SUMMARY
Socio-behavioural factors and pathogens associated with childhood diarrhoea are of global public health concern. Our survey in 696 children aged ⩽2 years in rural West Bengal detected rotavirus as sole pathogen in 8% (17/199) of diarrhoeic stool specimens. Other organisms were detected along with rotavirus in 11% of faecal specimens. A third of the children with rotavirus diarrhoea, according to Vesikari score, had severe illness. The top four rotavirus genotypes were G9P[4] (28%), G1P[8] (19%), G2P[4] (14%) and G8P[4] (8%). In the multivariate model, the practice of ‘drawing drinking water by dipping a pot in the storage vessel’ [adjusted odds ratio (aOR) 2·21, 95% confidence interval (CI) 1·03–4·74, P = 0·041], and ‘children aged ⩽6 months with non-exclusive breastfeeding’ (aOR 2·07, 95% CI 1·1–3·82, P = 0·024) had twice the odds of having diarrhoea. Incidence of rotavirus diarrhoea was 24/100 child-years in children aged >6–18 months, 19/100 child-years in children aged >18–24 months and 5/100 child-years in those aged ⩽6 months. Results have translational implications for future interventions including vaccine development.
Key words: Diarrhoea, enteric bacteria, helminths (worms), infectious disease, rotavirus
INTRODUCTION
Diarrhoea in young children and associated mortality continue to pose challenges to public health professionals throughout the world. However, distribution of diarrhoeal illness is not uniform and is disparately high in some settings in Asian and African countries. It is estimated that diarrhoea is responsible for the death of about 6–7 children aged <5 years every 5 minutes in the World Health Organization's (WHO) South-east Asia region [1]. Conducting epidemiological studies, to help understand the determinants of these illnesses in specific contexts and develop interventions, is therefore paramount.
The present study on diarrhoea was conducted against this backdrop from rural West Bengal – one of the eastern Indian states. It is important to note that in South and South-east Asian countries, India, Bangladesh, Indonesia, Myanmar and Nepal face similar challenges in controlling diarrhoeal diseases [2]. Specifically, rotavirus causes an estimated 122 000–153 000 deaths, 457 000–884 000 hospitalizations and 2 million outpatient visits in children aged <5 years in India annually. Moreover, the country spends Rs 2–3·4 billion (US$ 41–72 million) in medical costs with regard to treatment [3]. Factoring in the cost of illness for all-cause diarrhoea would clearly escalate these figures. Generating ground-level information on determinants of diarrhoea and circulating strains of rotavirus in particular in the face of this huge public health expenditure formed the basis of our investigation. As observations have suggested that co-infection with bacteria and viruses could decrease the immune response to live viral vaccines [4], identifying gut pathogens with or without rotavirus in diarrhoeic stool specimens of children constituted another objective of the current study. Such information might help to explain the results of future rotavirus vaccine initiatives.
We located our study in the district of South-24 Parganas of West Bengal and covered 22 villages (gram) catered by four sub-centres under Sonarpur Block. In India, blocks represent planning and development units of a district and a group of local village administrative bodies (locally called gram panchayats) fall under the jurisdiction of a block. A group of small villages or if a village is large – only one village – is administered by a gram panchayat – which is democratically elected by villagers and allows villagers to take part in local level decision making. On the other hand, a sub-centre is the most peripheral outpost of the Indian healthcare system. One sub-centre usually caters to the needs of about 5000 people in plain land and about 3000 people in hilly, tribal or backward areas.
Our study location had 27 613 individuals (adults and children) spread over a 9·2 km2 area; children aged ⩽2 years were the focus. The study was conducted during December 2010 to May 2011. Lack of recent information on determinants of diarrhoea in children of rural West Bengal with a special emphasis on rotavirus genotype distribution was addressed by the current investigation.
METHODS
Information collected from study participants
Following the setting up of field offices and completion of training of the project staff, regular home visits were made commencing on 21 January 2011. Each household was visited daily by the assigned field workers during the study. Occurrences of diarrhoea (if any) in children and its severity were recorded during these visits. Passage of ⩾3 loose stools in 24 h (recorded on a diary card) was considered as diarrhoea in the present study during analyses. However, if a mother of an exclusively breastfed child observed the passage of stools more frequently than usual (and/or recent change in consistency) [5], she was encouraged to seek clinical advice. Obtaining such medical assistance was facilitated through the establishment of a network with the local government healthcare system by the project field workers and field attendants. Information was collected from each eligible family within the study area by interviewer-administered questionnaire as well as direct observation (for variables as applicable) on sociodemographic profile, economic condition, existing practices regarding hand washing by adults, rubbish disposal, source of drinking water, its storage and usage as well as child feeding practices, etc.
Six hundred and seventy-seven families had 701 children aged ⩽2 years and were thus eligible. Of these, five families, each having one eligible child, declined to participate and were excluded from the study. Ten children were born in the first month after home visits started, 12 in the second, seven in the third and one in the last month. All of these newborns contributed person-time to the cohort and were followed until the end of the study period. During this 4-month period from 21 January to 21 May 2011, 79 children in the total cohort attained the age of 2 years and were not followed up further under the current study. Data thus obtained on 696 children from 672 families were used in the analysis. Approval from the institutional ethics and scientific advisory committee of the National Institute of Cholera and Enteric Diseases (NICED), Indian Council of Medical Research (ICMR) was obtained before initiation of the study.
Specimen collection, transportation and processing
Each eligible family was supplied with a sterile screw-capped wide-mouthed plastic container and plastic spoon for collection of stool specimen from children if they had diarrhoea. Labels on these containers allowed the marking of identification codes and date of collection. Each stool specimen was transported to the NICED laboratory for investigation on the same day as collection. Each of the eligible families having diarrhoeic children received feedback on laboratory investigation results. We used different assay techniques such as culturing bacteria, enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR). While parasitic pathogens were detected by ELISA, some of the viral pathogens were detected by PCR technique. Detailed information on these methods is given elsewhere [6].
Parasitological tests
In order to detect enteric parasites, faecal specimens were processed separately for microscopic and antigen detection. A highly sensitive antigen capture ELISA system (ELISA, Tech Lab, USA) was used for the detection of Giardia lamblia, Cryptosporidium parvum and Entamoeba histolytica.
Bacteriological methods
PCR targeting outer membrane protein (ompW) and a trans-membrane transcriptional activator protein (toxR) was performed for species confirmation of Vibrio cholerae and V. fluvialis, respectively [7, 8]. V. cholerae strains were serotyped using antisera prepared at NICED. Confirmed strains of V. parahaemolyticus, Shigella species and Salmonella species were serotyped using commercially available antisera (Denka Seiken, Japan; Bio-Rad, France, respectively).
Three different lactose-fermenting colonies were picked up from MacConkey agar plate and included in the multiplex PCR assay for detection of different diarrhoeagenic Escherichia coli that included enterotoxigenic E. coli (ETEC inclusive of both heat-labile and heat-stable enterotoxin producers), enteropathogenic E. coli (typical and atypical EPEC) and enteroaggregative E. coli (EAEC) [9]. Simplex PCR was performed for the detection of enteroinvasive E. coli (EIEC) and Shiga-toxin producing E. coli (STEC) [10, 11].
Virology investigation
All stool specimens were processed and the presence of human group A rotavirus antigen was confirmed by means of rotavirus solid-phase sandwich-type enzyme immunoassay (IDEIA™, Thermo Fisher, USA) according to the manufacturer's instructions. In addition viral RNA–polyacrylamide gel electrophoresis (RNA–PAGE) was performed to determine the electropherotype (long/short) of the untypable group A rotavirus strains.
Rotavirus RNA was extracted with a commercially available RNA extraction kit (QIAamp viral RNA Mini kit; Qiagen GmbH, Germany) according to the manufacturer's instructions. Reverse transcriptase–PCR (RT–PCR) was used to perform analysis of G and P genotypes on the basis of previously determined conditions. Multiplex semi-nested PCR-based G and P genotyping was conducted with several sets of G and P genotype-specific primer pairs [12]. The PCR products of VP7 and VP8 genes were purified with QIAquick PCR purification kit (Qiagen GmbH, Germany) and sequenced by using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, USA) and Big Dye Terminator Cycle Sequencing kit v. 3.1, according to the manufacturer's protocol.
Norovirus groups I and II, sapovirus and astrovirus were detected by RT–PCR using random primers for reverse transcription and specific primers for PCR. Different viruses were detected according to the appropriate amplicon sizes observed in agarose gel stained with ethidium bromide [13, 14]. Adenovirus was detected by the commercially available Rota-Adeno VIKIA kit (bioMérieux, France), which is a qualitative test based on immunochromatography in lateral flow format.
Data analysis
Information collected on several variables such as socio-demographic profile, economic status, education level of parents, hygienic practices, water use and feeding of children were examined to find if any of these were associated with occurrence of diarrhoea in children. A child with first diarrhoeal episode was considered as study outcome during such analyses. Factors thus identified as associated with diarrhoea and having biological plausibility (P⩽0·1), were used to build a multivariate logistic regression model. We maintained during analyses that the confidence interval would indicate the likely range of values for an association of independent variable with the study outcome in the population and P value would determine how likely it was that the observed association in the specimen was due to chance. As confidence interval and P value thus respectively provided quantitative and qualitative measure of association, we generated both these measures of uncertainty and presented them with results [15]. Microsoft Access 2007 (Microsoft Corp., USA) was used for data entry and creation of a database and SPSS v. 16 (IBM SPSS Inc., USA) was used for data analysis.
In order to calculate annualized incidence, the sum of child-years at-risk contributed by each eligible child in the study was used as denominator. Contribution of each child was calculated by subtracting the starting date of home visit or date of birth of a child (if the child was born during the study) from the end date of study or date of exit of a child (whichever occurred earlier). As a diarrhoeic child cannot be at risk of diarrhoea during the days s/he was actually suffering from it, the total number of days suffered at the time of such episodes was subtracted from the total observation time.
RESULTS
Profile of children suffering from diarrhoea
About one third (213/696, 30%) of the study children suffered from diarrhoea (125 males, 88 females). Fever was one of the presenting symptoms in 45% (95/213) of cases and a lesser proportion had vomiting (61/213, 29%). Fifteen percent of children (32/213) suffering from diarrhoea had accompanying skin rash. Parents of 6% (12/213) of children complained of the stool being tinged with blood. We compared the maximum number of stools passed within any 24-h period during diarrhoeal episodes between children who were exclusively breastfed and those who were not exclusively breastfed; no significant difference was observed between these groups – the former having a mean frequency of 5·5 (range 4, s.d. ± 1·5) and the latter being 5·4 (range 13, s.d. ± 2·4; 95% CI −0·6 to 0·8, P = 0·79). Examining this issue restricted only to children aged ⩽6 months similarly did not reveal any significant difference.
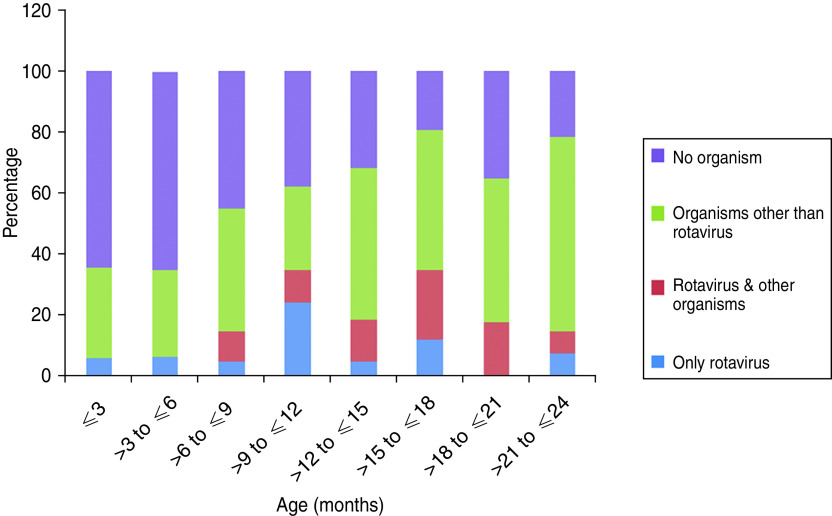
We were able to collect stool specimens from 199/213 cases. Out of the total 199 stool specimens examined, no organism could be detected from 83 (42%) specimens. Rotavirus was detected from 37 (19%) specimens, viruses other than rotavirus were detected from 33 (16%) and no virus was detected from 46 (23%) specimens. Figure 1 provides the age group distribution of data on detection of organisms from diarrhoeic stool specimens. Ova of helminths were detected by light microscopy in about 8% of diarrhoeic stool specimens (15/199) in which Hymenolepis nana (7/199) and Ascaris lumbricoides (7/199) contributed the most. Ova of Trichuris trichura was detected in only one case.
Fig. 1.
Distribution of organisms in diarrhoea cases in different age groups.
Rotavirus diarrhoea in children with and without co-infection
In 8% (17/199) of stool specimens, rotavirus was detected as the sole pathogen and presence of other organisms along with rotavirus was detected in one tenth (20/199) of the specimens; in total rotavirus was identified in 19% (37/199) of cases. Based on Vesikari score [16], one third of the children with rotavirus diarrhoea had severe illness. It is worth noting that in Vesikari's original work on the development of a scoring system for rotavirus diarrhoea severity, a frequency of ⩾3 loose stools in a 24-h period was used to define a case of diarrhoea in a cohort of newborn children.
While some of the specimens showed the presence of only other viruses along with rotavirus, in others, various combinations of viruses, bacteria as well as parasites, were found. In 2/20 specimens where rotavirus had co-infection, astrovirus and norovirus (G2) were present – one in each. In four other stool specimens rotavirus was associated with sapovirus in two (one also had ETEC and another E. histolytica) and adenovirus (along with Cryptosporidium) in one and norovirus (G1) in another (along with ETEC and G. lamblia).
No other virus along with rotavirus was identified in 14 specimens (14/20, 70%) where co-infections comprised of Campylobacter lari, C. jejuni, Cryptosporidium sp., E. histolytica, G. lamblia, H. nana and EPEC in different combinations.
Detection of viruses other than rotavirus
In 33/199 (16%) stool specimens, viruses other than rotavirus were detected. Non-rotavirus single viral pathogen was present in 8% (17/199) of stool specimens where sapovirus and adenovirus independently featured as the commonly found viruses (6/17, 35% for each). The next most common virus detected in this group was norovirus (4/17, 23%). In one case only astrovirus was present.
When co-infections present with viruses other than rotavirus in diarrhoeic stool specimens were examined, sapovirus was present in 4% (9/199) and was associated with astrovirus in four cases, G. lamblia in two, Salmonella 09 in one and helminths in the remaining two cases (one with H. nana and the other with A. lumbricoides). Norovirus, on the other hand, when present with co-infection was detected along with Cryptosporidium, ETEC or G. lamblia.
Organism profile in diarrhoeic stool specimens where viruses were not detected
In about a quarter of diarrhoeic stool specimens (46/199, 23%), viruses were not present and the most commonly detected in this group was G. lamblia (13/46, 28%) of which 10 specimens had G. lamblia as the sole pathogen. The next most common organism was EAEC (9/46, 19%) of which seven specimens had EAEC as the sole pathogen. When co-infections were present, EPEC and Cryptosporidium sp. co-featured with G. lamblia and EAEC.
Diarrhoea incidence and rotavirus strains
Incidence of diarrhoea by age is presented in Table 1. In children aged ⩽6 months, the incidence rate of rotavirus diarrhoea was 5/100 child-years (95% CI 1·3–12); in the >6 months to ⩽12 months age group incidence was 24/100 child-years (95% CI 13·8–41·4); in children aged >12 months to ⩽18 months the rate was 24/100 child-years (95% CI 14–36) and in the >18 months to ⩽24 months age group the incidence rate of rotavirus diarrhoea was 19/100 child-years (95% CI 7·5–39). Incidence of all-cause diarrhoea was highest in children aged >6 months to ⩽12 months.
Table 1.
Diarrhoea incidence rate in different age groups
| Age group (months) | Disease | No. of children* | No. of cases detected | Child-years contributed | Incidence/100 child-years (95% CI) |
|---|---|---|---|---|---|
| ⩽6 | All-cause diarrhoea | 205 | 65 | 63·1 | 102 (80·1–130·3) |
| Rotavirus diarrhoea | 203 | 3 | 63·0 | 5 (1·3–12·7) | |
| >6 to ⩽12 | All-cause diarrhoea | 159 | 59 | 51·7 | 114 (87·6–145·9) |
| Rotavirus diarrhoea | 157 | 13 | 51·4 | 25 (14·1–41·9) | |
| >12 to ⩽18 | All-cause diarrhoea | 193 | 54 | 63·0 | 86 (65·0–110·8) |
| Rotavirus diarrhoea | 188 | 15 | 61·7 | 24 (14·2–39·1) | |
| >18 to ⩽24 | All-cause diarrhoea | 139 | 35 | 32·1 | 109 (77·2–149·8) |
| Rotavirus diarrhoea | 134 | 6 | 30·8 | 19 (8·1–40·1) |
CI, Confidence interval.
While clinical confirmation sufficed for diarrhoea cases, rotavirus diarrhoea was confirmed by laboratory tests. As stool specimens could not be collected from all diarrhoea cases, the number of children in the same age group varied between calculation for diarrhoea incidence and that of rotavirus diarrhoea incidence.
G and P typing of rotavirus were undertaken on 36/37 stool specimens identifying presence of rotavirus. In decreasing proportion the distributions of G type were G9 (13, 36%), G1 (12, 33%), G2 (5, 14%) and G8 (3, 8%). The presence of G12 (2, 6%) and G3 (1, 3%) was much less. In decreasing proportion the distributions of P types were P[4] (20, 56%), P[8] (10, 28%) and P[10] (1, 3%). In five specimens P could not be typed. The top four G and P combinations in rotavirus genotypes in 36 stool specimens were G9P[4] (10, 28%), G1P[8] (7, 19%), G2P[4] (5, 14%) and G8P[4] (3, 8%).
Univariate and multivariate analyses
Univariate association between key risk factors and diarrhoea (study outcome) in children is presented in Table 2. Neither education of family head, education of parents, or presence of pets in the family was significantly associated with occurrence of diarrhoea in children (P > 0·05). Economic status of the families from where children were recruited as reflected by presence of cultivable land and the construction of the houses they lived in (mud wall, brick wall or cemented) also did not have an association with the study outcome. We observed the rubbish disposal practices of the respondent families, asked them about the presence of flies in the household and also about hand-washing practices by adults before eating. None of these variables were associated with study outcome. Mothers reporting breastfeeding were asked if they cleaned their breasts before feeding their children and this practice did not also have any association with diarrhoea in children. Sources used by families to collect drinking water were ‘1000 ft deep tube wells’, ‘other tube wells which were not that deep’ and ‘pipe water’. About 65% of the families reported using ‘1000 ft deep tube wells’. Distribution of ‘drinking water sources’ used by families did not differ significantly between children who had diarrhoea compared to those who did not. In order to examine the relationship between measles vaccination status and diarrhoea, we separated 241 children who were aged <9 months and therefore did not fall under the purview of measles vaccination according to the Indian Immunization Schedule. Of the remainder (n = 455) no difference was observed in occurrence of diarrhoea between recipients (101/375, 27%) and non-recipients (27/80, 34%) of measles vaccine [odds ratio (OR) 1·38, 95% confidence interval (CI) 0·82–2·32, P = 0·22].
Table 2.
Comparison of determinants between diarrhoeic and non-diarrhoeic children
| Variables | Total | Diarrhoea, n (%) | OR (95% CI) | P |
|---|---|---|---|---|
| Gender of the child | ||||
| Male | 355 | 125 (35) | 1·56 (1·1–2·2) | 0·007 |
| Female (ref.) | 341 | 88 (26) | ||
| Age group (months) | ||||
| ⩽6 | 205 | 65 (32) | 1·38 (0·8–2·2) | 0·192 |
| >6 to ⩽12 | 159 | 59 (37) | 1·75 (1·1–2·2) | 0·028 |
| >12 to ⩽18 | 193 | 54 (28) | 1·15 (0·7–1·9) | 0·57 |
| >18 to ⩽24 (ref.) | 139 | 35 (25) | ||
| Education of child's mother | ||||
| Illiterate | 30 | 9 (30) | 1·04 (0·4–2·6) | 0·935 |
| Can read (non-formal education) | 16 | 4 (25) | 0·8 (0·2–2·27) | 0·732 |
| Primary | 104 | 31 (30) | 1·03 (0·5–1·9) | 0·928 |
| Middle | 322 | 95 (29) | 1·01 (0·6–1·7) | 0·958 |
| Secondary | 135 | 48 (36) | 1·34 (0·7–2·4) | 0·324 |
| >Secondary (ref.) | 89 | 26 (29) | ||
| Education of child's father | ||||
| Illiterate | 41 | 11 (27) | 0·87 (0·4–1·9) | 0·739 |
| Can read (non-formal education) | 17 | 9 (53) | 2·67 (0·9–7·6) | 0·068 |
| Primary | 140 | 39 (28) | 0·91 (0·5–1·6) | 0·766 |
| Middle | 310 | 94 (30) | 1·03 (0·6–1·7) | 0·905 |
| Secondary | 97 | 33 (34) | 1·22 (0·7–2·3) | 0·523 |
| >Secondary (ref.) | 91 | 27 (30) | ||
| Pets in the family | ||||
| Present | 222 | 69 (31) | 1·03 (0·7–1·5) | 0·852 |
| Absent (ref.) | 474 | 144 (30) | ||
| Source of drinking water for adults | ||||
| Water from other sources | 240 | 79 (33) | 1·18 (0·8–1·7) | 0·337 |
| 1000 ft deep tube well (ref.) | 456 | 134 (29) | ||
| Vessel used for storing water | ||||
| Wide mouthed | 309 | 115 (37) | 1·75 (1·2–2·4) | 0·001 |
| Narrow mouthed (ref.) | 387 | 98 (25) | ||
| Pot dipped in drinking water vessel | ||||
| Yes | 276 | 108 (39) | 1·93 (1·4–2·7) | 0·001 |
| No (ref.) | 420 | 105 (25) | ||
| Hand-wash after child ablution | ||||
| Ash | 66 | 16 (24) | 0·7 (0·4–1·3) | 0·279 |
| Earth | 81 | 22 (27) | 0·8 (0·5–1·4) | 0·512 |
| Only water | 101 | 37 (37) | 1·3 (0·8–2·0) | 0·257 |
| Soap (ref.) | 448 | 138 (31) | ||
| Breastfeeding in older children | ||||
| Age >6 months and non-exclusive | 487 | 146 (30) | 0·43 (0·6–3·06) | 0·399 |
| Age >6 months and exclusive (ref.) | 4 | 2 (50) | ||
| Breastfeeding in younger children | ||||
| Age ⩽6 months and non-exclusive | 115 | 44 (38) | 2·04 (1·1–3·7) | 0·024 |
| Age ⩽6 months and exclusive (ref.) | 90 | 21 (23) | ||
| Defecation place of the child | ||||
| Inside room | 401 | 133 (33) | 2·73 (0·6–12·5) | 0·196 |
| Courtyard | 166 | 41 (25) | 1·8 (0·4–8·5) | 0·455 |
| Verandah | 116 | 37 (32) | 2·58 (0·5–12·2) | 0·233 |
| Outside the room (ref.) | 13 | 2 (15) |
OR, Odds ratio; CI, confidence interval.
In univariate analysis, occurrence of diarrhoea was significantly higher (OR 1·56, 95% CI 1·1–2·2, P = 0·007) in male children (125/355, 35%) compared to females (88/341, 26%) and the >6 months to ⩽12 months age group (Table 2). The other significantly associated factors in univariate analysis were ‘type of vessel used for storing drinking water’ and ‘practice of dipping a pot to draw drinking water from the storage vessel’. Practices regarding storage of drinking water and the method employed to draw water from these vessels were directly observed during household visits. When we compared occurrence of diarrhoea between exclusively breastfed children (23/94, 25%) and children who were not exclusively breastfed (190/602, 32%), no significant difference was observed (OR 1·42, 95% CI 0·9–2·3, P = 0·167). However, we were interested in examining the issue in age disaggregated groups without using the same reference of exclusive breastfeeding for different age groups of children. Through this analysis younger children (aged ⩽6 months) were identified as being at increased risk of diarrhoea (Table 2) when not on exclusive breastfeeding. No such association existed for older children (>6 months).
In the multivariate model (Table 3) children within the >6 months to ⩽12 months age group had about double the odds [adjusted odds ratio (aOR) 1·73, 95% CI 1·04–2·9, P = 0·036] of having diarrhoea. Male children compared to females had increased risk of diarrhoea as well (aOR 1·66, 95% CI 1·19–2·33, P = 0·003). The ‘practice in the family of dipping a pot to draw drinking water from the storage vessel’ had double the odds of being associated with diarrhoea in children (aOR 2·28, 95% CI 1·05–4·92, P = 0·036). Children aged ⩽6 months had similar odds of having diarrhoea when put on ‘non-exclusive breastfeeding’ (aOR 2·07, 95% CI 1·1–3·82, P = 0·024).
Table 3.
Adjusted odds ratios for variables entered in the multivariate logistic regression model
| Variables | aOR | (95% CI) | P value |
|---|---|---|---|
| Gender of the child | |||
| Male | 1·66 | (1·19–2·33) | 0·003 |
| Age group (months) | |||
| ⩽6 | 0·39 | (0·05–3·22) | 0·383 |
| >6 to ⩽12 | 1·73 | (1·04–2·9) | 0·036 |
| >12 to ⩽18 | 1·16 | (0·7–1·93) | 0·559 |
| Practice at point of use | |||
| Pot dipped in the vessel storing drinking water | 2·21 | (1·03–4·74) | 0·041 |
| Wide-mouthed vessel used for storing drinking water | 0·91 | (0·43–1·94) | 0·81 |
| Breastfeeding in younger children | |||
| Age ⩽6 months and non-exclusive | 2·07 | (1·1–3·82) | 0·024 |
| Education of child's father | |||
| Illiterate | 0·82 | (0·35–1·91) | 0·642 |
| Can read (non-formal education) | 1·95 | (0·66–5·81) | 0·229 |
| Primary | 0·76 | (0·41–1·39) | 0·366 |
| Middle | 0·93 | (0·55–1·57) | 0·776 |
| Secondary | 1·05 | (0·56–1·99) | 0·875 |
aOR, Adjusted odds ratio; CI, confidence interval.
DISCUSSION
Our study has highlighted an important issue – the role of intervention at the household and individual level to prevent diarrhoea in children – where people's participation remains central. Practices around managing drinking water at home, specifically ‘dipping a pot to draw water from storage vessel’ had twice the odds of being associated with diarrhoea in children in the study setting. It is important to note that the source of drinking water used by families did not have such an association. This raises a concern that inappropriate health behaviour such as poor domestic water handling may offset the gains achieved through structural and environmental changes such as installation of deep tube wells or supply of safe pipe-water. In this context, it is important to take note of an experiment conducted in family contacts of cholera patients in the mid-1980s. The study showed that ‘use of narrow-necked earthenware vessel (locally known as sorai) for storing drinking water at home’ was effective in reducing transmission of V. cholerae in the urban slums of eastern Kolkata [17]. Several other studies have shown a similar association [18] and clinical trials focusing on intervention at ‘point of use of water’ have generated encouraging results [19].
In order to provide a safe water supply and adequate drainage facilities to the entire urban and rural population of the country, which in turn would impact on the occurrence of diarrhoeal diseases and other waterborne health hazards, the ‘National Water Supply and Sanitation Programme’ was initiated in India in 1954. About two decades later the ‘Accelerated Rural Water Supply Programme’ came into being in 1972. The latest boost in these initiatives came from the United Nations Millennium Declaration adopted in September 2000 where India was one of the signatories. ‘Goal 7’ under this declaration [20] aimed to halve the proportion of people without sustainable access to safe drinking water by 2015.
The present investigation further highlighted the importance of promoting an often discussed public health intervention – exclusive breastfeeding – for the prevention of diarrhoea in children. However, intervention around exclusive breastfeeding requires an innovative communication approach with the aim of changing the attitudes and practices of parents. Based on a systematic review, the WHO clearly states that infants who are exclusively breastfed for 6 months experience less morbidity from gastrointestinal infection than those who receive mixed breastfeeding for 3 or 4 months, and no deficits have been demonstrated regarding growth in infants from either developing or developed countries who were exclusively breastfed for 6 months [21]. We generated corroborating evidence by showing that the children in our study who were aged ⩽6 months and were not exclusively breastfed had increased risk of having diarrhoea. It was of concern that more than half (115/205, 56%) of the children in the study, who were aged ⩽6 months, were not on ‘exclusive breastfeeding’ (Table 2).
Although previous studies have discussed the role of measles vaccine in reducing diarrhoea in children [22, 23] we did not find such an association in our cohort. It should, however, be appreciated that every year some 7·6 million children die before reaching their fifth birthday, many of them during their first year of life. Of these, 70% die due to one of five causes: diarrhoea, pneumonia, measles, malaria, malnutrition – often in combination [24].
Apart from ‘diarrhoea in general’, our study investigated rotavirus diarrhoea due to its impact on the health of children as well as the associated cost implications. In order to posit prevention efforts including vaccines in the local context, we estimated the incidence of rotavirus diarrhoea and identified circulating rotavirus genotypes in the community. This assumed importance in the light of research from other parts of West Bengal, showing the inability of exclusive breastfeeding to offer protection against rotavirus diarrhoea in children [25]. The highest incidence of rotavirus diarrhoea in our study was in the 6–18 months age group (24/100 child-years), which substantiates the recommendation for the introduction of rotavirus vaccine early in life. It was interesting to note that in the majority (32/49, 65%) of diarrhoeic stool specimens collected from children aged ⩽6 months (Fig. 1), we did not find any organism and when rotavirus diarrhoea was detected in this group, it was not present with other co-infections. However, immunogenicity and efficacy of rotavirus vaccines in poor developing countries could be reduced by practices such as consumption of breast milk at the time of immunization as researchers have detected higher titres of rotavirus-specific immunoglobulin A (IgA) and neutralizing activity in breast milk of mothers [26]. Issues around delaying breastfeeding at the time of immunization with oral live rotavirus vaccine and its overall impact as well as impact on immunogenicity and efficacy of the vaccine should therefore be reflected upon.
The global incidence estimate for symptomatic rotavirus infection generated through a systematic review and meta-analysis of 20 studies was similar to the rate we estimated for our population. The global pooled estimate was 0·31 symptomatic rotavirus infections per person-year of observation for children aged <2 years (95% credible interval 0·19–0·5), which fell to 0·24 (95% credible interval 0·17–0·34) when a study with the extremely high value of 0·84 reported for Mayan Indians in Guatemala was excluded [27].
Rotavirus detection in diarrhoeic stool specimens from children in India varies from 6% to 71% [28]. While most of the studies contributing to such variation recruited children from hospitals, a few were community based. Our study detected rotavirus in 19% of symptomatic children recruited from the rural community of West Bengal. The common genotype distribution identified (G9P[4], G1P[8], G2P[4] G8P[4]) was slightly diverse compared to the report generated by the Indian Rotavirus Strain Surveillance Network (G2P[4], G1P[8], G9P[8]), operating through hospitals [29]. A study from south India recognized similar diversity when comparing rotavirus genotypes identified from the hospital setting (G1P[8], G9P[8], G2P[4]) with the community setting (G1P[8], G2P[4], G1P[4]) [30]. Studies from the northeastern state of Manipur bordering Myanmar identified a similar distribution and diversity of rotavirus genotype (G1P[8], G2P[4], G12P[6], G9P[6]) as with other parts of the country [31]. While serotype-specific and serotype cross-reactive neutralizing antibodies would be required to offer protection in such an environment of heterogeneity, it would be necessary to regularly map rotavirus genotypes over an extended period of time in a location where new vaccines will be trialled so that the duration and breadth of protection offered by these vaccines can be assessed.
Strengths of the current study rested with direct observation of domestic water management practices, regular home visits to identify newly occurring cases of diarrhoea in children and extensive profiling of organisms in diarrhoeic stool specimens. Furthermore, had it not been for a demographic health survey team constituted by local community members, we would not have been able to accomplish the study, which gave rise to results of public health importance. However, the weakness of the study was its short duration, necessitated by limited availability of resources, which did not allow us to conduct the study over the full year and map seasonal variation in occurrence of diarrhoea in general and rotavirus diarrhoea in particular. Consequently, we had to annualize the incidence rates of rotavirus as well as all-cause diarrhoea. Despite these limitations, the present investigation has been able to identify some important translation points, which could be used for intervention development to control diarrhoea in children.
Inappropriate water handling at home by adults emerged from our study as an issue with regard to diarrhoea in children in rural West Bengal and calls for innovative public health intervention. People's participation will remain critical for the success of such endeavours. How to effectively promote exclusive breastfeeding for children aged ⩽6 months is another important area requiring attention. Finally, oral rotavirus vaccine administered early in life (before age 6 months) can be expected to reduce the incidence of symptomatic rotavirus infection in children in our study setting, which has the highest occurrence in the >6–18 months age group. However, vaccines that effectively prevent severe rotavirus gastroenteritis (RVGE) but are less efficacious against mild RVGE, low mortality from RVGE, unfavourable cost–benefit calculations for some vaccines, and concerns still existing over intussusception [32] are some of the issues that need to be addressed. Otherwise policy level uptake of rotavirus vaccine and its wider use in many countries, including India, will remain slow. Surveillance of rotavirus genotypes in the community should of course be continued in order to examine the duration and flexibility of protection offered by rotavirus vaccines (mono- or polyvalent) in the community and also to identify any change in intervention strategy that might be warranted.
ACKNOWLEDGEMENTS
The study was financially supported in the larger part by the Serum Institute India Limited (SIIL) and partly by the National Institute of Cholera and Enteric Diseases (Indian Council of Medical Research).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.World Health Organization. Urgent efforts needed to prevent deaths from diarrhoea and pneumonia. WHO, Geneva, 2010. (http://www.searo.who.int/linkfiles/press_Releases_year_2010_PR_1509.pdf). Accessed 10 April, 2011). [Google Scholar]
- 2.Ghimire M. Acute diarrhoea and respiratory infections: neglected and forgotten health problems? Communicable Diseases Newsletter 2008; 5: 1–2. [Google Scholar]
- 3.Tate JE, et al. Disease and economic burden of rotavirus diarrhoea in India. Vaccine 2010; 27 (Suppl. 5): F18–24. [DOI] [PubMed] [Google Scholar]
- 4.Cunliffe NA, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bulletin of the World Health Organization 1998; 76: 525–537. [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. The treatment of diarrhoea : a manual for physicians and other senior health workers 2010 (http://www.who.int/maternal_child_adolescent/documents/9241593180/en/). Accessed 15 May 2013.
- 6.Panchalingam S, et al. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clinical Infectious Diseases 2012; 55: S294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nandy RK, et al. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. Journal of Clinical Microbiology 2000; 38: 4145–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty R, et al. Species-specific identification of Vibrio fluvialis by PCR targeted to the conserved transcriptional activation and variable membrane tether regions of toxR gene. Journal of Medical Microbiology 2006; 55: 805–808. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TV, et al. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. Journal of Clinical Microbiology 2005; 43: 755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pal A, et al. Shiga-toxin producing Escherichia coli from healthy cattle in a semi-urban community in Calcutta, India. Indian Journal of Medical Research 1999; 110: 83–85. [PubMed] [Google Scholar]
- 11.Albert MJ, et al. Diarrhoeagenic Escherichia coli are not a significant cause of diarrhoea in hospitalised children in Kuwait. BMC Microbiology 2009; 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniguchi K, et al. Identification of human and bovine rotavirus serotypes by polymerase chain reaction. Epidemiology and Infection 1992; 109: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, et al. Detection of norovirus (GI, GII), sapovirus and astrovirus in fecal samples using reverse transcription single-round multiplex PCR. Journal of Virological Methods 2003; 114: 37–44. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharya R, Sahoo GC, Nayak MK. Molecular epidemiology of human astrovirus infections in Kolkata, India. Infection, Genetics and Evolution 2006; 6: 425–435. [DOI] [PubMed] [Google Scholar]
- 15.du Prel J-B, et al. Confidence interval or P-value? Deutsches Ärzteblatt International 2009; 106: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruuska T, Vesikari T. Rotavirus disease in Finish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scandinavian Journal of Infectious Diseases 1990; 22: 259–267. [DOI] [PubMed] [Google Scholar]
- 17.Deb BC, et al. Studies on interventions to prevent el tor cholera transmission in urban slums. Bulletin of the World Health Organization 1986; 64: 127–131. [PMC free article] [PubMed] [Google Scholar]
- 18.Clasen T, et al. Interventions to improve water quality for preventing diarrhea: systematic review and meta-analysis. British Medical Journal 2007; 334: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobsey MD, et al. Managing water in the home: accelerated health gains from improved water supply. Geneva: World Health Organization, 2002. [Google Scholar]
- 20.Waage J, et al. The millennium development goals: a cross-sectional analysis and principles for goal setting after 2015. Lancet 2010; 376: 991–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer M, Kakuma R. The optimal duration of exclusive breastfeeding: a systematic review. Geneva: World Health Organization, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Feachem M, Koblinsky M. Intervention for the control of diarrhoeal diseases among young children: measles immunization. Bulletin of the World Health Organization 1983; 61: 641–652. [PMC free article] [PubMed] [Google Scholar]
- 23.Deivanayagam N, et al. Measles associated diarrhoea and pneumonia in South India. Indian Pediatrics 1994; 31: 35–40. [PubMed] [Google Scholar]
- 24.World Health Organization. World Health Statistics, 2011.
- 25.Misra S, et al. A prospective study of rotavirus diarrhoea in children under 1 year of age. Clinical Paediatrics 2007; 46: 683–688. [DOI] [PubMed] [Google Scholar]
- 26.Moon SS, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Paediatric Infectious Diseases Journal 2010; 29: 919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilcke J, et al. Estimating the incidence of symptomatic rotavirus infections: a systematic review and meta-analysis. PLoS One 2009; 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broor S, Ghosh D, Mathur P. Molecular epidemiology of rotaviruses in India. Indian Journal of Medical Research 2003; 118: 59–67. [PubMed] [Google Scholar]
- 29.Kang G, et al. Multicenter, hospital-based surveillance of rotavirus disease and strains among Indian children aged <5 years. Journal of Infectious Diseases 2009; 200: 47–53. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee I, Ramani S, Primrose B. Comparative Study of the Epidemiology of rotavirus in children from a community-based birth cohort and a Hospital in South India. Journal of Clinical Microbiology 2006; 44: 2468–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee A, et al. Surveillance and molecular characterization of rotavirus strains circulating in Manipur, North-Eastern India: increasing prevalence of emerging G12 strains. Infection, Genetics and Evolution 2010; 10: 311–320. [DOI] [PubMed] [Google Scholar]
- 32.Vesikari T. Rotavirus vaccination: a concise review. Clinical Microbiology and Infection 2012; 18 (Suppl. 5): 57–63 [DOI] [PubMed] [Google Scholar]