SUMMARY
This study reports clinico-epidemiological features and viral agents causing acute encephalitis syndrome (AES) in the eastern Indian region through hospital-based case enrolment during April 2011 to July 2012. Blood and CSF samples of 526 AES cases were investigated by serology and/or PCR. Viral aetiology was identified in 91 (17·2%) cases. Herpes simplex virus (HSV; types I or II) was most common (16·1%), followed by measles (2·6%), Japanese encephalitis virus (1·5%), dengue virus (0·57%), varicella zoster virus (0·38%) and enteroviruses (0·19%). Rash, paresis and cranial nerve palsies were significantly higher (P < 0·05) with viral AES. Case-fatality rates were 10·9% and 6·2% in AES cases with and without viral aetiology, respectively. Simultaneous infection of HSV I and measles was observed in seven cases. This report provides the first evidence on viral aetiology of AES viruses from eastern India showing dominance of HSV that will be useful in informing the public health system.
Key words: Acute encephalitis syndrome, acyclovir, herpes simplex virus
INTRODUCTION
Acute encephalitis syndrome (AES) is a major public health problem worldwide because of its high morbidity and mortality. Although incidence of AES varies according to different studies, it is generally between 3·5 and 7·4/100 000 patient-years, and is higher in children aged <1 year and adults aged ⩾65 years [1]. Aetiology of AES can be infective or non-infective. The infective agents include a wide range of bacteria and viruses that can also vary according to the geographical location, climate and host factors [2]. Although viruses are assumed to be the major pathogens, most cases remain undiagnosed. Japanese encephalitis virus (JEV) has been attributed to the majority of AES outbreaks in South East Asia. About three billion people (60% of the world's population) live in JE endemic regions and JEV is a public health concern with around 35 000 cases each year, 10 000 deaths and about 30% of survivors developing permanent sequelae [3]. Herpes simplex virus (HSV) has been observed to be the major causative agent (1 case/250 000–500 000 population annually) in sporadic AES cases in Western countries with high (>70%) mortality in the absence of acyclovir therapy [4]. Besides JEV and HSV enteroviruses, West Nile virus (WNV), Chandipura virus (CHPV), equine encephalitis, tick-borne encephalitis, Nipah virus (NiV) and measles can be attributed to AES in different proportions.
Even though AES cases are frequently being reported in India, the actual contribution of viral encephalitis is unknown, because of problems in establishing laboratory diagnosis and the fact that a wide variety of CNS disorders, both infectious and non-infectious, may mimic the illness [5]. Available reports on outbreak investigations in India have shown viral aetiology in 6–100% of the samples tested, with JEV being the most commonly identified pathogen [6], other causative agents were CHPV [7], enteroviruses [8] and NiV [9]. Hospital-based investigations of AES cases in the country had confirmed viral pathogens in 6–86·7% of cases with either enteroviruses, JEV, dengue virus (DENV), CHPV, measles, varicella zoster virus (VZV), mumps or HSV as the attributed agents [6].
The above reports were mostly concentrated in Uttar Pradesh, West Bengal, Andhra Pradesh, Tamilnadu and Maharashtra states. In the state of Odisha, located on the eastern coast of India, the first outbreak investigation of AES suspected for viral aetiology was reported from Rourkela city in 1989, which showed JEV as the causative agent [10]. A subsequent report from a hospital-based study in children during 1992–1993 indicated JEV infection in 11·4% of AES cases where other viruses were not investigated [11]. Since then there is no information available on viral aetiology of AES from this region. This report provides the first information on the spectrum of viral infections associated with sporadic hospitalized AES cases in this eastern Indian region and will be useful for informing public health action. Observations on clinical presentations of AES cases will also support syndromic case management in situations lacking a laboratory investigation facility.
MATERIALS AND METHODS
Case enrolment and assessment
This study was performed in the eastern Indian region enrolling AES cases admitted to the tertiary-care referral hospitals in Odisha catering to patients from Odisha and neighbouring states (West Bengal, Bihar, Jharkhand). Sri Ram Chandra Bhanja Medical College and Hospital (SCBMCH) and Sardar Vallabh Bhai Patel Post Graduate Institute of Paediatrics (SVPPGIP) in Cuttack and the Capital Hospital, Kalinga Institute of Medical Sciences (KIMS), Kalinga Hospital, Sum Hospital and High Tech Medical College and Hospital in Bhubaneswar were taken as the source hospitals for study during the investigation period, i.e. from April 2011 to July 2012. For inclusion in the study an AES case was clinically defined as ‘a person of any age, at any time of year, with acute onset of fever and a change in mental status (including symptoms such as confusion, disorientation, coma, or inability to talk) and/or new onset of seizures (excluding simple febrile seizures). Other early clinical findings included an increase in irritability, somnolence or abnormal behaviour greater than that seen with usual febrile illness’ [12–14]. However, cases positive for malaria by either peripheral smear or immunochromatography test, presenting with fever and meningeal signs confirmed to be of bacterial origin, or diagnosed with any metabolic encephalopathies were excluded from investigation for viral aetiologies. Demographic and clinical observations were recorded in a predesigned format after enrolment of the cases. Samples (CSF and blood) were collected according to the standard procedure and laboratory investigations were performed. The patients were observed in the hospital until discharge and clinical manifestations were noted. In a subset of patients with viral and non-viral aetiology, neurological impairment and quality of life were assessed at 6 months after disease onset through a questionnaire administered to the subjects or their parents and relatives regarding the patient's recovery, activity and autonomy in daily life either by direct or telephone conversation. For this purpose every second patient diagnosed with viral aetiology was included following systematic random sampling. For each of above viral AES case, one patient with non-viral aetiology was selected from the patients enrolled in the corresponding week by simple random procedure. From these patients 40 viral AES cases responded, against whom 40 non-viral AES cases were also included in the survey. Any change in behaviour, impairment in speech, memory or concentration in study, need for anti-epileptic treatment, occurrence of seizures, need for speech, motor, or any other rehabilitation after discharge from the hospital were recorded. The patient's outcome was defined as ‘favourable outcome’, for those having good recovery or mild disability and ‘poor outcome’ for severe disability or death as described by Raschilas et al. [15].
The study was conducted under approval of the Human Ethical Committee of the institution following guidelines of Indian Council of Medical Research (ICMR), New Delhi. Written consent was obtained from the patients' parents, guardians/caregivers before enrolment and sample collection.
Laboratory investigation
Samples were subjected to either serological tests (antigen/antibody detection by ELISA) or PCR/RT–PCR depending upon the day of sample collection from onset of symptoms. Early phase samples (within 5 days of symptom onset) were subjected to PCR/RT–PCR and antigen detection by ELISA, whereas IgM antibodies were tested in late phase samples (>5 days after symptom onset). The above tests were performed on blood as well as CSF samples collected from the patients.
IgM antibodies were detected by using virus-specific ELISA kits [JEV and dengue kits from National Institute of Virology (NIV), Pune; HSV I and II from Novatech Immunodiagnostica, Germany; measles and VZV from De medi tech, Germany; enterovirus from Serion Immunodiagnostica, Germany]. Detection of NS1 antigen for DENV was made using the ELISA kit of Panbio, Australia. In each test run known positive standards with low and high titres and negative controls were used to interpret a positive or negative result/titre to avoid positivity. Any borderline test was repeated twice and the average titre was calculated for final interpretation.
Viral nucleic acid was extracted from 200 μl of specimen using either the DNA Extraction kit or Viral RNA kit (Qiagen, Germany). Extraction of DNA or RNA was performed according to the manufacturer's instructions with requisite in-house modifications. Either 80 μl DNA or 40 μl RNA was eluted at the end of the extraction procedure. Nucleic acid was either processed immediately for PCR amplification or stored at −80°C for further use.
Conventional PCR was conducted to detect HSV and VZV according to Read & Kurtz [16]. RT–PCR was performed as previously described for DENV [17], JEV [18], WNV [19], measles [20] and enteroviruses [21].
Diagnostic interpretation
Viral aetiology of encephalitis was considered either definitive or possible based upon the sample type and evidence of viral infection. Presence of viral genome by PCR/RT–PCR/culture of virus or detection of IgM antibodies in CSF were considered as ‘definitive’, while presence of viral genome by PCR/RT PCR from blood sample or detection of IgM antibodies in serum and absence of above viral markers in CSF was considered as ‘possible’.
Statistical analysis
Descriptive statistical methods were used to present the demographic characteristics of the patients. Fisher's exact test was used to compare the relevant data between groups of patients using GraphPad Prism v. 5 (GraphPad Software Inc., USA). HSV I or II IgM-positive and HSV PCR-positive samples were grouped as HSV infection for analysis because PCR-positive samples could not be classified further.
RESULTS
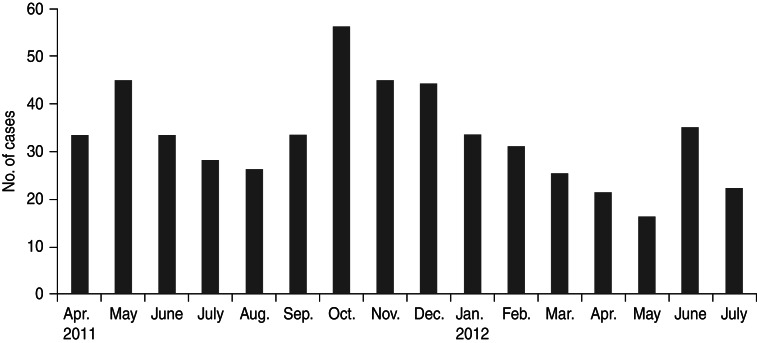
A total of 526 patients satisfying the case definition and exclusion criteria were enrolled from seven hospitals during the investigation period. These included 249 (47·3%) males and 131 (25%) females aged ⩽15 years, while 77 (14·6%) males and 71 (13·5%) females were aged >15 years. Median age of the enrolled patients was 9 years (interquartile range 16·25). Age and sex distribution of AES cases is shown in Table 1. Most (85%) of the patients were reported from a rural setting. The cases were found to be distributed throughout the year and monthly distribution is shown in Figure 1. Thirty-three percent of AES cases were enrolled during September–December 2012, which falls during the post-monsoon and early winter seasons in this region.
Table 1.
Age and sex distribution of acute encephalitis syndrome cases
| Age group | Total, n (%) | Male, n (%)/female, n (%) |
|---|---|---|
| 1 month–5 yr | 173 (32·8) | 117 (67·7)/56 (32·3) |
| 6−10 yr | 164 (31·2) | 110 (67)/54 (33) |
| 11–15 yr | 43 (8·2) | 22 (51·1)/21 (48·9) |
| 16–30 yr | 55 (10·5) | 30 (54·5)/25 (45·5) |
| 31–45 yr | 41 (7·8) | 19 (46·3)/22 (53·7) |
| 46–60 yr | 33 (6·3) | 20 (60)/13 (40) |
| >60 yr | 17 (3·2) | 8 (47)/9 (53) |
| Total | 526 (100) | 326 (62)/202 (38) |
Fig. 1.
Monthly case enrolment of acute encephalitis syndrome cases during the investigation period, April 2011 to July 2012.
Evidence for viral aetiology was noted in 91 (17·2%, 54 possible and 37 definitive) out of 526 enrolled patients. The laboratory investigation results are summarized in Table 2. Evidence of simultaneous infection with two viruses was observed in 22 (4·1%) patients. Seven of these had measles and HSV I infection, 14 had HSV I and II and one had dengue with JEV infection. The distribution of AES cases showing viral aetiologies in different age and sex groups is given in Table 3.
Table 2.
Laboratory investigation results (n = 526)
| Viral agent | Positive cases n (%) | IgM antibody positive | PCR/RT–PCR positive | ||
|---|---|---|---|---|---|
| CSF | Blood | CSF | Blood | ||
| HSV I | 39 (7·4) | 0 | 39 (possible) | 0 | 0 |
| HSV II | 25 (4·7) | 0 | 25 (possible) | 0 | 0 |
| HSV | 21 (4·0) | 0 | 0 | 21 (definitive) | 0 |
| Measles | 14 (2·6) | 7 (definitive) | 7 (possible) | 0 | 0 |
| JEV | 8 (1·5) | 7 (definitive) | 1 (possible) | 0 | 0 |
| Dengue | 3 (0·57) | 1 (definitive) | 2 (possible) | 0 | 1* (possible) |
| VZV | 2 (0·38) | 1 (definitive) | 1 (possible) | 0 | 0 |
| Enteroviruses | 1 (0·19) | 0 | 1 (possible) | 0 | 0 |
| Total | 113† | 16 | 76 | 21 | 1 |
HSV, Herpes simplex virus; JEV, Japanese encephalitis virus; VZV, varicella zoster virus.
The sample was also positive for NS1 antigen.
Dual infection in 22 patients: HSV I and HSV II (n = 14), measles and HSV I (n = 7); dengue and JEV (n = 1).
Table 3.
Age and sex distribution of the acute encephalitis syndrome (AES) cases showing viral aetiology
| Age group | No. of cases showing viral aetiology/AES cases (%) | ||
|---|---|---|---|
| Total | Male | Female | |
| 1 month–5 yr | 21/173 (12·1) | 14/117 (11·9) | 7/56 (12·5) |
| 6–10 yr | 22/164 (13·4) | 17/110 (15·4) | 5/54 (9·25) |
| 11–15 yr | 22/43 (55·8) | 9/22 (40·9) | 13/21 (61·9) |
| 16–30 yr | 12/55 (21·8) | 6/30 (20) | 6/25 (24) |
| 31–45 yr | 7/41 (17) | 3/19 (15·7) | 4/22 (18·1) |
| 46–60 yr | 4/33 (12·1) | 1/20 (5) | 3/13 (23) |
| >60 yr | 3/17 (17·6) | 1/8 (12·5) | 2/9 (22·2) |
| Total | 91/526 (17·3) | 51/326 (14·3) | 40/200 (20) |
Clinical features
The major signs/symptoms associated with fever were altered sensorium (97·3%), convulsion (46·7%), headache (30·9%), vomiting (32·6%) and meningeal signs (26·2%) in different combinations. Detailed clinical features are given in Table 4 while comparing the clinical presentations of AES cases of viral origin with others. Skin rash (15·3%), motor paresis (6·6%) and cranial nerve palsies (9%) were observed to be significantly higher (P < 0·05) in patients with viral AES compared to those of non-viral cause.
Table 4.
Clinical features of acute encephalitis syndrome (AES) cases showing viral or non-viral aetiology
| Clinical features | AES cases, n (%) | P value (viral aetiology vs. non-viral aetiology) | ||
|---|---|---|---|---|
| Total (n = 526) | With viral aetiology (n = 91) | Without viral aetiology (n = 435) | ||
| Fever | 526 (100) | 91 (100) | 435 (100) | n.a. |
| Headache | 163 (30·9) | 25 (27·4) | 138 (32) | 0·456 |
| Vomiting | 172 (32·6) | 37 (40·6) | 138 (32) | 0·112 |
| Convulsion | 246 (46·7) | 41 (45) | 205 (47) | 0·730 |
| Respiratory problem | 44 (8·3) | 8 (8·8) | 36 (8·2) | 0·836 |
| Abdominal pain | 27 (5·1) | 6 (6·6) | 21 (5) | 0·441 |
| Diarrhoea | 28 (5·3) | 7 (7·7) | 21 (5) | 0·301 |
| Rash | 40 (7·6) | 14 (15·3) | 24 (5·5) | 0·002 |
| Altered sensorium | 512 (97·3) | 88 (96·7) | 424 (97·4) | 0·718 |
| Meningeal signs | 138 (26·2) | 28 (31) | 110 (25·2) | 0·295 |
| Paresis | 15 (2·2) | 6 (6·6) | 9 (2%) | 0·030 |
| Cranial nerve palsies | 12 (2·2) | 8 (9) | 4 (1) | 0·0001 |
| Death during hospital stay | 37 (7) | 10 (10·9) | 27 (6·2) | 0·1150 |
n.a., Not applicable.
Out of 71 patients diagnosed (50 possible, 21 definitive) with HSV encephalitis meningeal signs were observed in 26 (36·6%), hemiparesis in four (4·7%) and cranial nerve palsies in five (5·9%) cases. Both meningeal signs and cranial nerve palsies were observed in four cases. Two HSV cases had cranial nerve palsies as well as hemiparesis. Meningeal signs were observed in three measles encephalitis cases (two of which were also positive for HSV I). One case of measles had both quadriparesis and bulbar palsy. Paraparesis was observed in one varicella zoster encephalitis case. Meningeal signs were observed to be significantly higher (P = 0·02) in HSV-infected (26/71) patients than non-HSV viral AES (2/20) cases but presence of rash was significantly higher (P = 0·01) in non-HSV (7/20) than HSV-infected cases (7/71).
Outcome
The patients were treated in hospital with antibiotics, antimalarials and supportive medications following standard hospital guidelines. Diagnosed cases of HSV infection were treated with acyclovir. Out of 526 AES cases, 37 (7%) patients died during their hospital stay of which ten had a viral infection (Case-fatality rate of AES, 10·9%). Mean gap between hospital admission and death was 6 days (s.d. ± 3). Out of 489 patients discharged from hospital, 80 patients (40 with viral AES, 40 with non-viral aetiology) were followed up at 6 months post-disease onset and information collected as necessary. Sixty-six (82%) patients had favourable outcome and 14 (18%) had poor outcome. Favourable outcome included complete recovery (n = 23), mild loss of attention/concentration in study (n = 41) and easy irritability (n = 23). Thirty-six (90%) patients with the diagnosis of viral AES had favourable outcome whereas four (10%) had poor outcome as death occurred at home. Thirty (75%) of AES cases not showing viral aetiology had favourable outcome and ten (25%) had poor outcome. Poor outcome included two (5%) deaths and severe neurological disability in eight subjects. The patients who had poor outcome at 6 months follow-up were observed to have poor Glasgow Coma Scale (GCS) score (GCS <6) during admission.
DISCUSSION
Previous investigations of outbreaks and sporadic hospitalized patients presenting with an encephalitis syndrome revealed viral infections as a major aetiology. However, aetiological diagnosis of AES cases still represents a diagnostic challenge [22]. It is of note that a large review of over 1500 patients presenting to healthcare facilities in California, USA, showed that only 16% had a confirmed aetiological agent (the majority of these were viral); 13% had a suspected agent and 8% had non-infectious cause identified (autoimmune disease and vasculitis being common). Aetiology was not found in nearly two thirds of cases referred to specialist units [23]. Of the confirmed viral aetiologies, the type of viruses identified in different parts of the world also differs considerably. HSV was shown to be the dominant viral agent for encephalitis in China (15%, 2012) [24], Vietnam (63%, 2012) [25], UK (22·1%, 2003; 19%, 2010) [26, 27], Norway (19%, 2013) [28], Spain (71·4%, 2013) [29], and France (21·7%, 2009) [30]. JEV was the predominant cause of AES from Uttar Pradesh (23%, 1990) [31] and northeastern states (Assam, 2004; Arunachal Pradesh, 2011) [32, 33] of India, and Cambodia (31%, 2002) [34].
Enteroviruses were reported as the major cause of AES from Uttar Pradesh, India in the present decade outbreak investigations in 2006, and a prospective study during 2009–2010 in Uttar Pradesh [8, 35], and also from China (15·4%, 1996) [36], and USA (25%, 2006) [23]. A study from China (1996) had shown enteroviruses (15·4%), mumps (7·2%), rubella (6·1%), JEV (5·1%), HHV-6 (2%), HSV (2%) and EBV (1%) as the viral aetiologies of encephalitis in children aged between 7 months and 13 years [36]. VZV was found to be the most common aetiology (25%) of encephalitis from a population-based study on AES in Finland (1989); where mumps (8·4%), HSV (7·3%) and measles (4·2%) were the other agents identified [37]. Besides the above viruses, NiV (West Bengal, 2006) [9] and CHPV (Gujarat, 2005; Maharashtra, 2010) [38, 39] were reported as emerging viral infections that caused encephalitis outbreaks in Indian states.
The present study reports the first information on the viral aetiology of AES covering a wide range of neurotropic viruses in the eastern Indian region. This investigation on 526 AES patients admitted to referral hospitals of Odisha, has shown viral aetiology in 17·2% of patients; HSV was dominant (16·1%) and other viruses were measles (2·6%), JEV (1·5%), DENV (0·57%), VZV (0·38%) and enteroviruses (0·19%). This proportion seems to be lower compared to many reports of viral aetiology, but was similar to the recent report from USA [23]. This can be explained on the basis of case definition and other possible aetiologies.
In this study the case definition of AES was adopted from the WHO guidelines [13]. The same guidelines have also been adopted by the national vector-borne disease control programme (NVBDCP), India [14]. The case definition was tested by Solomon et al. who described that this case definition can miss some cases of AES [12] that present only with meningism, paresis or headache. By contrast, this case definition can include some of the cases of bacterial or viral meningitis that can present with a short lasting or ill-defined altered sensorium. This could have broadened the denominator, hence reducing the proportion of viral AES cases. The relatively low identification of viral aetiologies can be due to possibilities such as: (i) the case definition used was more sensitive but less specific, (ii) delay in investigation because of late admission at referral hospitals in case of sporadic AES cases, and (iii) greater chance of viral identification in an outbreak setting due to planned and timely investigation along with better case selection due to closeness of case presentations. However, despite the above limitations the present report is important when considering the inherent difficulties of viral diagnosis.
This is the also first report that shows HSV as the dominant viral aetiology of sporadic encephalitis syndromes in India and indicates that HSV can be an important cause of AES both in tropical and cold countries, although it seems to be prominent in low-temperature zones.
Measles was the second viral aetiology (n = 14, 2·6%) in the study. All measles patients had cutaneous rash as the initial presentation before CNS involvement. HSV was found to be associated with measles in seven (50%) subjects. This supports the possibility raised by Orren et al. that measles infection can increase susceptibility to severe HSV infection [40].
Seven cases of JEV infection were identified during the study. These patients belonged to five coastal districts of Odisha and all were isolated cases without clustering. Almost all the cases were reported during the post-monsoon period. Paddy fields, post-monsoon period and low socioeconomic status have been shown to be epidemiological factors for transmission of JEV [3]. Although favourable ecological condition are apparent in many parts in the studied area there have been no reports of JEV infection in the state of Odisha since 1993 [11]. This could be due to lack of attention of the public health system for JEV investigation especially during AES outbreaks or that the ecological triad for JEV infection has not been established in the region because of unexplained reasons.
In this report dengue infection was found in three patients with AES, who were referred during a dengue outbreak encountered in the region during September–November 2011. These patients had thrombocytopenia along with CNS involvement. One of them died, even in the absence of haemorrhagic manifestation. Although precise pathogenesis of dengue-associated CNS manifestation remains unclear [41], this report adds to the potential of dengue infection for encephalitis that is being reported with increasing frequency in endemic areas [42, 43].
Two cases (0·38%, one confirmed and one possible case of varicella zoster encephalitis) were identified in this study. These patients recovered from exanthematous illness but developed encephalitis within 2 weeks. Neurological complications (0·01–0·03%) like cerebellar ataxia and encephalitis caused by VZV have been observed from India previously [44]. This suggests that although encephalitis is rare, patients recovering from rash due to VZV should be placed under follow-up to avoid mortality.
Only one possible case of enterovirus was identified in the study, even though enteroviruses are among the well established causes of aseptic meningitis and encephalitis in young children [5]. Virus detection from stool samples, which has not been included in the current investigation, could have added to the diagnosis.
This investigation on the aetiology of hospitalized AES patients has shown evidence of viral infection in 17·2% of cases and provided the first information from the area. However, it is limited by the fact that other possible aetiologies have not been addressed. Autoimmune encephalitis is increasingly identified as a cause of AES with advances in laboratory detection of a variety of antibodies, e.g. anti-N-methyl-d-aspartic acid receptor (anti-NMDAR), anti-LGI1, anti-α-amino-3-hydroxy-5-methyl-4-isoxazo-lepropionic acid (anti-AMPA), anti-gamma-amino butyric acid (anti-GABA), intracellular antigens anti-Hu and anti-M2, etc. [22, 45]. Autoantibody studies and MRI scans would have identified some of the non-infectious aetiologies that are also important for management of the treatable condition. Acute encephalitis-like presentations of Plasmodium falciparum malaria also cannot be ruled out as a cause because microscopy and rapid diagnostic tests can miss a significant proportion of falciparum infections and investigation by PCR could have supported the diagnosis in the malaria-endemic area [46, 47].
ACKNOWLEDGEMENTS
The authors extend their sincere thanks to Indian Council of Medical Research, New Delhi for financial support. Thanks are also due to the concerned physicians of the referral hospitals and all participating patients.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Granero DJ, Crowcroft NS. The epidemiology of acute encephalitis. Neuropsychological Rehabilitation 2007; 17: 406–428. [DOI] [PubMed] [Google Scholar]
- 2.Tan LV, et al. Viral aetiology of encephalitis in children in southern Vietnam: results of a one-tear prospective descriptive study. PLoS Neglected Tropical Disease 2010; 4: e854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anuradha SK, et al. Epidemiological aspects of Japanese encephalitis in Bellary, Karnataka, India. International Journal of Biological and Medical Research 2011; 2: 691–695. [Google Scholar]
- 4.Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogen. Lancet 2002; 359: 507–514. [DOI] [PubMed] [Google Scholar]
- 5.Karmarkar SA, et al. A study of acute febrile encephalopathy with special reference to viral aetiology. Indian Journal of Paediatrics 2008; 75: 801–805. [DOI] [PubMed] [Google Scholar]
- 6.Joshi R, et al. Changing landscape of acute encephalitis syndrome in India: a systematic review. National Medical Journal of India 2012; 5: 212–220. [PubMed] [Google Scholar]
- 7.Tandale BV, et al. Chandipura virus: a major cause of acute encephalitis in children in North Telangana, Andhra Pradesh, India. Journal of Medical Virology 2008; 80: 118–124. [DOI] [PubMed] [Google Scholar]
- 8.Sapkal GN, et al. Enteroviruses in patients with acute encephalitis, Uttar Pradesh, India. Emerging Infectious Disease 2009; 15: 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadha MS, et al. Nipah virus associated encephalitis outbreak, Siliguri, India. Emerging Infectious Disease 2006; 12: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vajpayee A, et al. Investigation of an outbreak of japanese encephalitis in Rourkela city (Orissa) during 1989. Journal of Communicable Disease 1991; 23: 18–21. [PubMed] [Google Scholar]
- 11.Devi PS, Behera PL, Swain A. Japanese encephalitis in Orissa. Indian Paediatrics 1996; 33: 702–703. [PubMed] [Google Scholar]
- 12.Solomon T, et al. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bulletin of World Health Organization 2008; 86: 178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Japanese encephalitis surveillance standards (http://www.path.org/files/WHO_surveillance_standards_JE.pdf).
- 14.Government of India. (http://nvbdcp.gov.in/Doc/Revised%20guidelines%20on%20AES_JE.pdf).
- 15.Raschilas F, et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clinical Infectious Disease 2002; 35: 254–260. [DOI] [PubMed] [Google Scholar]
- 16.Read SJ, Kurtz JB. Laboratory diagnosis of common viral infections of central nervous system by using a single multiplex PCR screening assay. Journal Clinical Microbiology 1999; 37: 1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti RS, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcription polymerase chain reaction. Journal Clinical Microbiology 1992; 30: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pujhari SK, et al. A novel mutation (S227 T) in domain II of the envelope gene of JEV circulating in north India. Epidemiology and Infection. Published online: 30 July 2010. doi: 10.1017/S0950268810001937. [DOI] [PubMed] [Google Scholar]
- 19.Shi PY, et al. High throughput detection of west nile virus RNA. Journal Clinical Microbiology 2001; 39: 1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremer JR, et al. Measles virus genotyping by nucleotide specific multiplex PCR. Journal Clinical Microbiology 2004; 42: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger D, et al. Reverse transcription multiplex PCR for differentiation between polio and enteroviruses from clinical environmental samples. Journal of Clinical Microbiology 1995; 33: 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingfield T, et al. Autoimmune encephalitis: a case series and comprehensive review of the literature. Quarterly Journal of Medicine 2011; 104: 921–931. [DOI] [PubMed] [Google Scholar]
- 23.Glaser CA, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clinical Infectious Disease 2006; 43: 1565–1577. [DOI] [PubMed] [Google Scholar]
- 24.Wang XX, et al. Analysis of epidemiology and the main clinical symptom of viral encephalitis in Gansu, 2009–2011. Chinese Journal of Preventive Medicine 2012; 46: 10999–10102. [PubMed] [Google Scholar]
- 25.Taylor WR, et al. The spectrum of CNS infection in an adult referral hospital in Hanoi, Vietnam. PLoS One. Published online: 30 Aug 2012. doi: 10.1371/journal.pone.0042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davison KL, et al. Viral encephalitis in England, 1989–1998: what did we miss? Emerging Infectious Disease 2003; 9: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granerod J, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infectious Disease 2010; 10: 835–44. [DOI] [PubMed] [Google Scholar]
- 28.Quist-Paulsen E, et al. Infectious encephalitis: a description of Norwegian cohort. Scandinavian Journal of Infectious Disease 2013; 43: 179–185. [DOI] [PubMed] [Google Scholar]
- 29.De Ory F, et al. Viral infection of CNS in Spain: a prospective study. Journal of Medical Virology 2013; 85: 554–562. [DOI] [PubMed] [Google Scholar]
- 30.Mailles A, Stahl JP. Infectious encephalitis in France in 2007: a national prospective study. Clinical Infectious Disease 2009; 49: 1838–1847. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R, et al. Virological investigation of acute encephalopathy in India. Archives of Disease in Childhood 1990; 65: 1227–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phukan AC, et al. Japanese encephalitis in Assam, north east India. South East Asian Journal of Tropical Medicine and Public Health 2004; 35: 618–622. [PubMed] [Google Scholar]
- 33.Khan SA, et al. Japanese encephalitis epidemiology in Arunachal Pradesh, a hilly state in north east India. Asian Pacific Journal of Tropical Disease 2011; 1: 119–122. [Google Scholar]
- 34.Srey VH, et al. Aetiology of encephalitis syndrome among hospitalized children and adults in Takeo, Cambodia, 1999–2000. American Journal of Tropical Medicine and Hygiene 2002; 66: 200–207. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, et al. Molecular epidemiological study of enteroviruses associated with encephalitis in children from India. Journal of Clinical Microbiology 2012; 50: 3509–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, et al. Viral aetiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Paediatric Infectious Disease Journal 1996; 15: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 37.Rantala H, Uhari M. Occurrence of childhood encephalitis: a population-based study. Paediatric Infectious Disease Journal 1989; 8: 426–430. [PubMed] [Google Scholar]
- 38.Chadha M, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. American Journal of Tropical Medicine and Hygiene 2005; 73: 566–570. [PubMed] [Google Scholar]
- 39.Gaurav YK, et al. Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian Journal of Medical Research 2010; 132: 395–399. [PubMed] [Google Scholar]
- 40.Orren A, et al. Increased susceptibility to herpes simplex virus infections in children with acute measles. Infection and Immunity 1981; 31: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon T, et al. Neurological manifestations of dengue infection. Lancet 2000; 355: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 42.Cam BV, et al. Prospective case control study of encephalopathy in children with dengue hemorrhagic fever. American Journal of Tropical Medicine and Hygiene 2001; 65: 848–851. [DOI] [PubMed] [Google Scholar]
- 43.Kumar R, et al. Dengue encephalopathy in children in Northern India: clinical features and comparison with non dengue. Journal of Neurological Sciences 2008; 269: 41–48. [DOI] [PubMed] [Google Scholar]
- 44.Girija AS, Rafeeque M, Abdurehman KP. Neurological complications of chickenpox. Annals of Indian Academy of Neurology 2007; 10: 240–246. [Google Scholar]
- 45.Zuliani L, et al. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. Journal of Neurology, Neurosurgery and Psychiatry 2012; 83: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranjit MR. The epidemiology of malaria in Orissa. ICMR Bulletin 2006; 36: 29–38. [Google Scholar]
- 47.Golassa L, et al. Detection of substantial number of submicroscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malaria Journal 2013; 12: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]