (HANNA Instruments®, UK). Properly chlorinated water was defined as being above a threshold of 0·2 mg/l free chlorine [10]. The presence of Escherichia coli was assessed using chromogenic medium AquaCHROM™ (CHROMagar™, France). After adding a fixed dose of chromogenic medium to a 100 ml water sample, the sample was incubated at room temperature for 24 h. The sample appearance was interpreted as follows: green or blue-green = presence of E. coli; yellow = presence of non-E. coli coliforms; colourless = absence of E. coli and non-E. coli coliforms.
Statistical methods
Data were entered using EpiData v. 3.1 (EpiData, Denmark) and analysed using Stata v. 11 (StataCorp, USA).
Matched odds ratios (ORs) were calculated using conditional logistic regression as a measure of cholera risk. Matched ORs, 95% confidence intervals (95% CIs) and P values were estimated with the case/control status as outcome variable and with exposure variables as explanatory variables, and interpreted with a bilateral test. Statistical significance was defined as P < 0·05.
A score for socioeconomic status was constructed by determining whether or not the family owned specific items (radio, television, refrigerator, oven, washing machine, water storage recipient, car, animals), and by education level of the interviewee and the head of the family (main provider of household income). Details on how this score was determined are presented in the supplementary online Appendix.
Multivariate conditional logistic regression analysis was performed as described by Hosmer & Lemeshow [11]. Models incorporated those variables that showed a significance level of P < 0·2 in univariate analysis, as well as those generally considered to have public health relevance for cholera (level of free chlorine and presence of E. coli in home-stored drinking water). The likelihood-ratio test was used to evaluate the contribution of each variable to the model; relevant first-degree interactions were also analysed.
Ethics
The two studies were implemented in collaboration with the Haitian Ministry of Public Health and Population and they adhered to the principles governing biomedical research involving human subjects, as defined by the Declaration of Helsinki. Protocols were validated by the Haitian Ethics Committee.
Written consent was obtained from participants or a parent/guardian. Privacy and confidentiality of data was ensured during and after conducting the surveys.
RESULTS
Univariate analysis
Direct and indirect contacts with cholera patients
Compared to controls, cases in Carrefour (but not Gonaives) had more frequent exposure to direct contact with cholera patients (living with, visiting or caring for). Sharing latrines with someone suffering from diarrhoea was significantly associated with the risk of getting cholera for both locations (Table 1).
Table 1.
Univariate conditional logistic regression in relation to direct and indirect contact with a cholera patient, quality of drinking water and food consumption by study site, Haiti 2011
| Gonaives | Carrefour | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure (%) | OR | 95% CI | Exposure (%) | OR | 95% CI | |||
| Controls | Cases | Controls | Cases | |||||
| Direct and indirect contact | ||||||||
| Cholera case in the household since beginning of the epidemic | 17·8 | 20·0 | 1·1 | 0·6–2·1 | 7·2 | 17·8 | 2·7* | 1·2–5·8 |
| Visiting someone suffering from cholera | 15·6 | 11·2 | 0·7 | 0·3–1·5 | 8·4 | 23·0 | 3·0** | 1·5–6·2 |
| Caring for someone suffering from diarrhoea or cholera | 13·3 | 4·4 | 0·3* | 0·1–0·9 | 4·4 | 13·5 | 3·2* | 1·3–8·3 |
| Sharing latrine with someone suffering from diarrhoea | 16·9 | 32·5 | 2·1* | 1·2–3·8 | 13·5 | 34·2 | 3·8*** | 1·8–8·1 |
| Quality of drinking water stored at home | ||||||||
| Residual free chlorine in drinking water >0·2 mg/l | 15·6 | 11·5 | 0·7 | 0·3–1·5 | 60·7 | 63·2 | 1·1 | 0·6–1·9 |
| Presence of non-E. coli coliforms | 78·2 | 82·9 | 1·3 | 0·6–2·5 | 33·9 | 29·9 | 0·7 | 0·4–1·4 |
| Presence of E. coli | 21·2 | 31·7 | 1·8 | 0·9–3·2 | 20·2 | 18·2 | 0·8 | 0·4–1·7 |
| Always chlorinate water before drinking (self-reported) | 48·9 | 34·4 | 0·5* | 0·3–0·9 | 75·6 | 65·6 | 0·6 | 0·3–1·1 |
| Ate a meal away from home at least once in week before illness | 14·4 | 42·2 | 7·6*** | 3·3–17·4 | 28·3 | 47·8 | 2·5** | 1·4–4·5 |
| Places where meal was eaten | ||||||||
| Restaurant | 2·8 | 5·6 | 2·2 | 0·6–8·4 | 4·0 | 5·6 | 1·5 | 0·4–5·6 |
| School† | 4·4 | 14·4 | 15·6** | 2·0–124·3 | 0·6 | 0·0 | — | — |
| Street vendor | 2·8 | 8·9 | 3·2* | 1·0–9·8 | 16·5 | 25·6 | 1·7 | 0·9–3·2 |
| Market | 1·7 | 12·2 | 19·1** | 2·4–149·1 | 0·6 | 2·2 | 4·0 | 0·4–44·1 |
| Parent's/friend's house | 2·8 | 8·9 | 6·3* | 1·3–30·7 | 4·0 | 7·8 | 2·0 | 0·7–5·7 |
| Buying fresco from street vendor | n.a. | n.a. | — | — | 27·4 | 42·7 | 2·0* | 1·1–3·3 |
OR, Odds ratio; CI, confidence interval; n.a., not available.
In Gonaives the odds ratio for this variable could not be calculated due to the absence of pairing with unexposed cases. The odds ratio presented here was calculated by randomly re-coding an exposed case as unexposed.
P< 0·05, ** P < 0·01, *** P < 0·001 (two-tailed tests).
Water and food consumption
Most households in both locations had access to drinking water from protected water sources such as the town water system or private vendors. No significant difference was found in terms of household drinking-water source between cases and controls (Table 1); however, always drinking chlorinated water was protective in both studies (significantly associated in Gonaives and almost significantly associated in Carrefour).
Eating a meal away from home at least once during the week before illness was significantly more frequent on cases than controls in both studies (OR 7·6 and 2·5 in Gonaives and Carrefour, respectively) (Table 1). This was a frequent risk factor as it was reported by 42·2% and 47·8% of cases in Gonaives and Carrefour, respectively. In Gonaives, the most common location of these meals were school, street vendors, and parents’/friends' houses. Investigators collected detailed information about the types of food consumed over the previous week, including fish, seafood, meat, milk, vegetables and fruit, but found no differences in consumption habits between the two groups (data not shown).
Hygiene conditions and hygienic behaviours
A large proportion of households used a latrine in their yard and shared it with other households (more frequently in Carrefour). Soap and water were rarely available at the latrine site, although this was not significantly associated with risk in either location. The use of soap for hand washing and use of individual dishes (rather than a communal serving dish) at meals, was less frequent in cases than controls, although statistical significance was reached only in Gonaives.
In both locations, compared to controls, cases more frequently used non-chlorinated water for washing dishes.
Exposure to information on cholera prevention
Radio was the most common means of receiving information on cholera prevention, but no difference was found between cases and controls in either location. Controls more frequently reported exposure to prevention information from training sessions in Gonaives, at church in Carrefour and via television in both locations (Table 2).
Table 2.
Univariate conditional logistic regression for hygiene conditions and behaviours by study site, Haiti 2011
| Gonaives | Carrefour | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure (%) | OR | 95% CI | Exposure (%) | OR | 95% CI | |||
| Controls | Cases | Controls | Cases | |||||
| Type/location of toilets | ||||||||
| Toilet inside house | 4·8 | 7·2 | Ref. | 24·9 | 13·6 | Ref. | ||
| Toilet/latrine in garden | 1·2 | 0·0 | — | — | 1·2 | 2·5 | 3·4 | 0·4, 26·8 |
| Latrine in courtyard | 86·7 | 79·5 | 0·7 | 0·2–2·1 | 67·1 | 71·6 | 1·8 | 0·9–3·8 |
| Latrine belonging to neighbour | 7·2 | 12·0 | 1·3 | 0·3–5·2 | 6·4 | 6·2 | 1·6 | 0·4–5·4 |
| Shallow pit in yard | 0·0 | 0·0 | — | — | 0·6 | 4·9 | 11·9* | 1·2–113·9 |
| Other | 0·0 | 1·2 | — | — | 0·0 | 1·2 | — | — |
| Persons using the toilet/latrine | ||||||||
| Only household members | 74·1 | 72·8 | Ref. | 62·3 | 49·4 | Ref. | ||
| Several households | 25·9 | 25·9 | 1·0 | 0·5–1·9 | 36·0 | 45·6 | 1·8 | 1·0–3·3 |
| Anybody | 0·0 | 1·2 | — | — | 1·7 | 5·1 | 5·8 | 1·0–33·4 |
| Latrines were overflowing | 21·7 | 30·8 | 1·3 | 0·7–2·4 | 4·3 | 8·8 | 1·9 | 0·6–6·3 |
| Water available for hand washing at site of latrines | 5·1 | 4·4 | 0·8 | 0·3–2·7 | 8·6 | 2·3 | 0·3 | 0·1–1·2 |
| Soap available for hand washing at site of latrines | 4·5 | 2·2 | 0·5 | 0·1–2·2 | 9·8 | 5·8 | 0·7 | 0·3–1·8 |
| Use of soap for hand washing | 83·1 | 68·5 | 0·4** | 0·2–0·8 | 91·5 | 85·2 | 0·5 | 0·2–1·1 |
| Use of individual place setting to eat | 84·9 | 68·9 | 0·3** | 0·1–0·6 | 91·3 | 86·7 | 0·6 | 0·3–1·4 |
| Using untreated water to wash dishes | 38·9 | 52·2 | 2·1* | 1·2–3·8 | 19·6 | 35·6 | 3·0** | 1·5–6·2 |
| Sources of information on cholera prevention | ||||||||
| Television | 32·8 | 20·0 | 0·4** | 0·2–0·8 | 61·7 | 42·2 | 0·4** | 0·2–0·7 |
| Radio | 75·0 | 66·7 | 0·6 | 0·3–1·1 | 61·7 | 51·1 | 0·6 | 0·3–1·0 |
| Door-to-door | 48·9 | 54·4 | 1·4 | 0·7–2·5 | 27·8 | 28·9 | 1·1 | 0·6–1·9 |
| Theatre | 0·6 | 2·2 | 4·0 | 0·4–44·1 | 0·6 | 0·0 | — | — |
| Posters | 2·8 | 2·2 | 0·8 | 0·2–4·1 | 11·7 | 10·0 | 0·8 | 0·3–2·1 |
| Town crier/sound track | 3·3 | 5·6 | 2·1 | 0·5–9·5 | 8·3 | 10·0 | 1·3 | 0·5–3·2 |
| Training session | 17·2 | 5·6 | 0·3** | 0·1–0·7 | 20·6 | 16·7 | 0·7 | 0·4–1·5 |
| School | 19·4 | 17·8 | 0·9 | 0·4–1·9 | 8·3 | 10·0 | 1·3 | 0·5–3·2 |
| Church | 11·7 | 5·6 | 0·4 | 0·1–1·2 | 11·7 | 3·3 | 0·2* | 0·1–0·8 |
| Other sources of information | 4·4 | 8·9 | 1·3 | 0·4–4·1 | 2·8 | 8·9 | 3·7* | 1·1–12·3 |
OR, Odds ratio; CI, confidence interval.
P < 0·05, ** P < 0·01, *** P < 0·001 (two-tailed tests).
Social and economic status
In both Gonaives and Carrefour, the education levels of the interviewee and his/her head of family were lower in cases than in controls (same trend in both locations, significant only in Carrefour). Cases had fewer household members than controls (same trend in both locations, although significant only in Gonaives), and were less likely to own a television, refrigerator and car (significant in both locations). Socioeconomic score was significantly lower for cases than for controls in both locations (Table 3).
Table 3.
Univariate conditional logistic regression for social and economic status by study site, Haiti 2011
| Gonaives | Carrefour | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposure (%) | OR | 95% CI | Exposure (%) | OR | 95% CI | |||
| Controls | Cases | Controls | Cases | |||||
| Type of home dwelling | ||||||||
| Concrete | n.a. | n.a. | — | — | 80·6 | 63·3 | Ref. | |
| Wood or iron sheeting | n.a. | n.a. | — | — | 13·9 | 21·1 | 2·3* | 1·1, 5·1 |
| Tent or plastic sheeting | n.a. | n.a. | — | — | 5·6 | 15·6 | 3·4** | 1·5, 8·3 |
| Number of household members† | ||||||||
| 1–3 | 8·9 | 22·2 | Ref. | 15·2 | 21·3 | Ref. | ||
| 4–5 | 22·8 | 26·7 | 0·5 | 0·2–1·1 | 35·4 | 33·7 | 0·6 | 0·3–1·3 |
| 6–8 | 33·9 | 36·7 | 0·4* | 0·2–1·0 | 31·5 | 30·3 | 0·6 | 0·3–1·4 |
| ⩾9 | 34·4 | 14·4 | 0·2*** | 0·1–0·4 | 18·0 | 14·6 | 0·5 | 0·2–1·3 |
| Household owns | ||||||||
| Goats | 13·3 | 15·6 | 1·2 | 0·6–2·4 | 2·8 | 1·1 | 0·4 | 0·0–3·4 |
| Pigs | 3·9 | 16·7 | 5·5** | 2·0–15·1 | 0·6 | 2·3 | 4·0 | 0·4–44·1 |
| Chickens | 25·0 | 21·1 | 0·8 | 0·5–1·5 | 19·6 | 18·0 | 0·9 | 0·5–1·8 |
| Other animals | 19·4 | 17·8 | 0·9 | 0·4–1·8 | 39·7 | 26·7 | 0·5* | 0·3–0·9 |
| Household owns at least one | ||||||||
| Radio | 77·8 | 70·0 | 0·7 | 0·4–1·2 | 73·3 | 60·0 | 0·5* | 0·3–0·9 |
| Television | 71·1 | 46·7 | 0·4*** | 0·2–0·7 | 68·3 | 53·3 | 0·5* | 0·3–0·9 |
| Refrigerator | 21·7 | 11·1 | 0·4* | 0·2–0·9 | 31·7 | 21·1 | 0·6 | 0·3–1·1 |
| Oven | 6·1 | 3·3 | 0·5 | 0·2–2·0 | 12·2 | 6·7 | 0·5 | 0·2–1·3 |
| Washing machine | 1·1 | 2·2 | 2·0 | 0·3–14·2 | 1·7 | 0·0 | — | — |
| Water storage tank | 7·8 | 3·3 | 0·4 | 0·1–1·5 | 3·3 | 2·3 | 0·7 | 0·1–3·3 |
| Car | 14·4 | 3·3 | 0·2* | 0·1–0·7 | 13·3 | 3·4 | 0·2* | 0·1–0·8 |
| Socioeconomic score‡ (mean) | 1·45 | 1·13 | 0·5** | 0·3–0·8 | 1·82 | 1·32 | 0·4*** | 0·3–0·6 |
OR, Odds ratio; CI, confidence interval; n.a., not available.
Odds ratios for trend: Gonaives (0·83, 95% CI 0·75–0·92), Carrefour (0·94, 95% CI 0·85–1·04).
Socioeconomic score includes educational level of the interviewee and of the head of the family as well as ownership of radio, television, refrigerator, oven, washing machine, water storage tank, car and animals).
P < 0·05, ** P < 0·01, *** P < 0·001 (two-tailed tests).
Multivariate analysis
In Gonaives the multivariate analysis indicated eating meals outside the home [adjusted OR (aOR) 35·9], owning pigs (aOR 10·3) and sharing latrines (aOR 3·5) to be the strongest and most significant risk factors. The presence of E. coli in the family drinking water, which approached the threshold of significance in univariate analysis, became significant in the multivariate analysis. Interactions between the presence of E. coli and chlorine levels, between owning pigs and socioeconomic level, and between participant's age and the presence of E. coli were explored, but none were statistically significant. Receiving cholera prevention messages either via television (aOR 0·2) or through training sessions (aOR 0·2) was protective (Table 4).
Table 4.
Multivariate analysis of risk factors associated with cholera illness in Gonaives, Haiti 2011
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Ate a meal away from home at least once in week before illness | 7·6*** | 3·3–17·4 | <0·001 | 35·9*** | 7·9–163·4 | <0·001 |
| Household owns pigs | 5·5** | 2·0–15·1 | 0·001 | 10·3** | 2·3–46·6 | 0·002 |
| Sharing latrine with someone suffering from diarrhoea | 2·1* | 1·2–3·8 | 0·013 | 3·5* | 1·3–9·5 | 0·016 |
| Presence of E. coli in drinking water stored at home | 1·8 | 0·9–3·2 | 0·074 | 3·5* | 1·2–10·0 | 0·021 |
| Chlorine level >0·2 mg/l in drinking water stored at home | 0·7 | 0·3–1·5 | 0·376 | 0·5 | 0·2–1·9 | 0·341 |
| Always chlorinate water before drinking | 0·5* | 0·3–0·9 | 0·019 | 0·3 | 0·1–1·0 | 0·060 |
| Receiving information on cholera prevention via television | 0·4* | 0·2–0·8 | 0·015 | 0·2* | 0·1–0·8 | 0·021 |
| Receiving information on cholera prevention in training session | 0·3** | 0·1–0·7 | 0·009 | 0·2* | 0·0–0·9 | 0·035 |
| Number of member in household (ref. 1–3 members) | ||||||
| 4–5 | 0·5 | 0·2–1·1 | 0·099 | 0·5 | 0·2–1·7 | 0·291 |
| 6–8 | 0·4 | 0·2–1·0 | 0·044 | 0·7 | 0·2–2·6 | 0·595 |
| ⩾9 | 0·2*** | 0·1–0·4 | <0·001 | 0·1** | 0·0–0·5 | 0·004 |
| Socioeconomic score | 0·5** | 0·3–0·8 | 0·001 | 0·5* | 0·3–1·0 | 0·036 |
OR, Odds ratio; CI, confidence interval.
P < 0·05, ** P < 0·01, *** P < 0·001 (two-tailed tests)
The multivariate analysis in Carrefour confirmed as significant the main factors identified by univariate odds ratios. Three significant variables measuring direct or indirect contact with someone suspected of having cholera (sharing a latrine with someone suffering from diarrhoea, visiting a cholera patient, and caring for someone suffering from diarrhoea or cholera) were highly collinear and were therefore analysed separately in three models with the three variables interchanged. The aORs were 3·2 for sharing a latrine, 3·7 for visiting a cholera patient, and 3·8 for caring for someone suffering from diarrhoea or cholera. Using untreated water for washing dishes (aOR 3·2) remained a significant risk factor, while receiving cholera prevention messages via television or in church was protective in all three models (Table 5).
Table 5.
Multivariate analysis of risk factors associated with cholera illness in Carrefour, Haiti 2011
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Sharing latrine with someone suffering from diarrhoea† | 3·8*** | 1·8–8·1 | <0·001 | 3·2* | 1·3–7·7 | 0·011 |
| Using untreated water for washing dishes | 3·0** | 1·5–6·2 | 0·002 | 3·2** | 1·4–7·3 | 0·006 |
| Ate a meal away from home at least once in week before illness | 2·5** | 1·4–4·5 | 0·001 | 1·8 | 0·9–3·7 | 0·117 |
| Presence of E. coli in drinking water stored at home | 0·8 | 0·4–1·7 | 0·582 | 1·5 | 0·5–4·3 | 0·489 |
| Chlorine level >0·2 mg/l in drinking water stored at home | 1·1 | 0·6–1·9 | 0·845 | 1·0 | 0·5–2·4 | 0·920 |
| Receiving information on cholera prevention via television | 0·4** | 0·2–0·7 | 0·002 | 0·4** | 0·2–0·9 | 0·027 |
| Receiving information on cholera prevention at church | 0·2* | 0·1–0·8 | 0·027 | 0·1** | 0·0–0·5 | 0·003 |
| Socioeconomic score | 0·4*** | 0·3–0·6 | <0·001 | 0·5** | 0·3–0·8 | 0·002 |
OR, Odds ratio; CI, confidence interval.
Two other variables measuring contacts with suspected cholera cases (visiting someone suffering from cholera and caring for someone suffering from diarrhoea or cholera) were collinear with sharing the latrines with someone suffering from diarrhoea. We built separate models replacing sharing the latrines with these two variables; the odds ratios were 3·7 (95% CI 1·2–11·9) for visiting someone suffering from cholera and 3·8 (95% CI 1·5–9·5) for caring someone suffering from diarrhoea or cholera.
P < 0·05; ** P < 0·01; *** P < 0·001 (two-tailed tests).
Statistically significant risk factors common to the two locations were sharing latrines and low socioeconomic level. Information on cholera prevention via television was a common preventive factor.
DISCUSSION
Studies performed in the early phase of the cholera epidemic in Haiti identified contaminated water as a major risk factor in transmission of cholera [4–6]. Waterborne transmission was consistent with the rapid and explosive spread of the epidemic across Haiti and probably with the following peaks which coincided with the rainy seasons. Our findings show that, in addition to contaminated water, other factors related to direct and indirect inter-human contacts may play a major role in continued transmission during the inter-peak periods.
Apart from the association with pig ownership, which requires further investigation and clarification, all other risk factors identified in our studies were already known. Nevertheless, they provide potentially valuable information for decision makers in Haiti. In particular, we stress the importance of control measures during lull periods, when prevention efforts are typically scaled down and the population tends to lose the perception of the risk of getting the disease.
The quality of drinking water was far from optimal in both locations. Self-reported chlorination of drinking water was a protective factor in Gonaives, but adequate chlorine concentration in home-stored drinking water was not. This contradictory result may have multiple explanations. It is possible that the chlorination was incorrectly done, or that the presence of chlorine went undetected due to the delay between chlorination and sample collection (the latter information was not recorded). Alternatively, it might reflect interviewees' reluctance to admit that they had not followed proper hygiene or clean water recommendations. In Carrefour highly chlorinated drinking water was more frequent in households of cases than controls, a finding that may reflect excessive caution by family members after someone in the household falls ill. In either case, it is clear that poor water quality was common, as shown by the high proportion of water samples found to be contaminated with E. coli in households of both cases and controls, and that the quality of drinking water needs to be improved.
Direct and indirect contacts, such as helping or visiting a person suffering from diarrhoea [12–14], sharing latrines [15, 16] or a low socioeconomic status [16, 17] are risk factors that have already been described in other cholera epidemic or endemic contexts. In Haiti, it remains unclear whether the risk of cholera via direct contact reflects a lack of means (soap, chlorine, water), insufficient knowledge of essential hygiene measures, or both. The Haitian Ministry of Public Health and Population, together with other interested parties, distributed cleaning kits to caregivers of patients admitted to CTCs to limit transmission within patients' homes; although well-intentioned, this effort may have little impact since most intra-household transmission would have already occurred by the time of the distribution.
In our studies the investigation of household latrines did not go beyond whether the latrine was overflowing and whether soap and water were present, so it remains unclear whether the observed elevated risk was directly linked with contaminated latrines or, again, with insufficient knowledge of essential hygiene measures. Nevertheless, as most households lacked soap for hand washing, prevention efforts should focus on making soap and chlorine available. Considering that sharing a latrine with neighbours is common in Haiti, outreach campaigns should specifically address this issue by reinforcing the importance of cleaning latrines after use and of providing decontamination of shared latrines.
Selling food and beverages in the streets and markets, a common activity in developing countries has also been identified as a key factor in cholera transmission in other contexts [18–21]. In Gonaives this factor was notable for both the strength of the association and the high proportion of associated cases suggesting that food consumed in the market or at school was highly implicated in cholera transmission. Since these studies were conducted, street vendors in several Haitian cities (Dessalines, Gros Morne) have been given information about cholera transmission, along with supplies of chlorine, soap and hand washing buckets, which were well-received by both vendors and customers. These and other preventive measures should be strongly encouraged until more permanent hygiene and sanitation measures are in place.
In Carrefour, conditions specific to post-earthquake victims, such as living in a tent or a dwelling made of plastic sheeting, were associated with increased risk. Early in the epidemic, displaced populations had relatively sufficient access to clean water and improved sanitation. However, since then, some displaced people have been relocated and aid agencies have reduced their services inside the camps. Two surveys by the Dinepa (National Water Board) Observatory [22, 23] showed that already by the end of 2011 there had been an alarming decrease in access to safe drinking water, and that there was poor maintenance of latrines and hand-washing facilities in the surveyed camps.
The association with owning pigs was highly unexpected. Although V. cholerae has been detected in stool samples of animals, including pigs [24], to our knowledge this is the first time that owning animals has been associated with risk of contracting cholera. Pig ownership may be a proxy indicator for a risk factor we did not investigate and merits further investigation. It may be worth including this potential risk factor in further studies on cholera transmission.
Both studies show that insufficient practice of essential hygiene measures is an important issue to tackle in Haiti, but also that targeted information campaigns can help reduce cholera incidence. Visual messages on television, the persuasive appeal of a church leader, and the personal motivation required to attend a training session, may enhance the likelihood that people will implement the suggested hygiene measures. Prevention information through various means was widespread in Haiti during acute transmission phases, but gradually decreased as the peak subsided. Prevention campaigns can effectively make an impact to reduce cholera incidence and should remain active during low transmission periods.
These studies involve some limitations. One is the low specificity of the Crystal VC test [25], leading to inadvertent inclusion of some non-cholera patients among cases. Another is that the selection of controls was based on self-reports of no prior history of cholera. The two misclassifications above, however, would only have weakened the results, i.e. hidden weak associations such as using soap, a protective factor demonstrated by other studies [26, 27]. In addition, we cannot exclude that some controls had an asymptomatic form of cholera, which occurs frequently [28, 29] and is potentially transmissible [30]. However, the risk factors we evaluated apply only to symptomatic cholera.
We have presented evidence that in addition to contaminated water, human-to-human and mediated transmission through food handling or sharing latrines, may play a substantial role in the maintenance of V. cholerae during the lull between periods of peak caseloads in Haiti. Reinforcing efforts to raise public awareness of risk reduction measures and to improve hygiene, clean food and safe water practices are effective interventions for cholera control that should be implemented also during lull periods. Such interventions are, however, difficult to implement and maintain especially when the perception of the risk of getting the disease decreases. Specific plans for low transmission periods should be also foreseen as a promising approach to reducing or eliminating circulating V. cholerae, thereby averting the occurrence of future outbreaks in Haiti.
Supplementary Material
Supplementary information supplied by authors.
ACKNOWLEDGEMENTS
This work was supported by the French Operational Centre of Médecins Sans Frontières. The authors acknowledge Dr Jode Préval Pierre and Dr Manice Cupidon (Direction Sanitaire de l'Artibonite) for their valuable comments on the questionnaire, Dr Elsie Lafosse (Direction d'Epidémiologie, des Laboratoires et de la Recherche, Port-au-Prince) for her contributions to the design and submission of the study protocol, and Dr Patricia Kahn for help with preparation of the manuscript.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268813002562.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Jenson D, Szabo V. Cholera in Haiti and other Caribbean regions, 19th century. Emerging Infectious Diseases 2011; 17: 2130–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministère de la Sante Publique de de la Population. Report of 31 March 2013 (http://www.mspp.gouv.ht/site/index.php?option=com_content&view=article&id=120&Itemid=1). Accessed 15 April 2013.
- 3.Piarroux R, et al. Understanding the cholera epidemic, Haiti. Emerging Infectious Diseases 2011; 17: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunkle SE, et al. Epidemic cholera in a crowded urban environment, Port-au-Prince, Haiti. Emerging Infectious Diseases 2011; 17: 2143–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill VR, et al. Toxigenic Vibrio cholerae O1 in water and seafood, Haiti. Emerging Infectious Diseases 2011; 17: 2147–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor KA, et al. Risk factors early in the 2010 cholera epidemic, Haiti. Emerging Infectious Diseases 2011; 17: 2136–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Direction des Statistiques Démographiques et Sociales. Population des différentes unités géographiques (Département, Arrondissement, Commune, Section communale). In: Institut Haïtien de Statistique et D'Informatique. Population totale, population de 18 ans et plus menages et densités estimées en 2009. Port-au-Prince, 2009, pp. 9–51. [Google Scholar]
- 8.IOM Haiti. Displacement tracking matrix v. 2.0, update 31 July 2011 (http://www.iomhaitidataportal.info/dtm). Accessed 15 April 2013.
- 9.Lowther SA, et al. Feasibility of satellite image-based sampling for a health survey among urban townships of Lusaka, Zambia. Tropical Medicine & International Health 2009; 14: 70–78. [DOI] [PubMed] [Google Scholar]
- 10.Unicef. Guidelines on chlorination practices 2003. (http://www.hpsl.lk/docs/watsan/Guidelines_on_chlorination_(English).pdf). Accessed 15 April 2013.
- 11.Hosmer DW, Lemeshow S. Applied Logistic Regression, 2nd edn. New York: Wiley-Interscience Publications, 2000. [Google Scholar]
- 12.Siddiqui FJ, et al. Consecutive outbreaks of Vibrio cholerae O139 and V. cholerae O1 cholera in a fishing village near Karachi, Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006; 100: 476–482. [DOI] [PubMed] [Google Scholar]
- 13.Nelson EJ, et al. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. Proceedings of the National Academy of Sciences USA 2007; 104: 19091–19096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sur D, et al. The burden of cholera in the slums of Kolkata, India: data from a prospective, community based study. Archives of Disease in Childhood 2005; 90: 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shultz A, et al. Cholera outbreak in Kenyan refugee camp: risk factors for illness and importance of sanitation. American Journal of Tropical Medicine and Hygiene 2009; 80: 640–645. [PubMed] [Google Scholar]
- 16.Sasaki S, et al. Spatial analysis of risk factor of cholera outbreak for 2003–2004 in a peri-urban area of Lusaka, Zambia. American Journal of Tropical Medicine and Hygiene 2008; 79: 414–421. [PubMed] [Google Scholar]
- 17.Emch M. Diarrheal disease risk in Matlab, Bangladesh. Social Science & Medicine 1999; 49: 519–530. [DOI] [PubMed] [Google Scholar]
- 18.Gunn RA, et al. Cholera in Bahrain: epidemiological characteristics of an outbreak. Bulletin of the World Health Organization 1981; 59: 61–66. [PMC free article] [PubMed] [Google Scholar]
- 19.Koo D, et al. Epidemic cholera in Guatemala, 1993: transmission of a newly introduced epidemic strain by street vendors. Epidemiology and Infection 1996; 116: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ries AA, et al. Cholera in Piura, Peru: a modern urban epidemic. Journal of Infectious Diseases 1992; 166: 1429–1433. [DOI] [PubMed] [Google Scholar]
- 21.Moren A, et al. Practical field epidemiology to investigate a cholera outbreak in a Mozambican refugee camp in Malawi, 1988. Journal of Tropical Medicine and Hygiene 1991; 94: 1–7. [PubMed] [Google Scholar]
- 22.DINEPA Observatory. Indicators, ratios and performance measurements of WASH in temporary accommodation sites October 2011 (http://www.dinepa.gouv.ht/wash_cluster/index.php?option=com_rokdownloads&view=file&Itemid=57&id=1584:111124-enquete-wash-sites-hebergement-temporaire-oct-2011). Accessed 15 April 2013.
- 23.DINEPA Observatory. Indicators, ratios and performance measurements of WASH in temporary accommodation sites November 2011 (http://www.dinepa.gouv.ht/wash_cluster/index.php?option=com_rokdownloads&view=file&Itemid=57&id=1586:enquete-epah-novembre-2011). Accessed 15 April 2013.
- 24.Keshav V, Potgieter N, Barnard T. Detection of Vibrio cholerae O1 in animal stools collected in rural areas of the Limpopo Province. Young Water Professionals Special Edition 2010; 36: 167–171. [Google Scholar]
- 25.Harris JR, et al. Field evaluation of crystal VC Rapid Dipstick test for cholera during a cholera outbreak in Guinea-Bissau. Tropical Medicine & International Health 2009; 14: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 26.DuBois AE, et al. Epidemic cholera in urban Zambia: hand soap and dried fish as protective factors. Epidemiology and Infection 2006; 134: 1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quick RE, et al. Epidemic cholera in rural El Salvador: risk factors in a region covered by a cholera prevention campaign. Epidemiology and Infection 1995; 114: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemens JD, et al. Evidence that inactivated oral cholera vaccines both prevent and mitigate Vibrio cholerae O1 infections in a cholera-endemic area. Journal of Infectious Diseases 1992; 166: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 29.King AA, et al. Inapparent infections and cholera dynamics. Nature 2008; 454: 877–880. [DOI] [PubMed] [Google Scholar]
- 30.Van de Linde P, Forbes G. Observations on the spread of cholera in Hong Kong, 1961–63. Bulletin of the World Health Organization 1965; 32: 515–530. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268813002562.
click here to view supplementary material