SUMMARY
Q fever is a notifiable disease in Germany. The majority of the reported cases are related to outbreaks. The objective of our study was to evaluate the general role of Q fever in community-acquired pneumonia (CAP). We investigated respiratory samples and sera from 255 patients with CAP, who were enrolled into a CAPNETZ cohort in summer 2005. Altogether, our data showed a significant prevalence of Q fever as CAP (3·5%). If a patient's condition leads to a diagnostic test for Chlamydophila sp., Mycoplasma sp. or Legionella sp., then a Q fever diagnostic test should also be included. In particular, ELISA as a first diagnostic step is easy to perform. PCR should be performed at an early stage of the disease if no antibodies are detectable. Because of our highly promising findings we suggest performing PCR in respiratory samples.
Key words: Community-acquired pneumonia, Coxiella burnetii, endocarditis, pneumonia, Q fever, zoonoses
INTRODUCTION
Outbreaks of acute debilitating influenza-like illness are a common appearance in Q fever. Between 80 and 400 cases per year are reported in Germany, with 40–80% of these related to outbreaks [1, 2]. Based on the unspecific symptoms of Q fever and infrequent use of advanced diagnostic techniques, sporadic cases are often missed. In a retrospective study, 12 (0·76%) out of 1569 acute Q fever patients subsequently developed chronic infection [3]. Chronic Q fever mainly presents as infectious endocarditis or vascular infection with high mortality [4].
Q fever is a worldwide zoonosis caused by Coxiella burnetii, a small Gram-negative intracellular coccobacillus. The common transmission route is inhalation of infected aerosolized particles carried over distances of up to 2–5 km or by direct contact with birth products of infected ruminants [5]. A peculiarity utilized in the testing of Q fever is the differentiation between specific antibodies against the complete lipopolysaccharide (LPS), termed phase I (Ph 1) and the truncated form, termed phase II (Ph 2). Acute Q fever is serologically confirmed by antibodies against Ph 2 LPS and chronic infections by antibodies against Ph 1 LPS [6]. Several commercial and in-house polymerase chain reactions (PCRs) have been established to close the diagnostic gap of 1–3 weeks between onset of clinical illness and detectable antibodies in sera in recent years [7]. Sera, due to its easy availability, are mainly investigated.
In 2001 the German network for community-acquired pneumonia (CAPNETZ) was implemented. Patients with community-acquired pneumonia (CAP), treated as outpatients or inpatients were enrolled at eight different sites in Germany [8]. Acute-phase serum, urine, blood culture and respiratory samples were collected at the time of enrolment. All samples underwent a standardized extensive microbiological work-up (for details see [8]). However, testing for C. burnetii by PCR or serology was not included in this work-up. Medical history, clinical data and results of the microbiological investigations were collected in one common database. This compilation gave us the opportunity to evaluate the role of Q fever in CAP. As the majority of reported Q fever cases in Germany occur during the warm season, we investigated cases enrolled between May and September in 2005.
METHODS
We investigated the respiratory samples and sera from all patients included in the CAPNETZ study between May and September 2005. Inclusion criteria of the CAPNETZ study are age >18 years, a pathological chest X-ray, fever and one of the following symptoms: cough, purulent sputum or pathological sounds on auscultation. All samples were frozen at –80°C after sampling and transported to the Central Service Unit of the CAPNETZ study. From there the materials were transported to our institute on dry ice. As the specimens were first investigated for other respiratory pathogens a prompt DNA extraction from the respiratory samples was performed and together with the serum stored at –80°C. We maintained the specimens at –25°C during our investigation.
We screened all sera by an enzyme-linked immunosorbent assay (ELISA; Virion/Serion, Germany). The cut-off value for ELISA was calculated on the basis of the standard curve corrected by the mean of the extinction of the standard serum according to the manufacturer's instructions. Quantitative analysis was only evaluated for Ph 2 IgG antibodies and was measured in U/ml. A result >30 U/ml was considered positive (equivocal: 20–30 U/ml). Positive and borderline ELISA results were verified by an indirect immunofluorescence antibody test (IFAT; BIOS/Focus, USA). For detection of IgM antibodies, sera were pretreated with GullSORB and tested at dilutions of 1:10, 1:40, 1:80 and 1:160. A titre of 1:80 for Ph2 IgM antibodies with or without a Ph2 IgG antibody titre of ⩾1:64 was considered positive.
All sera and respiratory samples were further investigated by nested PCR (nPCR) according to Fenollar et al. [9]. The PCR samples were analysed using gel electrophoresis. Species confirmation was performed by Sanger sequencing. Before starting sequencing, PCR amplicons were extracted from agarose gel by using the DNA Agarose Gel Extraction kit (Jena Bioscience, Germany). Next, 5 pmol primer and the Big Dye Termination Cycle Sequencing kit (Applied Biosystems, USA) were used for sequencing reactions in a profile of 25 cycles, including 5 s at 95°C, 10 s at 58°C and 1 min at 60°C. A precipitation with 1·5 m sodium acetate/0·125 m EDTA (pH 8), was used to remove unincorporated nucleotides. Sequencing was performed by using the automatic sequencer ABI Prism® 3130 (Applied Biosystems). All positive nPCR results were additionally tested by real-time PCR (rtPCR) (Adiagene AES, bioMérieux, France) using Light Cycler® 2·0 (Roche, Switzerland) technology.
For the patients with positive results by rtPCR and nPCR, or with antibody constellation of an acute Q fever, the clinical picture, duration of illness, laboratory results, results of additional microbiological investigations, antibiotic treatment and follow-up data were collected from the database.
RESULTS
Our study group comprised 255 patients with CAP [131 (51%) men, 124 (49%) women]. The data and specimen collection occurred in eight different CAPNETZ centres, most located in the north and central parts of Germany with moderate risk for Q fever and one (Ulm) in the high endemic area of Germany.
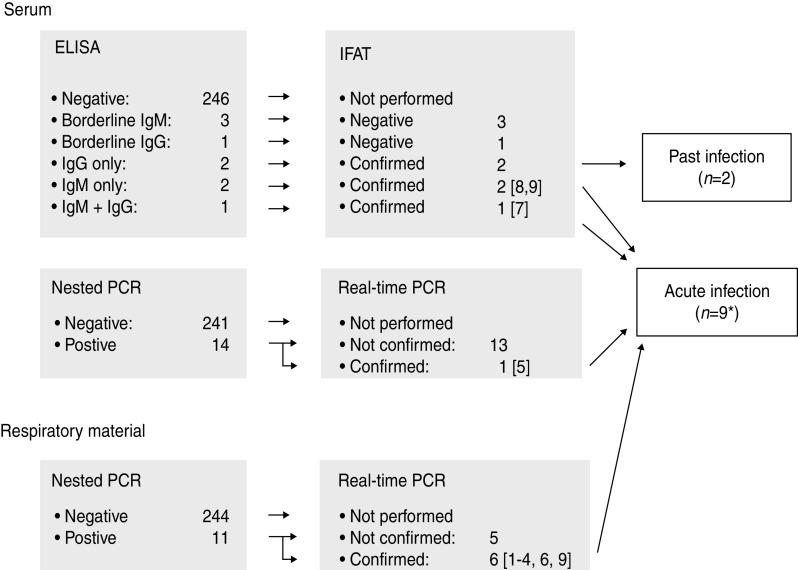
The screening of the sera using ELISA revealed a positive result in five and a borderline result for C. burnetii-specific antibodies in four cases. The positive results included a detection of solitary Ph 2 IgM antibodies (n = 2), a combination of Ph 2 IgM and IgG antibodies (n = 1) and Ph 2 IgG antibodies alone (past infection) (n = 2). All positive results, but none of the borderline results by ELISA, could be confirmed using the IFAT (Fig. 1).
Fig. 1.
Flow chart of the diagnostic procedures. * IgM antibodies and a positive PCR result in the respiratory sample were found together in one patient.
Sera as well as respiratory samples of all 255 patients were investigated by PCR. The nPCR revealed an amplification product in 10 sera and in 11 respiratory samples. All amplicons were confirmed by sequencing. Real-time PCR revealed a positive PCR product in 1/10 positive sera by nPCR and in 6/10 respiratory samples positive by nPCR. Altogether three patients presented an antibody constellation of an acute Q fever, one together with a positive PCR result in the respiratory sample. C. burnetii was found by both nPCR and rtPCR in one serum and six respiratory samples (Fig. 1).
These nine cases of acute C. burnetii infection correspond to 3·5% of the investigated patients and are summarized in Table 1. Including the positive results by nPCR alone we detected C. burnetii infection in 7·8% (20/255) of cases. In two patients the nPCR revealed a positive result in both serum and respiratory samples. Those results were considered non-relevant because they could not be confirmed by rtPCR. In 3/9 cases summarized in Table 1 an extra pathogen was identified. All specimens of patients diagnosed with acute Q fever were collected during the first week of illness by two of the eight centres involved (Berlin and Ulm). In Ulm (4/44) and in Berlin (5/95) investigated cases were positive. Five of the nine cases had to be treated as inpatients. Empirical antibiotic treatment covered C. burnetii in 6/9 cases (case nos. 2, 3, 6–9). The remaining three patients also recovered from pneumonia.
Table 1.
Cases with C. burnetii infection
| Case no. | Age (yr) | Location | Day of sampling* | History, symptoms | Inpatient or outpatient | CRP (mg/l) | WBC (/μl) | Co-infection | Antibiotics |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Ulm | 1 | COPD, dyspnoea, pleurodynia | Inpatient | 18·2 | 13 500 | None | Aminopenicillin + β-lactamase inhibitor i.v. |
| 2 | 44 | Ulm | 7 | Cough, dyspnoea | Outpatient | 5·6 | 7200 | M. pneumoniae† | Levofloxacin |
| 3 | 60 | Berlin | 1 | Cough, pleurodynia | Inpatient | 352 | 24 400 | None | Azithromycin + third-generation cephalosporin |
| 4 | 77 | Berlin | 8 | COPD, dyspnoea | Inpatient | 216·6 | 33 230 | S. pneumoniae‡ | Aminopenicillin + β-lactamase inhibitor i.v. |
| 5 | 31 | Ulm | 4 | Cough | Inpatient | 175·3 | 6300 | None | Aminopenicillin + β-lactamase inhibitor i.v. |
| 6 | 20 | Ulm | 5 | Cough, dyspnoea | Outpatient | 22·9 | 7000 | M. pneumoniae§ | Ketolides |
| 7 | 39 | Berlin | 7 | Cough, dyspnoea | Outpatient | 33·9 | 7500 | None | Clarithromycin |
| 8 | 47 | Berlin | 2 | COPD, dyspnoea, pleurodynia | Outpatient | 205 | 9200 | None | Moxifloxacin |
| 9 | 44 | Berlin | 2 | Cough, dyspnoea, pleurodynia | Inpatient | 267·9 | 5570 | None | Aminopenicillin + β-lactamase inhibitor i.v. + clarithromycin |
CRP, C-reactive protein; WBC, white blood cell count; COPD, chronic obstructive pulmonary disease.
Duration between onset of clinical symptoms and collection of specimen.
Detected by PCR in respiratory sample.
106 c.f.u./ml in respiratory sample.
IgM detection by ELISA and immunoblot.
DISCUSSION
In the present study we found evidence of C. burnetii infection by PCR and/or antibody detection in 3·5% of patients with CAP acquired in the warm season. The detection rate would have been 7·8% if the results by nPCR, and not confirmed by rtPCR, had been taken into account.
However, the positive results found solely by nPCR are difficult to evaluate. They could be caused by a higher sensitivity of nPCR compared to rtPCR. This assumption is supported by our finding of positive nPCRs in the corresponding specimens of two patients. Furthermore, repeated melting and freezing of samples could have led to the degradation of DNA. In order to account for possible cross-contamination only positive results of nPCR, confirmed by rtPCR, were defined as truly positive (Fig. 1).
Nevertheless, our results are in the range of existing prevalence data of acute Q fever in CAP patients found in Israel (5·8%) and Japan (2·5%) [10, 11]. A higher rate (18·5%) was found for the Basque Country [12].
These results classify C. burnetii equal to other respiratory pathogens, e.g. Legionella spp. and are even higher than the annual rate found for C. pneumoniae (0·9%) in the CAPNETZ cohort [13]. Unfortunately, awareness of Q fever is much lower. Late antibody appearance, widespread use of antibody detection tests with low sensitivity, e.g. complement fixation reaction, as well as the often self-limiting course of the disease may account for this observation. The improvement of diagnostic tools for Q fever, e.g. PCR for investigation of serum, and commercially available ELISAs provide new methods with sufficient sensitivity.
From our data and the study by Takahashi et al. [10], respiratory samples appear to be promising specimens for detection of C. burnetii in the first week after onset of the disease. In both studies PCR revealed more positive results in respiratory samples than in serum. However, in three cases with C. burnetii detection in the respiratory sample a concomitant pathogen (M. pneumonia detected by PCR once and by IgM antibody detection once, as well as S. pneumoniae isolated with 106 c.f.u./ml sputum) was identified. In these cases, the clinical significance of C. burnetii is debatable and may reflect co-infection or super infection of a carrier.
All identified cases were located in two centres, Berlin and Ulm. In Berlin we found 5/95 (5·2%) investigated cases and in Ulm 4/44 (8·9%). The area surrounding Ulm is known for a high prevalence of Q fever, which corresponds with our results. However, the city of Berlin is not considered an endemic area with only a few sporadic cases per year [2].
Doxycycline is the most effective treatment for acute Q fever. Other antibiotics that can be used are moxifloxacin, clarithromycin, trimethoprim/sulfamethoxazole, and rifampin [14]. More than half of our cases were treated with an antibiotic therapy for C. burnetii (Table 1). Due to the self-limiting character of acute Q fever inappropriate treatment may not have an impact on short time of recovery from pneumonia but increases the risk for chronic infection.
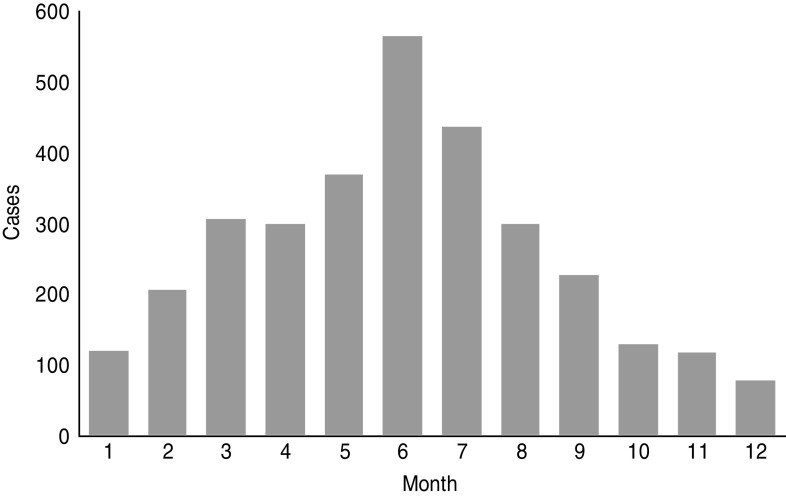
Reported cases suggest a lower incidence of Q fever in winter with half the number found in summer (Fig. 2). Because of this seasonality and the higher incidence of CAP in winter, the annual rate of Q fever in CAP should be less than the 3·5% found in our study. According to cumulative data from CAPNETZ comprising more than 10 000 patients aged >10 years, only 30–35% of all patients were enrolled between May and September. Therefore the overall proportion of C. burnetii in CAP per year is 2%. According to German guidelines, patients with hospitalized CAP receive an empirical treatment for C. burnetii (β-lactamase with or without macrolide or fluoroquinolone) [15]. However, the recommended first-line treatment for outpatients is amoxicillin [15], and at least half of the CAP patients are treated as outpatients [16]. Even if half of the Q fever pneumonia cases are adequately treated – which probably reduces the risk for chronic disease – a large portion of Q fever without pneumonia exists and may develop as chronic disease, most often manifesting as endocarditis.
Fig. 2.
Monthly distribution of reported Q fever cases (acute) 2001–2013 (Robert Koch-Institute: SurvStat, http://www3.rki.de/SurvStat, deadline 5 July 2013).
A main limitation of the present study is the lack of convalescent sera. The combination of PCR and antibody detection reveals only a sensitivity of 77% for investigation of the first serum sample [17]. Based on our data, additional investigation of respiratory samples leads to a higher detection rate.
ACKNOWLEDGEMENTS
This work was supported by the Federal Ministry of Education and Research Germany (K.B., D.F., E.S., grant no, 01 KI 0735), (K.B., D.F., E.S., M.W.P., grant no. 01 KI 1001C), (M.W.P., G.U.R., grant no. 01KI07145 2001–2011), (M.W.P., grant no. 01 KI 1204). We thank Juliana Schrimpf for technical support in the laboratory.
APPENDIX. Members of the CAPNETZ Study Group (except the authors)
S. Krüger, D. Frechen (Aachen); W. Knüppel, I. Armari (Bad Arolsen); D. Stolz (Basel); N. Suttorp, H. Schütte, A. Tessmer, P. Martus (Berlin, Charité); T. Bauer, J. Hecht (Berlin); W. Pankow, A. Lies, D. Thiemig (Berlin-Neukölln); B. Hauptmeier, S. Ewig, D. Wehde, M. Suermann (Bochum); M. Prediger, G. Zernia (Cottbus); J. Rademacher, G. Barten, L. Gosman, W. Kröner (Hannover); R. Bals (Homburg/Saar); C. Kroegel (Jena); K. Dalhoff, S. Schütz, R. Hörster, (Lübeck); W. Petermann, H. Buschmann, R. Kröning, Y. Aydin (Paderborn); T. Schaberg, I. Hering (Rotenburg/Wümme); R. Marre, C. Schumann (Ulm); H. von Baum (Ulm, Med. Microbiology); T. Illmann, M. Wallner (Ulm); O. Burghuber, G. Rainer (Wien) and all study nurses.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Anon. Q fever: increased incidence in spring 2008 [in German]. Epidemiologisches Bulletin 2008; 25: 5. [Google Scholar]
- 2.SurvStat. (http://www3.rki.de/SurvStat). Accessed 3 July 2013.
- 3.Fenollar F, et al. Risks factors and prevention of Q fever endocarditis. Clinical Infectious Disease 2001; 33: 312–316. [DOI] [PubMed] [Google Scholar]
- 4.Botelho-Nevers E, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. European Journal of Clinical Microbiology and Infectious Diseases 2007; 26: 635–640. [DOI] [PubMed] [Google Scholar]
- 5.Maurin M, Raoult D. Q fever. Clinical Microbiology Reviews 1999; 12: 518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peacock MG, et al. Serological evaluation of Q fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infection and Immunity 1983; 41: 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneeberger PM, et al. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clinical and Vaccine Immunology 2010; 17: 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welte T, Suttorp N, Marre R. CAPNETZ community-acquired pneumonia competence network. Infection 2004; 32: 234–238. [DOI] [PubMed] [Google Scholar]
- 9.Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. Journal of Clinical Microbiology 2004; 42: 4919–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi H, et al. Prevalence of community-acquired respiratory tract infections associated with Q fever in Japan. Diagnostic Microbiology and Infectious Disease 2004; 48: 247–252. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman D, et al. Q-fever pneumonia in the Negev region of Israel: a review of 20 patients hospitalised over a period of one year. Journal of infection 1995; 30: 135–140. [DOI] [PubMed] [Google Scholar]
- 12.Capelastegui A, et al. Etiology of community-acquired pneumonia in a population-based study: link between etiology and patients characteristics, process-of-care, clinical evolution and outcomes. BMC Infectious Diseases 2012; 12: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellinghausen N, et al. Low prevalence of Chlamydia pneumoniae in adults with community-acquired pneumonia. International Journal of Medical Microbiology 2006; 296: 485–491. [DOI] [PubMed] [Google Scholar]
- 14.Anderson A, et al. Diagnosis and management of Q fever – United States, 2013: recommendations from CDC and the Q Fever Working Group. Morbidity and Mortality Weekly Report 2013; 62: 1–30. [PubMed] [Google Scholar]
- 15.Hoffken G, et al. Guidelines of the Paul-Ehrlich-Society of Chemotherapy, the German Respiratory Diseases Society, the German Infectious Diseases Society and of the Competence Network CAPNETZ for the Management of Lower Respiratory Tract Infections and Community-acquired Pneumonia. Pneumologie 2010; 64: 149–154. [DOI] [PubMed] [Google Scholar]
- 16.Schnoor M, et al. Approaches to estimate the population-based incidence of community acquired pneumonia. Journal of infection 2007; 55: 233–239. [DOI] [PubMed] [Google Scholar]
- 17.Boden K, et al. Diagnosis of acute Q fever with emphasis on enzyme-linked immunosorbent assay and nested polymerase chain reaction regarding the time of serum collection. Diagnostic Microbiology and Infectious Disease 2010; 68: 110–116. [DOI] [PubMed] [Google Scholar]