SUMMARY
This study reports the epidemiology of respiratory syncytial virus (RSV) in hospitalized children in Cyprus over three successive seasons (2010–2013) and the association between prevalent genotypes and disease severity. RSV infections had a circulation pattern from December to March. Most RSV-positive children (83%) were aged <2 years. Genotyping of RSV isolates showed that during the first winter season of the study (2010–2011), the only RSV genotype circulating was GA2 (RSV-A), followed by genotype BA (RSV-B) in the next winter season with only few sporadic cases of GA2. During the last winter season of the study (2012–2013) the newly emerged RSV genotype ON1 (RSV-A) was virtually the only circulating genotype. Children infected with genotype ON1 suffered a significantly milder illness compared to infections with genotypes GA2 and BA with a higher percentage of BA-infected children requiring oxygen. Our findings are in contrast to the majority of published reports that suggest RSV-A causes more severe illness than RSV-B. Therefore, further investigation of the association between RSV genotypes and disease severity is required, as it might affect treatment strategies in the future.
Key words: Respiratory infections, respiratory syncytial virus, virology (human) and epidemiology
INTRODUCTION
Human respiratory syncytial virus (RSV) is currently considered the leading cause of lower respiratory tract infections in the paediatric population, especially in infants and young children. It has been estimated that over one third of all infants aged <1 year admitted to hospital with severe bronchiolitis or pneumonia, are infected by RSV [1]. Infections usually occur during the winter months in temperate countries or during the rainy season in tropical areas.
RSV is an enveloped virus with a negative-sense single-stranded RNA genome that encodes for 11 proteins and is classified in the Pneumovirus genus of the family Paramyxoviridae. Its major surface glycoproteins, the attachment (G) and the fusion (F) proteins are mainly involved in virus attachment to cell receptors and mediation of cell membrane fusion, respectively [2]. Two groups, RSV-A and RSV-B, have been described based on the variability of the G glycoprotein, especially its second hypervariable region located at the C-terminal. To date, several genotypes of each RSV group have been described; for RSV-A: GA1–GA7 [3, 4], SAA1 [5], NA1, NA2 [6] and ON1 [7]; for RSV-B: GB1–GB4 [4], SAB1–SAB3 [5] and BA1–BA10 [8, 9].
The main clinical manifestations during primary RSV infection vary widely in individuals, including upper respiratory tract infections, fever, otitis media, lower respiratory tract infections that can vary broadly from mild manifestations to life-threatening bronchiolitis and pneumonia, death in rare cases, post-infection abnormalities in respiratory function that can persist through adolescence, and possible sensitization to asthma [10]. RSV causes an atypical host response to infection with attributes of altered innate and inadequate immune memory responses. The G and F surface proteins induce neutralizing antibody production. In addition to humoral immune response, T-cell responses also play an important role in viral clearance. However, immunity wanes over time, therefore, re-infection during subsequent seasons is common [11].
Data regarding RSV infections in not only Cyprus but also the whole Eastern Mediterranean are rather modest and incomplete, therefore, the aim of the present study was to examine the molecular epidemiology of RSV infections in paediatric patients over three consecutive winter seasons in Cyprus (November 2010 to March 2013). Previous studies have investigated the association between RSV subtypes A and B and disease severity, but none has distinguished between individual RSV genotypes. To our knowledge, our study is the first to investigate the possible association between RSV pathogenicity and individual RSV genotypes.
MATERIALS AND METHODS
Clinical specimens
Between November 2010 and March 2013, nasopharyngeal swab samples were collected from children aged <12 years, who had been hospitalized for acute respiratory tract infection in the Department of Paediatrics of Archbishop Makarios Hospital in Nicosia, Cyprus. The samples were collected using the BD Universal Viral Transport 3 ml Collection kit (Becton Dickinson Co. Ltd, USA) and sent to the Department of Molecular Virology of the Cyprus Institute of Neurology and Genetics. Selection of patients, based on clinical symptoms of infection, was performed by clinicians. Written informed consent was obtained from parents. The study was approved by the Cyprus National Bioethics Committee.
Nucleic acid extraction and RSV detection
Viral RNA was extracted from 400 μl samples using the iPrep PureLink Virus kit on an iPrep purification instrument (Invitrogen, USA). Five microlitres of the extracted RNA were added to 20 μl of a Real-time RT–PCR master mix containing 0·9 μm forward primer 5′-GGCAAATATGGAAACATACGTGAA-3′ [12], 0·3 μm reverse primer 5′-TCTTTTTCTAGGACATTGTAYTGAACAG-3′ and 0·2 μm probe 5′-CTTCACGAAGGCTCCACRTACACAGC-3′ (TAMRA-labelled). One-Step Real-time RT-PCR (Invitrogen SuperScript™ III Platinum® One-Step Quantitative kit) was performed on a 7500 Real-time PCR system (Applied Biosystems, USA).
PCR amplification and sequencing of the second hypervariable region of the G gene
cDNA was synthesized using random primers and SuperScript III Reverse Transcriptase (Invitrogen). The second hypervariable region of the G protein gene of RSV was the target for the external and the semi-nested PCRs. External PCR was performed with the forward primer ABG490 and the reverse primer F164, whereas semi-nested PCR was performed with group A-specific forward primer AG655 and group B-specific forward primer BG517 along with reverse primer F164 as described previously [13].
The semi-nested PCR products were analysed by electrophoresis on a 1% agarose gel and the amplicons were cut and purified by DNA Gel Extraction kit (Millipore, USA). DNA was then subjected to Sanger sequencing (CEQ 8000, Beckman Coulter, USA) using the semi-nested forward primers and the reverse primer F164 as sequencing primers.
Disease severity evaluation and statistical analysis
Severity score was defined by clinicians based on the respiratory rate (breaths/min), arterial oxygen saturation in room air, presence of retractions or nasal flaring, respiratory sounds and feeding capacity as described previously [14]. Scores on the severity index ranged from a minimum of 0 to a maximum of 2 and an average score for each RSV genotype was calculated. The Mann–Whitney test was used to assess the significance of the differences in the mean severity scores between RSV genotypes and Fisher's exact test was used to assess the significance of oxygen need with regard to RSV genotype.
RESULTS
Age distribution and seasonality of RSV infections
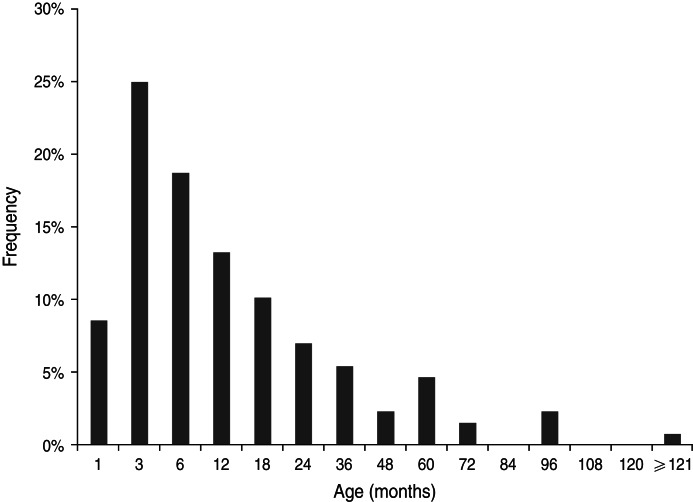
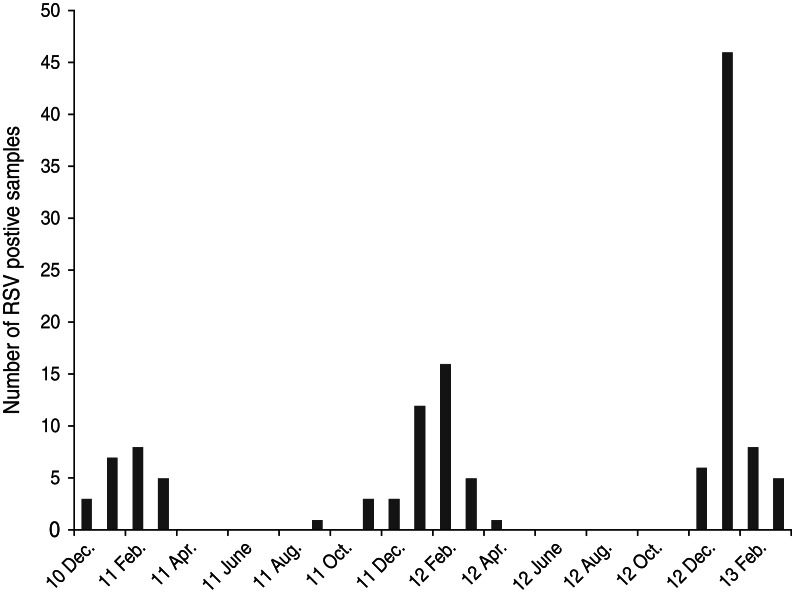
Of a total of 391 nasopharyngeal swab samples, RSV was detected in 96 samples as a single pathogen and in a further 32 samples as a co-infecting pathogen in mixed infections along with other respiratory viruses, mainly rhinovirus and bocavirus (data not shown). The age distribution of RSV-positive children, as shown in Figure 1, was: <1 month (11, 9%); 1–3 months (32, 25%); 3–6 months (24, 19%); 6–12 months (17, 13%); 12–18 months (13, 10%); 18–24 months (9, 7%); 24–36 months (7, 5%); and >36 months (15, 12%). With regard to RSV seasonality in Cyprus, there was a clear circulation pattern from December to March in winter seasons of 2010–2011 and 2012–2013, whereas the RSV season was extended from November to April during the 2011–2012 season (Fig. 2).
Fig. 1.
Age distribution of children hospitalized with respiratory syvcytial virus infection in Cyprus (December 2010 to March 2013).
Fig. 2.
Number of respiratory syncytial virus (RSV)-positive samples per month from December 2010 to March 2013.
Prevalent RSV genotypes
Within-season RSV genotype heterogeneity in Cyprus is low, with just one or two genotypes prevalent in each RSV season. During the 2010–2011 season only genotype GA2 was present, while in the next season (2011–2012) BA emerged as the most prevalent genotype with only a few sporadic cases of GA2. The situation changed completely during the last RSV season (2012–2013), when the newly emerged ON1 genotype was essentially the only genotype circulating with just one case of GA2 (Table 1).
Table 1.
Prevalent RSV genotypes in Cyprus over three epidemic seasons, December 2010 to March 2013
| GA2 | BA | ON1 | Untyped | Total | |
|---|---|---|---|---|---|
| 2010/2011 | 22 | 0 | 0 | 1 | 23 |
| 2011/2012 | 5 | 32 | 0 | 2 | 39 |
| 2012/2013 | 1 | 0 | 55 | 6 | 62 |
| Total | 28 | 32 | 55 | 9 | 124 |
Association between RSV genotype and disease severity
Table 2 shows that patients infected with RSV genotype BA had a mean severity score of 0·91, which was not significantly different from the mean severity score (0·93) of GA2-infected patients (P = 0·952). On the other hand, the mean score of children suffering from an infection with the recently characterized genotype ON1 (0·49) was significantly lower than that of those infected with either BA (P = 0·001) or GA2 (P = 0·007). These results were further supported by the percentage of patients that required oxygen supplementation. Thirty-four percent of BA-infected children required oxygen, whereas the percentage fell to 13% for children infected with genotype ON1 (P = 0·027) (Table 3). GA2-infected patients did not require significantly less oxygen supplementation compared to BA-infected patients (P = 0·57). There was no significant difference between the mean age of patients within each genotype subgroup (mean age was 14·7, 12·8 and 13·3 months for genotypes GA2, BA and ON1, respectively).
Table 2.
Association between RSV genotype and disease severity. Number of patients per severity score per prevalent RSV genotype
| RSV genotype | No. of patients per severity score | Mean severity score | ||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| BA | 6 | 23 | 3 | 0·91 |
| GA2 | 8 | 14 | 6 | 0·93 |
| ON1 | 29 | 25 | 1 | 0·49 |
Table 3.
Association between RSV genotype and oxygen need. Number and frequency of RSV-positive patients per prevalent RSV genotype requiring oxygen supplementation
| RSV genotype | No. of patients | Oxygen need, n (%) |
|---|---|---|
| BA | 32 | 11 (34) |
| GA2 | 28 | 7 (25) |
| ON1 | 55 | 7 (13) |
DISCUSSION
The present study is the first to have investigated the molecular epidemiology of RSV in Cyprus and its results, as far as the age distribution and the seasonality of RSV are concerned, have confirmed epidemiological data from other countries. The majority (66%) of RSV-positive children in our study were aged <1 year and this percentage increased to 83% when children aged <2 years were taken into consideration. In temperate climates, annual outbreaks generally occur during late autumn, winter and early spring months. In Cyprus, during the last three winter seasons, RSV infections began in December and declined in March, with the exception of the 2011–2012 season, which began as early as November and waned in April, most likely due to a colder and longer winter.
The severity of RSV infections is multifactorial as it depends both on host and viral factors. Host characteristics that increase the risk of severe RSV lower respiratory tract illness include infants with chronic lung disease (e.g. bronchopulmonary dysplasia), congenital heart disease and prematurity (<35 weeks gestation), immunocompromised patients and the elderly, particularly those with cardiopulmonary disease. In addition, lower socioeconomic status, exposure to tobacco smoke, household crowding, lack of breastfeeding and low levels of passively acquired maternal antibodies have also been associated with greater disease burden [15]. Less well defined is the influence of viral factors, such as viral load and RSV genotype on the severity of the infection. A study by Fodha et al. has shown that RSV-infected infants with severe disease had higher nasopharyngeal viral loads compared to infants with non-severe disease [16].
Published reports that have compared the two RSV subtypes in terms of disease severity produced controversial results. A number of studies failed to reveal significant differences; however, some investigators concluded that infection with either group A or B can be associated with more severe illness. In the majority of previous studies, RSV subtype A infection resulted in more severe disease in hospitalized infants [17, 18] in contrast to one study where RSV subtype B infection was shown to have produced more severe disease than RSV subtype A, in terms of length of hospital stay, use of respiratory support, and the presence of an infiltrate by chest radiograph [19].
These discrepancies could be attributed to differences in study design, which in most cases were retrospective, disease definition, inclusion criteria or genotyping methodology. It is also possible that strain virulence may be variable between epidemics. Furthermore, herd immunity against previously circulating subtypes or genotypes and pre-existing neutralization titres in mothers of infected children could have altered the severity of disease.
In the present study, we have investigated the association between the prevalent RSV genotypes in three consecutive winter seasons in Cyprus (2010–2013) and the severity of the illness and our findings have added more confusion to the conflicting results of previous investigations. Young children and infants infected with RSV A genotype ON1, suffered a significantly milder illness compared to infections with RSV A genotype GA2 and RSV B genotype BA. Disease severity score from infection with genotype BA was similar to that of GA2 infection. The observed differences in disease severity between RSV genotypes were not age-related.
Genotype BA was discovered in Buenos Aires, Argentina in 1999 and since then, it has almost completely replaced all other circulating B genotypes. BA contains a 60-nucleotide duplication in the second hypervariable region of the G protein, which is the target for genotype-specific neutralizing antibodies, and such changes in structure might alter the immunogenicity and pathogenicity of the virus [8]. The predominance of genotype BA over other subtype B genotypes suggests a potential selective advantage. This might also be the case with the novel genotype ON1 that was discovered in 2011 in Ontario, Canada. ON1 contains a 72-nucleotide duplication in the same region of G protein as BA does but further molecular analysis is needed to determine a possible future predominance of genotype ON1 over other subtype A genotypes [7, 20].
Despite its importance as a respiratory pathogen, there is currently no licensed vaccine for prevention of RSV infection, although many candidate vaccines are either under development or in clinical trials. The current RSV vaccine strategies include virus attenuation and recombinant technology for the presentation of viral antigens [21]. The treatment of RSV, for the time being, is largely supportive and multiple regimens are being used including bronchodilators, corticosteroids, antiviral agents, nasal suctioning and decongestants. However, none of these treatments has had a significant impact on symptoms or the course of the illness. The main management strategies are maintenance of hydration and oxygenation [22]. The use of palivizumab (Synagis™; MedImmune, USA), a humanized murine monoclonal antibody directed against RSV, is indicated for children in high-risk groups as a preventive measure and results in a reduced rate of RSV-associated hospitalizations [23].
As more therapeutic and prophylactic modalities against RSV are becoming available or being developed, possible differences in disease severity between genotypes may in the future have an effect on treatment strategies. For that reason, studies on the molecular epidemiology of RSV should be continued in order to establish whether determination of the RSV genotype is helpful in guiding therapy.
ACKNOWLEDGEMENTS
The work was in part co-funded by the European Regional Development Fund and the Republic of Cyprus through the Research Promotion Foundation (Project ΥΓΕΙΑ/ΔΥΓΕΙΑ/0609(ΒΙΕ/25).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Pancer K, et al. Infections caused by RSV among children and adults during two epidemic seasons. Polish Journal of Microbiology 2011; 60: 253–258. [PubMed] [Google Scholar]
- 2.Goto-Sugai K, et al. Genotyping and phylogenetic analysis of the major genes in respiratory syncytial virus isolated from infants with bronchiolitis. Japanese Journal of Infectious Diseases 2010; 63: 393–400. [PubMed] [Google Scholar]
- 3.Peret TC, et al. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. Journal of General Virology 1998; 79: 2221–2229. [DOI] [PubMed] [Google Scholar]
- 4.Peret TC, et al. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. Journal of Infectious Diseases 2000; 181: 1891–1896. [DOI] [PubMed] [Google Scholar]
- 5.Venter M, et al. Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. Journal of General Virology 2001; 82: 2117–2124. [DOI] [PubMed] [Google Scholar]
- 6.Shobugawa Y, et al. Emerging genotypes of human respiratory syncytial virus subgroup A among patients in Japan. Journal of Clinical Microbiology 2009; 47: 2475–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eshaghi A, et al. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: a novel genotype with a 72 nucleotide G gene duplication. PLoS One 2012; 7: e32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trento A, et al. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. Journal of General Virology 2003; 84: 3115–3120. [DOI] [PubMed] [Google Scholar]
- 9.Dapat IC, et al. New genotypes within respiratory syncytial virus group B genotype BA in Niigata, Japan. Journal of Clinical Microbiology 2009; 48: 3423–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scagnolari C, et al. Evaluation of viral load in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. Medical Microbiology and Immunology 2012; 201: 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshansky CM, et al. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiology 2009; 4: 279–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry AM, et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS One 2010; 5: e15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullender WM, Sun L, Anderson LJ. Analysis of respiratory syncytial virus genetic variability with amplified cDNAs. Journal of Clinical Microbiology 1993; 31: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scagnolari C, et al. Upregulation of interferon-induced genes in infants with virus-associated acute bronchiolitis. Experimental Biology and Medicine (Maywood, NJ) 2007; 232: 1355–1359. [DOI] [PubMed] [Google Scholar]
- 15.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Research 2011; 162: 80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fodha I, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. Journal of Medical Virology 2007; 79: 1951–1958. [DOI] [PubMed] [Google Scholar]
- 17.Walsh EE, et al. Severity of respiratory syncytial virus infection is related to virus strain. Journal of Infectious Diseases 1997; 175: 814–820. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos NG, et al. Does respiratory syncytial virus subtype influences the severity of acute bronchiolitis in hospitalized infants? Respiratory Medicine 2004; 98: 879–882. [DOI] [PubMed] [Google Scholar]
- 19.Hornsleth A, et al. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatric Infectious Disease Journal 1998; 17: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 20.Prifert C, et al. Novel respiratory syncytial virus a genotype, Germany, 2011–2012. Emerging Infectious Diseases 2013; 19: 1029–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudraraju R, et al. Respiratory syncytial virus: current progress in vaccine development. Viruses 2013; 5: 577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panitch HB. Respiratory syncytial virus bronchiolitis: supportive care and therapies designed to overcome airway obstruction. Pediatric Infectious Disease Journal 2003; 22: S83–87; discussion S87–88. [DOI] [PubMed] [Google Scholar]
- 23.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatric Infectious Disease Journal 2011; 30: 510–517. [DOI] [PubMed] [Google Scholar]