SUMMARY
This study analysed the spatio-temporal distribution and propagation of hand-foot-and-mouth disease (HFMD) in Shenzhen from 2008 to 2010. Specifically, we examined the epidemiological data, temporal distribution and spatial distribution, and then the relationship between meteorological, social factors and the number of reported HFMD cases was analysed using Spearman's rank correlation. Finally, a geographically weighted regression model was constructed for the number of reported HFMD cases in 2009. It was found that three independent variables, i.e. the number of reported HFMD cases in 2008 and, annual average temperature and precipitation, had different spatial impacts on the number of reported HFMD cases in 2009. In addition, these variables accounted for the propagation mechanism of HFMD in the centre and east of Shenzhen, where the high incidence rate areas are located. These results will be of great help in understanding the spatio-temporal distribution of HFMD and developing approaches to prevent this disease.
Key words: Geographically weighted regression, hand-foot-and-mouth disease (HFMD), spatio-temporal distribution, Shenzhen
INTRODUCTION
Hand-foot-and-mouth disease (HFMD) is a common viral illness of infants and children aged <10 years, characterized by 3–4 days of fever and the development of a vesicular enanthem on the buccal mucosa, gums, and palate and a papulovesicular exanthem on the hands, feet, and buttocks [1]. In most cases, this illness is self-limiting, and patients can recover completely. However, some patients infected with the EV71 virus may develop severe complications including meningoencephalitis, myocarditis, or even death [2]. HFMD is caused by viruses that belong to the Enterovirus genus. This group of viruses includes polioviruses, coxsackieviruses, echoviruses, and enteroviruses, of which coxsackievirus A16 (CVA16), coxsackievirus A10 (CVA10), and human enterovirus 71 (HEV71) are the major causative agents of HFMD. HFMD spreads from person to person by direct contact with the infectious virus, which is found in nose and throat secretions, saliva, blister fluid, and stools of infected individuals [3].
Large outbreaks of HFMD have attracted global attention. In 1998, an epidemic of enterovirus 71 infection caused HFMD and herpangina (HA) in thousands of people in Taiwan, some of who died from these infections [4]. In addition, Singapore experienced a HFMD outbreak during the latter part of 2000; from 14 September to 14 November 2000, a total of 3526 cases of HFMD were reported, and 652 patients were clinically suspected of having HFMD [5]. In 2008, another epidemic affected South East Asia including mainland China, Taiwan, Singapore, and Hong Kong [6–11]. In mainland China, the first HFMD case was reported in Shanghai in 1981. Since then, HFMD cases have been reported in Beijing, Hebei province, Tianjin, Fujian province, Jilin province, Shandong Province, Hubei province, Qinghai province and Guangdong province [12]. Recent outbreaks of HFMD have significantly threatened public health, and these outbreaks also cause social welfare and economic problems. In order to minimize the spread of HFMD, some child daycare centres and kindergartens are ordered to close during the outbreak period, although these closures cause problems for working parents who have to find alternative care for their children. In addition, parents are requested to keep their children indoors and stay away from crowded places during outbreaks. Thus, HFMD causes significant problems for society, the economy and public health.
Previous studies on HFMD have generally focused on the description and assessment of local outbreaks, the incidence and prevalence, the demographic distribution with regard to age, sex, season or clinical characteristics and potential cures [13]. In recent years, researchers have begun to analyse the temporal and spatial distribution of various diseases such as diarrhoea, influenza, and others [14–17]. The seasonal features of paediatric diarrhoeal mortality and the distinct spatial pattern of the timing of the peak mortality rate in Mexico were studied, and it was found that the direction and timing of the annual waves were related to the mean monthly precipitation and mean daily temperature [14]. In addition, Gabriel et al. investigated temporal, spatial, spatio-temporal and genetic variation in human Campylobacter jejuni infections, and their analysis showed statistically significant seasonal variation, spatial clustering and small-scale spatio-temporal clustering in the overall pattern of incidence [15]. There is also some literature identifying relationships between meteorological factors and various infections [10, 18–22], in which temperature and precipitation have been most frequently studied. Urashima et al. established seasonal models to predict fluctuations in rates of HA and HFMD associated with weather conditions and calendar months in Tokyo, Japan. These models predicted that warmer climate conditions lead to an increased number of HA and HFMD cases [18]. The relative risks between weekly HFMD cases and temperature and rainfall were estimated for 2001–2008 using time-series Poisson regression models allowing for over-dispersion, and it was found that weekly temperature and rainfall showed statistically significant associations with HFMD incidence at time lag of 1–2 weeks [21]. Ma et al. examined the relationship between meteorological parameters and HFMD activity, and their sensitivity analysis showed that HFMD consultation rates were mostly affected by relative humidity and least affected by wind speed [10]. Besides meteorological factors, social factors also have a non-negligible impact on the propagation of infectious diseases, including HFMD, such as the regional population, medical conditions, and transport [23]. The development of spatial technologies including remote sensing and geographical information systems has also greatly contributed to epidemic studies [24].
However, no attempts have yet been made to study the spatio-temporal distribution and propagation of HFMD in Shenzhen, including the quantitative analysis of space. The objective of this study was therefore to assess various epidemiological features of HFMD, identify the spatio-temporal evolutionary patterns, and study the propagation mechanisms, with the goal of establishing public health personnel plan interventions to effectively reduce the incidence and complications associated with HFMD.
STUDY AREA
Shenzhen is a major city in Guangdong province, Southern China. Although Shenzhen is located in the subtropical region of China, approximately at the Tropic of Cancer, this city has a warm, monsoon-influenced, humid subtropical climate due to the Siberian anticyclone. The annual average temperature is 22·5°C, and the annual average precipitation is >1900 mm. Shenzhen contains 55 streets that are administered by seven administrative districts, including eight streets in Futian district, 10 streets in Luohu district, eight streets in Nanshan district, four streets in Yantian district, 10 streets in Baoan district, 13 streets in Longgang district, and two streets in Guangming new district. The study area is shown in Figure 1.
Fig. 1.

Study area. (a) Location of Guangdong province in China. (b) Location of Shenzhen in Guangdong province. (c) Administrative map of Shenzhen, showing Futian district, Luohu district, Nanshan district, Yantian district, Baoan district, Longgang district, and Guangming new district.
The population of Shenzhen is unique, with the majority of those in the city being migrant workers. The local government estimated that by 2007, the overall population of Shenzhen had reached 14 million people, of which around 60% were migrant labour workers from other regions of China. Migrant workers generally have a lower income and poorer socio-demographic characteristics compared to the permanent residents of the city [25]. In addition, Zhang et al. [26] found that there were differences in health outcomes experienced between the different groups in the population. This poses problems for developing and implementing legislation and policy to support the healthcare system, particularly for action to guarantee equity and equality of health services delivery. Good initiatives have been started and priority has been given to the health insurance system for migrant employee. Early in 2004, Shenzhen's government issued a regulation concerning the development of a healthcare system to cater for migrant workers, From 1 March 2005, an experimental Co-operative Healthcare Service System for Migrant Workers (CHSMW) was initiated to provide coverage for services by contracting specific designated healthcare providers. In June 2006, CHSMW formally developed into the Medical Insurance System for Migrant Employees (MISM). This new system was open to all migrant workers in the city and is compulsory for employers. To echo the national reform theme, a health blueprint of Shenzhen was drafted and finalized in 2006. The highest priority is being given to the establishment of the hospital-based community health centre (CHC) network to ensure the provision of high-quality, community-based basic medical and public health services [26].
METHODS
Epidemiological data
HFMD has been upgraded to a Class C communicable disease since 2008. An implication of this classification is that nationwide cases of HFMD have been officially reported to the National Diseases Reporting System (NDRS), and the Chinese Center for Disease Control and Prevention (Chinese CDC). The clinical criteria for diagnosis of HFMD cases were provided in a guidebook published by the Chinese Ministry of Health in 2010. Patients with the following symptoms are defined as having HFMD: fever, papules, and herpetic lesions on the hands or feet, rashes on the buttocks or knees, inflammatory flushing around the rashes and little fluid in the blisters, or sparse herpetic lesions on oral mucosa. Clinical diagnoses of HFMD are strictly examined and verified by various levels of CDC. At the provincial level, at least 10 cases were serotyped each month. Serotyping and sequencing were performed at provincial surveillance laboratories with quality control from the Chinese CDC [27, 28]. For this study, HFMD data was obtained from the Chinese CDC for the period between 1 January 2008 and 31 December 2010. The recorded information included basic patient information (e.g. name, gender, age, date of birth), spatial information (e.g. patient's address, reporting company), and temporal information (e.g. patient's onset date, diagnosis date). We selected patient onset date as the temporal information and patient address as the spatial information.
When we analysed the temporal distribution of reported HFMD cases, we tested the correlation using Pearson's rank correlation with SPSS software v. 16.0 (SPSS Inc., USA). Two-tailed analysis was used for statistical tests and P < 0·05 was considered statistically significant. When we analysed the spatial distribution of reported HFMD cases, incidence rate refers to the frequency of new infected cases during a given time interval, which is equivalent to the number of new cases divided by the population during a given time interval. Although raw incidence rate data may not effectively reflect true risks, incidence rate represented in spatial units for areas of high population density is more reliable due to reduced randomness. Conversely, the incidence rate in spatial units with a lower population density is not reliable. To solve this problem, we re-estimated the incidence rate using Bayesian adjustments to uncover the true risks for an exposed population [12, 29]. We also searched for any association between the number of reported HFMD cases and meteorological and social factors using Spearman's rank correlation. Spearman's rank correlation is a non-parametric measure of statistical dependence between two variables, and it is non-parametric in that its exact sampling distribution can be obtained without requiring knowledge of the probability distribution of variables.
Meteorological data
Meteorological data collected in 2009 was obtained from the Meteorological Bureau of Shenzhen Municipality. There are a total of 54 meteorological stations in Shenzhen, including eight in Futian district, six in Luohu district, seven in Nanshan district, seven in Yantian district, 11 in Baoan district, 13 in Longgang district, and two in Guangming new district. The Meteorological Bureau of Shenzhen Municipality publishes daily data regarding temperature (°C) and precipitation (mm) for each meteorological station. In addition, this source also publishes a Shenzhen climate bulletin each year, in which the annual average temperature and precipitation are included. The annual average temperature and precipitation are calculated by averaging the daily data. By interpolating the annual average temperature and precipitation for meteorological stations, we were able to obtain a distribution map of the annual average temperature and precipitation in Shenzhen. Finally, the annual average temperature for each street was calculated by averaging the annual temperature distribution map for each street, and the same calculations were performed for the annual average precipitation for each street.
Social data
In this study, the social data included the road and drainage density, population, and number of hospitals. Both the scale of the road data and river data in Shenzhen were 1:1000, and these data were obtained in 2008. Road density (RD) refers to the ratio of the road length (RL) divided by the study area (A), and drainage density (DD) refers to the ratio of the length of the river (L) divided by the study area (A); these values can be obtained using equation (1) and equation (2), respectively.
| (1) |
| (2) |
The study period for HFMD ranged from 2008 to 2010. This was about the time of the sixth census in 2010 in China, so the population data were mainly obtained from the sixth census, which was published by the administrative districts of Shenzhen. The number of hospitals was recorded in 2008.
Geographically weighted regression (GWR) model
Traditional linear regression is often used for the analysis of quantitative geography. As a general technique for investigating the linkage between geographical variables, this method has featured in countless publications. The well-known components of this type of regression model include X, which is a matrix containing a set of independent or predictor variables, and y, which is a vector of the dependent or response variables [30, 31]. The relationship between these variables is modelled as follows:
| (3) |
where β is a vector of regression coefficients and εi ∼ N(0, σ2). The inclusion of two strongly collinear independent variables in a regression model may lead to an erroneous conclusion [32]; therefore, we need to eliminate the collinear variables in the independent variables before we establish the regression model.
However, there may be situations when the nature of such models is not fixed in space, which is referred to as spatial non-stationarity. To include spatial heterogeneity in the model, Fotheringham and colleagues proposed the GWR model, which extends the traditional regression model by allowing local variations in rates of change such that the coefficients in the model are specific to a given location, rather than serving as global estimates [30, 33]. This regression equation is shown as equation (4)
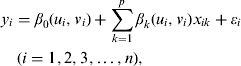 |
(4) |
where (ui, νi) is the coordinate at location i, βk(ui, νi) is the value of the kth parameter at location i, εi ∼ N(0, σ2), and Cov(εi, εj) = 0(i ≠ j). If βk(u1, v1) = βk(u2, v2) = … = βk(un, vn), the GWR model will be converted to the ordinary linear regression model. The advantage of the GWR model is that, once the model has been calibrated, it is possible to map the variation in the original regression parameters and gain some understanding of the spatial patterns in the association between predictor and response variables [33]. A geographical surface of models is derived with associated goodness-of-fit statistics and localized parameter estimates such as R2, standard error, and t values. We used the GWR coefficient values to explore the spatial variability of relationships between reported HFMD cases and meteorological and social factors. For the purpose of comparison, the traditional linear regression model was also derived and compared to the GWR model output using the global R2. Lin & Wen used Ordinary Least Squares (OLS) and GWR models to analyse spatial relationships and identify the geographical heterogeneities by using the information of entomology and dengue cases, and they demonstrated that a GWR model could be used to geographically differentiate the relationships of dengue incidence with immature mosquito and human densities [34]. Grillet et al. used local spatial statistics and GWR to determine the spatial pattern of malaria incidence and persistence in northeastern Venezuela. The GWR model greatly improved predictions of malaria risk compared to OLS regression models [35].
In this study, we eliminated the collinearity in all independent variables, and then constructed the traditional linear regression model for the number of reported HFMD cases in 2009 using the selected explanatory variables. In addition, we excluded the explanatory variables which had little impact on the regression model. Finally, we used GWR tools in ArcGIS 9.3 (ESRI, USA) to construct the GWR model to study the spatial varying relationships between the number of reported HFMD cases and explanatory variables.
RESULTS
Epidemiological data analysis
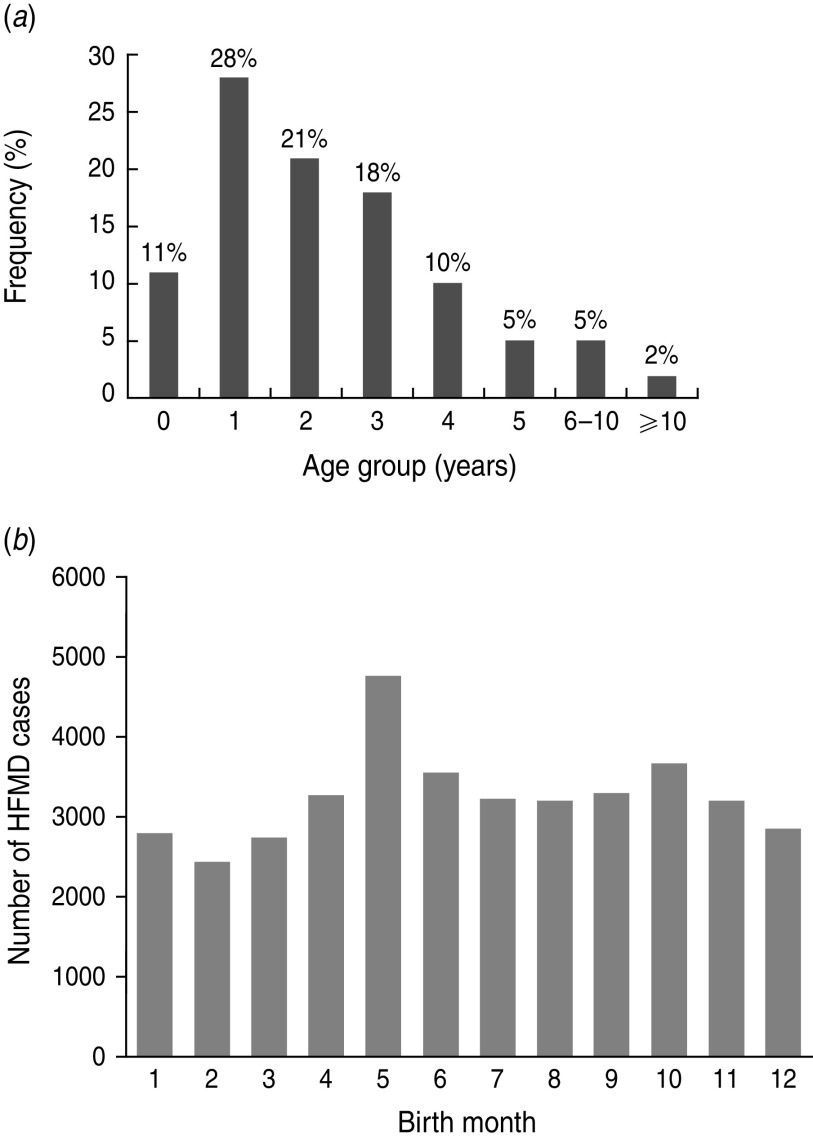
We analysed a total of 39 046 HFMD cases from 2008 to 2010 in Shenzhen, including 24 674 male patients and 14 372 female patients. The male/female ratio of reported HFMD cases was 1·72:1. Figure 2a shows the age distribution of the reported HFMD cases, with the age of affected individuals typically ranging from 0 to 4 years (88% of cases). According to the fifth census data of Shenzhen in 2000 the male/female ratio of the population in the 0–14 years age group was 1·35:1, and the population in age groups 0, 1–4, 5–9, 10–14 was 47 200, 184 400, 203 400, 160 300, respectively. The male/female ratio of reported HFMD cases was not significantly different compared to the demographics of the population. However, the population in the 5–9 years age group was highest, while the largest age group of reported HFMD cases was 1–4 years. Figure 2b shows the birth month distribution of reported HFMD cases, with a peak observed during the summer season, especially in May, and a trough observed in February. The ratio of reported HFMD cases for 2008 to 2010 in spring, summer, autumn, winter was: 2008 (359, 5461, 823, 554), 2009 (2318, 3435, 2086, 1095), 2010 (5431, 10 279, 5048, 2157).
Fig. 2.
Basic information of reported hand-foot-and-mouth disease (HFMD) cases. (a) Age distribution of reported HFMD cases, and age of affected individuals typically ranging from 0 to 4 years. (b) Birth month distribution of reported HFMD cases, with a peak observed in summer. Birth month means the month in which HFMD patients were born.
Temporal distribution
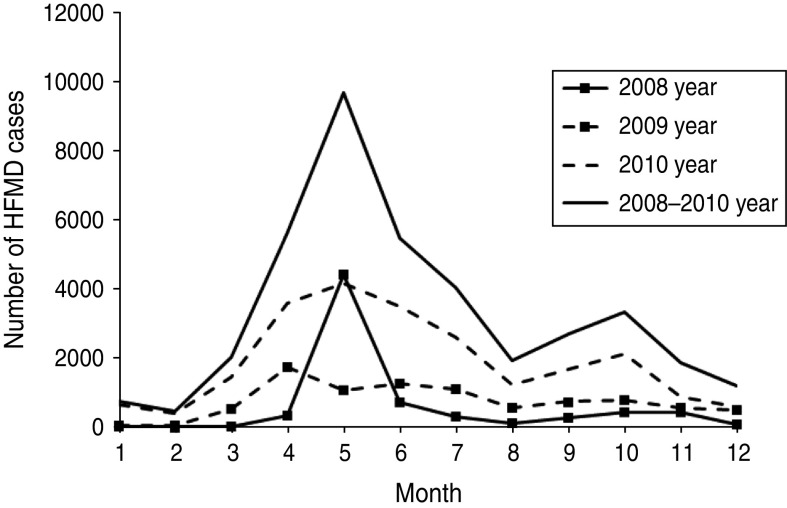
The number of yearly reported HFMD cases in 2008, 2009, and 2010 was 7197, 8934, and 22 915, respectively. Figure 3 shows the monthly increase in the number of reported HFMD cases, and it was found that a seasonal peak occurred in April, May, and June of each year, with a smaller peak also observed in October and November. When comparing Figure 2b and Figure 3, it was clear that the trends of these two curves were similar. Furthermore, after analysing the correlation of the number of reported HFMD cases per month between birth and onset, we found that these factors were significantly positively correlated (ρ = 0·9, P < 0·01). Therefore, the time of birth had an impact on the outbreak of reported HFMD cases.
Fig. 3.
Temporal distribution of reported hand-foot-and-mouth disease cases, with a seasonal peak in April, May, June of each year.
Spatial distribution
Figure 4 shows the incidence rate map of reported HFMD cases from 2008 to 2010 after Bayesian adjustments were made for the 55 streets in Shenzhen. It was found that the areas with high incidence rates were located in the centre and east of Shenzhen, particularly Donghu street (Luohu district) and Longgang street and Pingdi street (Longgang district). In contrast, the incidence rate was low in the west region of Shenzhen.
Fig. 4.
Incidence rate map of reported hand-foot-and-mouth disease (HFMD) cases from 2008 to 2010 after Bayesian adjustments for the 55 streets, and areas with high incidence rates located in Donghu street, Longgang street, and Pingdi street.
Analysis of explanatory variables
The relationships between various factors and the number of reported HFMD cases in 2009 are shown in Table 1. It was found that the number of reported HFMD cases in 2009 was positively associated with the annual average temperature, number of hospitals, population, and number of reported HFMD cases in 2008, whereas this number was negatively associated with the annual average precipitation, RD and DD. We examined correlations between independent variables and found that population, number of reported HFMD cases in 2008, and number of hospitals were highly correlated with each other (correlation coefficient >0·6). Hence, we selected the number of reported HFMD cases in 2008, RD, DD, annual average temperature and precipitation as explanatory variables for further analysis.
Table 1.
Spearman's rank correlation between meteorological and social factors and the number of reported HFMD cases in 2009
| Factors | Spearman's rank correlation coefficient | P value |
|---|---|---|
| Annual average temperature (°C) | 0·260 | 0·053 |
| Annual average precipitation (mm) | −0·301 | 0·024 |
| Number of hospitals | 0·590 | 0·000 |
| Population | 0·573 | 0·000 |
| Number of reported HFMD cases in 2008 | 0·760 | 0·000 |
| Drainage density | −0·024 | 0·862 |
| Road density | −0·117 | 0·390 |
HFMD, Hand-foot-and-mouth disease.
GWR model
In this study, we constructed a traditional linear regression model for the number of reported HFMD cases in 2009 based on the number of reported HFMD cases in 2008, RD, DD, annual average temperature and precipitation, with an R2 value of 0·57. As shown in Table 1, the correlations between the number of reported HFMD cases in 2009 and RD and DD were quite low. Therefore, we excluded these two factors, and the regression model constructed after excluding these two factors is given in equation (5), with an R2 value of 0·55. As a result, RD and DD had little impact on the regression model and could therefore be excluded from the model. In equation (5), Num refers to number of reported HFMD cases in 2008, Temp represents annual average temperature, and Rain stands for annual average precipitation. The coefficients of the linear regression model are shown in Table 2.
| (5) |
As previously stated, the above traditional regression model ignores the spatial dependence and heterogeneity of spatial data. In this study, we also constructed the GWR model for the 55 streets in Shenzhen. The independent variables included Num, Temp, and Rain. The output of the GWR model is shown in Figure 5 and Table 3, and this model was used to include spatial patterns in the association between these three independent variables and the dependent variable. The goodness of fit of this GWR model with an R2 value of 0·83, is much better than that of the traditional linear regression model, and sigma (the estimated standard deviation for the residuals), is 80·14.
Table 2.
The coefficients of the linear regression model
| Independent variable | Dependent variable | Unstandardized coefficients | Standard error of the unstandardized coefficients | P value |
|---|---|---|---|---|
| No. of reported HFMD cases in 2008 | No. of reported HFMD cases in 2009 | 0·996 | 0·147 | 0·000 |
| Annual average temperature | No. of reported HFMD cases in 2009 | 37·093 | 11·886 | 0·003 |
| Annual average precipitation | No. of reported HFMD cases in 2009 | −0·242 | 0·118 | 0·045 |
HFMD, Hand-foot-and-mouth disease.
Fig. 5.
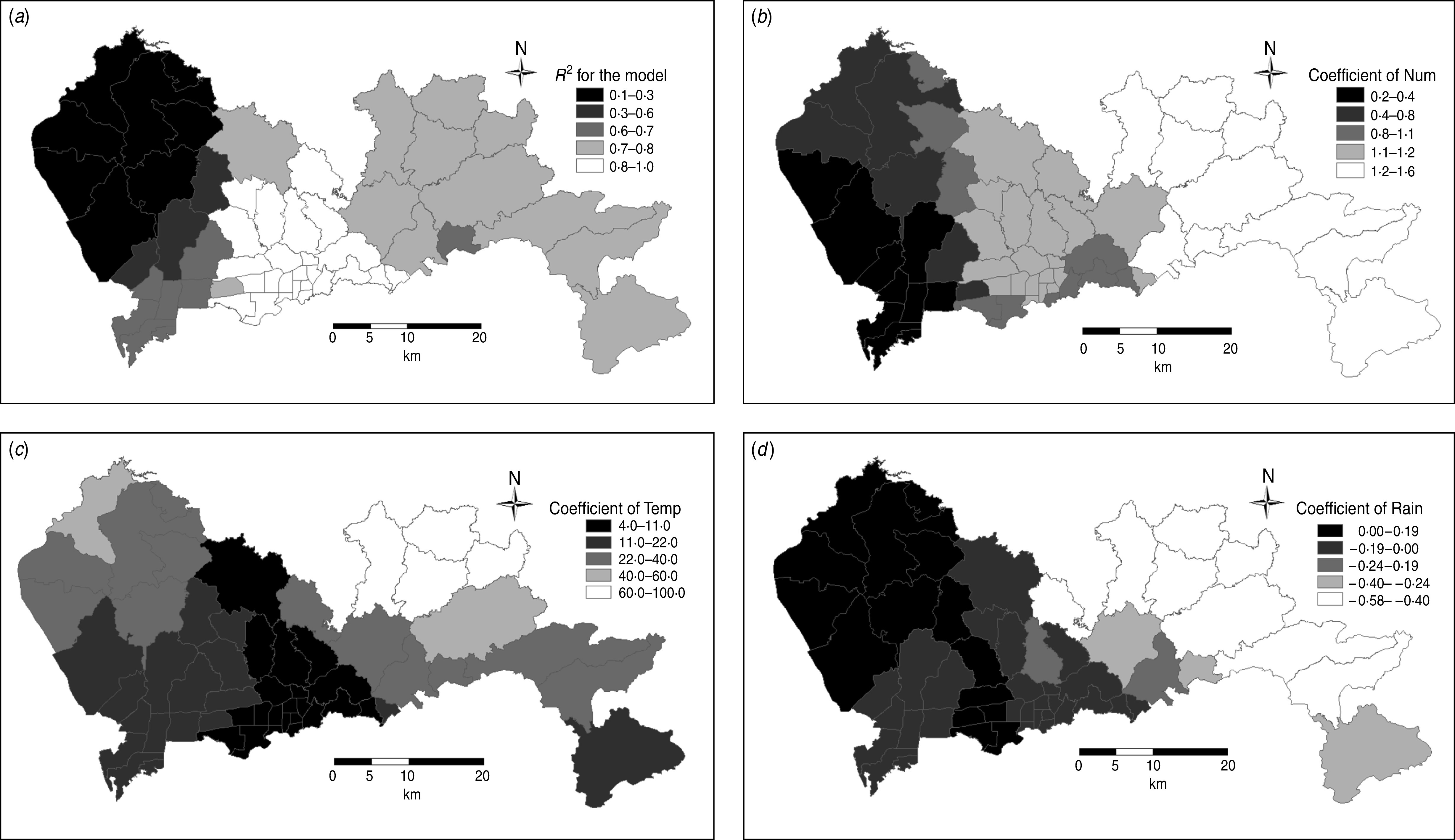
Coefficient distribution of determination of the model and independent variable in the model. (a) Coefficient of determination for the model showing differences in the goodness of fit. (b) Coefficient of number of reported hand-foot-and-mouth disease (HFMD) cases in 2008 showing the spatial impact of the number of reported HFMD cases in 2008 on the number of reported HFMD cases in 2009. (c) Coefficient of annual average temperature showing the spatial impact of the annual average temperature on the number of reported HFMD cases in 2009. (d) Coefficient of annual average precipitation showing the spatial impact of the annual average precipitation on the number of reported HFMD cases in 2009.
Table 3.
Parameter estimation and test results of the geographically weighted regression model
| Model parameter | Value |
|---|---|
| Residual squares | 44 925·56 |
| Effective number | 3·00 |
| Sigma | 80·14 |
| Akaike's Information Criterion | 124·92 |
| R2 | 0·83 |
| Adjusted R2 | 0·78 |
In the traditional regression model, the coefficient of annual average precipitation was negative. However, this value was positive for some areas and negative for other areas in the GWR model from Figure 5d; even if the signs of the variable coefficients in these two methods were the same, different areas demonstrated different values. As shown in Figure 5b, the coefficient for annual average temperature differed across the entire study area, as did the coefficient of the number of reported HFMD cases in 2008. Therefore, the independent variables had different impacts on the number of reported HFMD cases spatially.
The spatial distribution of R2 in Figure 5a revealed differences in the goodness of fit. For example, the maximum value of R2 (range 0·8–1·0) was detected in the centre of Shenzhen (including Luohu district, part of Yantian district, part of Baoan district, and part of Longgang district), while the minimum value of R2 (range 0·1–0·3) was located in the northwest region of the city (including Guangming new district and part of Baoan district). In the centre and east of Shenzhen the R2 value was >0·6, which was also greater than that calculated with the traditional regression model. However, in the west of Shenzhen, the R2 value was lower than that calculated with the traditional regression model. This result revealed that the three factors, i.e. the number of reported HFMD cases in 2008 and the annual average temperature and precipitation, accounted for the propagation mechanism of HFMD in the centre and east of Shenzhen. However, in the west of Shenzhen, the propagation of HFMD was also affected by other factors, such as the propagation of HFMD from nearby regions and preventive measures implemented by the government.
Figure 5b shows the spatial impact of the number of reported HFMD cases in 2008 on the number of reported HFMD cases in 2009. This impact demonstrated good clustering and an increasing trend for from west to east. Figure 5c shows the the coefficient distribution of the annual average temperature in the GWR model, which was found to be relatively discrete. The impact of the annual average temperature on the number of reported HFMD cases was greatest in the northeast and lowest in the centre of Shenzhen. Figure 5d shows the coefficient distribution of the annual average precipitation in the GWR model, and the number of reported HFMD cases was positively correlated with the annual average precipitation in the centre and east of Shenzhen, but negatively correlated in some regions in the west of Shenzhen. Moreover, the impact of precipitation on the number of reported HFMD cases was greater in the east than in the west. Therefore, when the annual average precipitation was positively correlated with the number of reported HFMD cases, the annual average precipitation had a lower impact on the number of reported HFMD cases.
DISCUSSION
HFMD has been upgraded to a Class C communicable disease since 2008 in China. The analysis of HFMD from aspects of spatio-temporal distribution and propagation will be of great help for the prevention and treatment of this disease. In this study we analysed the age distribution of reported HFMD cases from 2008 to 2010 and found reported HFMD cases targeting both children and adults, particularly children aged <4 years. According to the temporal distribution of reported HFMD cases, the number of reported HFMD cases continues to grow each year. A seasonal peak of HFMD occurs in April, May, and June of each year, which is similar to the results of Cao et al. [12], because host behaviour probably varies with season [36]. For example, people are more likely to go outside in summer than in winter, and when the frequency of person-to-person contact increases, this can facilitate the transmission of enteroviruses through respiratory droplets, open and weeping skin vesicles, or direct contact with contaminated objects and environmental surfaces [16]. After analysing the correlation of the number of reported HFMD cases per month between birth and onset, these factors were found to be significantly positively correlated (ρ = 0·9, P < 0·01). Therefore, we found that birth time has an impact on the outbreak of reported HFMD cases, which is also similar to the results of Cao et al. [12]. According to the spatial distribution of reported HFMD cases from 2008 to 2010, the high incidence rate areas were located in the centre and east of Shenzhen, indicating that studies concerning the propagation mechanism of HFMD in these areas are quite important.
Previous studies have demonstrated the relationships between climate factors and various infections, including HFMD [10, 13, 18–21, 37]. Under a regression model, temperature, relative humidity, wind speed, and precipitation were related to reported HFMD cases in Hong Kong [10]. Similarly, weekly temperature and rainfall showed statistically significant association with HFMD incidence at a time lag of 1–2 weeks in Singapore [21]. Similar to these studies, we analysed the relationships between reported HFMD cases and meteorological parameters. In addition, we took social factors into account, for example, RD and DD. To sum up, we analysed the correlations between the number of reported HFMD cases in 2009 and the annual average temperature and precipitation, number of hospitals, population, number of reported HFMD cases in 2008, RD and DD. We found that the annual average temperature and precipitation, population, number of hospitals, and number of reported HFMD cases in 2008 was significantly correlated with the number of reported HFMD cases in 2009. Moreover, the traditional regression model was mainly used to predict HFMD cases in the previous studies. In order to include spatial heterogeneity in the model, we constructed a GWR model to study the spatial varying relationships between number of reported HFMD cases and explanatory variables. The output of the model demonstrated that the independent variables had different impacts on the number of reported HFMD cases spatially. First, the output of the model revealed that the number of reported HFMD cases in 2009 was positively associated with the annual average temperature and number of reported HFMD cases in 2008 for the entire study area. Second, the impact of the number of reported HFMD cases in 2008 on the number of reported HFMD cases in 2009 demonstrated an increasing trend when moving from west to east; the impact of the annual average temperature on the number of reported HFMD cases was greatest in the northeast. Third, the impact of the annual average precipitation on the number of reported HFMD cases was positive for some regions in the west of Shenzhen, in which the annual average precipitation had a low impact on the number of reported HFMD cases, but this impact was negative for other regions.
Figure 5a shows that in the centre and east of Shenzhen, the three independent variables in the model provide a good explanation for the propagation of HFMD. As stated above, the high incidence rate areas were located in the centre and east of Shenzhen, which indicates that the GWR model was quite suitable for the high incidence rate areas in Shenzhen. However, the R2 value was relatively low in the west because, apart from the meteorological and social factors previously mentioned, the HFMD outbreak was associated with other factors such as immunity of the susceptible population, economic conditions in different regions, characteristics of different enteroviruses, medical treatment, and containment by the government.
The limitation of this study is the underreporting of HFMD cases in clinics and hospitals. First, migrant workers constitute the majority of the population in Shenzhen. These workers generally have a lower income and poorer socio-demographic characteristics compared to the permanent residents of the city. Although efforts are being made to improve the healthcare system, some migrant workers have problem receiving public health services. Second, some HFMD cases do not attend hospital to seek healthcare, because their symptoms are not serious.
The GWR model demonstrates clearly that using HFMD data from the past combined with meteorological factors helps to explain the propagation of HFMD, which will be useful to researchers and practitioners. They can take these variables into account when studying the propagation mechanism of HFMD, and then develop the forecasting systems for HFMD. However, some social factors including RD and DD have little impact on the regression model in this study. Nevertheless, it is better to consider these social factors in other study areas.
ACKNOWLEDGEMENTS
This paper has been supported by the National Natural Science Foundation of China (grant no. 41171330); National High Technology Research and Development Program of China (863 Program) (grant no. 2013AA12A302). The authors thank Shenzhen Center for Disease Control and Prevention for providing the diagnosed HFMD data. The authors also thank all the people that gave help during this study.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Grist NR, Bell EJ, Assaad F. Enteroviruses in human disease. Progrès en virologie médicale 1978; 24: 114–157. [PubMed] [Google Scholar]
- 2.Frydenberg A, Starr M. Hand, foot and mouth disease. Australian Family Physician 2003; 32: 594–595. [PubMed] [Google Scholar]
- 3.Podin Y, et al. Sentinel surveillance for human enterovirus 71 in Sarawak, Malaysia: lessons from the first 7 years. BMC Public Health 2006; 6: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho M, et al. An epidemic of enterovirus 71 infection in Taiwan. New England Journal of Medicine, 1999; 341: 925–935. [DOI] [PubMed] [Google Scholar]
- 5.Shah V A, et al. Clinical characteristics of an outbreak of hand, foot and mouth disease in Singapore. Annals of the Academy of Medicine, Singapore 2003; 32: 381–387. [PubMed] [Google Scholar]
- 6.Ding NZ, et al. Appearance of mosaic enterovirus 71 in the 2008 outbreak of China. Virus Research 2009; 145: 157–161. [DOI] [PubMed] [Google Scholar]
- 7.Huang SW, et al. Reemergence of Enterovirus 71 in 2008 in Taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. Journal of Clinical Microbiology 2009; 47: 3653–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. International Journal of Infectious Diseases 2010; 14: e1076–e1081. [DOI] [PubMed] [Google Scholar]
- 9.Ma E, et al. Epidemic of enterovirus 71 in 2008 – its public health implication to Hong Kong. International Journal of Infectious Diseases 2010; 14: 775–780. [DOI] [PubMed] [Google Scholar]
- 10.Ma E, et al. Is hand, foot and mouth disease associated with meteorological parameters? Epidemiology and Infection 2006; 138: 1779–1788. [DOI] [PubMed] [Google Scholar]
- 11.Zeng M, et al. Epidemiology of hand, foot, and mouth disease in children in Shanghai 2007–2010. Epidemiology and Infection 2012; 140: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 12.Cao ZD, et al. An epidemiological analysis of the Beijing 2008 hand-foot-mouth epidemic. Chinese Science Bulletin 2010; 55: 1142–1149. [Google Scholar]
- 13.Wang JF, et al. Hand, foot and mouth disease: spatiotemporal transmission and climate. International Journal of Health Geographics 2011; 10: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso W, et al. Spatio-temporal patterns of diarrhoeal mortality in Mexico. Epidemiology and Infection 2012; 140: 91–99. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel E, et al. Spatio-temporal epidemiology of Campylobacter jejuni enteritis, in an area of Northwest England, 2000–2002. Epidemiology and Infection 2010; 138: 1384–1390. [DOI] [PubMed] [Google Scholar]
- 16.Charland K, et al. Effect of environmental factors on the spatio-temporal patterns of influenza spread. Epidemiology and Infection 2009; 137: 1377–1387. [DOI] [PubMed] [Google Scholar]
- 17.Fenton S, et al. Spatial and spatio-temporal analysis of Salmonella infection in dairy herds in England and Wales. Epidemiology and Infection 2009; 137: 847–857. [DOI] [PubMed] [Google Scholar]
- 18.Urashima M, Shindo N, Okabe N. Seasonal models of herpangina and hand-foot-mouth disease to simulate annual fluctuations in urban warming in Tokyo. Japanese Journal of Infectious Diseases 2003; 56: 48–53. [PubMed] [Google Scholar]
- 19.Lowen AC et al. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathogens 2007; 3: 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan PKS, et al. Seasonal influenza activity in Hong Kong and its association with meteorological variations. Journal of Medical Virology 2009; 81: 1797–1806. [DOI] [PubMed] [Google Scholar]
- 21.Hii YL, Rocklöv J, Ng N. Short term effects of weather on hand, foot and mouth disease. PLoS ONE 2011; 6: e16796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H. A study on the spread model and spatial distribution of HFMD based on GIS – a case study of Ningbo (dissertation). Ningbo, Zhenjiang, China: Ningbo University, 2010, 17 pp. [Google Scholar]
- 23.Chang CY, et al. The novel H1N1 Influenza A global airline transmission and early warning without travel containments. Chinese Science Bulletin 2010; 55: 3030–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao CX, et al. Epidemic risk analysis after the Wenchuan Earthquake using remote sensing. International Journal of Remote Sensing 2010; 31: 3631–3642. [Google Scholar]
- 25.Mou J, et al. Health care utilisation amongst Shenzhen migrant workers: does being insured make a difference? BMC Health Services Research 2009; 9: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, et al. Public health services in Shenzhen: a case study. Public Health 2011; 125: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, et al. Effect of meteorological variables on the incidence of hand, foot, and mouth disease in children: a time-series analysis in Guangzhou, China. BMC Infectious Diseases 2013; 13: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinese Ministry of Health. Hand, Foot and Mouth Disease Control and Prevention Guide, 2010. (http://www.moh.gov.cn/mohyzs/s3586/201004/46884.shtml). Accessed 13 August 2013.
- 29.Anselin LY, Kim YW, Syabri I. Web-based analytical tools for the exploration of spatial data. Journal of Geographical Systems 2004; 6: 197–218. [Google Scholar]
- 30.Brunsdon CF, Fotheringham AS, Charlton M. Geographically weighted regression. Journal of the Royal Statistical Society: Series D (The Statistician) 1998; 47: 431–443. [Google Scholar]
- 31.Nelder JA, Wedderburn RWM. Generalized linear models. Journal of the Royal Statistical Society, Series A 1972; 135: 370–384. [Google Scholar]
- 32.Næs T, Mevik BH. Understanding the collinearity problem in regression and discriminant analysis. Journal of Chemometrics 2001; 15: 413–426. [Google Scholar]
- 33.Fotheringham AS, Charlton M, Brunsdon C. The geography of parameter space: an investigation of spatial non-stationarity. International Journal of Geographical Information Systems 1996; 10: 605–627. [Google Scholar]
- 34.Lin C-H, Wen T-H. Using geographically weighted regression (GWR) to explore spatial varying relationships of immature mosquitoes and human densities with the incidence of dengue. International Journal of Environmental Research and Public Health 2011; 8: 2798–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grillet M-E, et al. Disentangling the effect of local and global spatial variation on a mosquito-borne infection in a neotropical heterogeneous environment. American Journal of Tropical Medicine and Hygiene 2010; 82: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerging Infectious Diseases 2001; 7: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu MG, et al. Determinants of the incidence of hand, foot and mouth disease in China using geographically weighted regression models. PLoS ONE 2012; 7: e38978. [DOI] [PMC free article] [PubMed] [Google Scholar]