SUMMARY
The frequency of sporadic cases of hepatitis E in humans in developed countries has increased in recent years. The consumption of raw or undercooked pig liver-based products has been identified as an important source of human infection. The question of possible massive human exposure to this zoonotic agent has been raised by the high prevalence of hepatitis E virus (HEV) in swine herds. However, little is known about the epidemiology of HEV on pig farms. A retrospective study, based on a previous prevalence study of 185 farms, was conducted on 90 farms located in Western France, randomly selected from this database, to identify factors associated with the presence of HEV in pig livers and HEV seroprevalence in slaughter-age pigs. At least one HEV RNA-positive liver was found in 30% of the sampled farms while seroprevalence in slaughter-age pigs at the farm level reached almost 75%. Different factors were associated with the two conditions. The risk of having HEV-positive livers was increased by early slaughter, genetic background, lack of hygiene measures and surface origin of drinking water. High HEV seroprevalence was associated with mingling practices at the nursery stage and hygiene conditions. These results can be used to determine on-farm measures to reduce within-farm HEV spread and infection of slaughter-age pigs.
Key words: Hepatitis E, retrospective study, risk factors, swine, zoonosis
INTRODUCTION
Hepatitis E virus (HEV) is responsible for large outbreaks of acute enterically transmitted hepatitis E in humans, similar to hepatitis A but with more serious consequences [1]. Although hepatitis E in most human cases is self-limiting, some fulminant lethal cases (1–2% of cases) still occur. In pregnant women, the risk of fulminant hepatitis E can reach 25% [2]. Chronicity, particularly in immunocompromised patients, is also increasingly reported [3–5]. HEV was discovered in the late 1970s during an epidemic in Kashmir Valley and first identified in the early 1980s [6]. Retrospective studies indicated that it was also responsible for human outbreaks in endemic areas, i.e. in countries with poor sanitary conditions, such as tropical and subtropical regions of Asia and Africa in the 1950s [7]. More recently, many sporadic cases of hepatitis E, not linked with travel in endemic regions, have been reported in developed countries, e.g. USA, Europe and Japan [8–11]. In France, an increase in the number of locally acquired cases of hepatitis E was registered between 2002 (nine cases) and 2011 (249 cases) by the national reference centre [12, 13].
In 1997, Meng et al. detected genetic similarities between a novel porcine virus (i.e. swine HEV) and a human strain of HEV [14]. This further suggested the possible involvement of porcine HEV in human cases of hepatitis E. As a result, many studies of HEV infection have been conducted in different animal productions and have shown that HEV infects a great number of species, particularly swine, which constitute its main reservoir [14–17]. A direct link has been reported between the consumption of contaminated products and autochthonous cases of hepatitis E following the consumption of raw deer meat [18], uncooked wild boar meat [19] or raw pig liver sausages, also known as figatelli [20], although the exact proportion of cases strictly associated with the consumption of porcine products remains unknown. A close similarity has been shown between swine and human HEV sequences collected during the same period but with no geographical proximity, suggesting that the consumption of certain pork products, such as raw liver, was a major source of autochthonous HEV infection [21]. The risk of exposure of human populations to HEV was assessed in several countries utilizing prevalence surveys, which revealed high HEV prevalence in swine herds at the farm level [22–25] and non-negligible proportions of infected livers at the slaughterhouse [22, 24, 26–28]. Furthermore, large variations in viral prevalence [22] and seroprevalence [23–25] were reported between farms at the individual level, suggesting the existence of specific on-farm conditions which can modify the dynamics of HEV infection. In addition, high within-farm seroprevalences were significantly associated with a higher probability of delivering fattening pigs with HEV-positive livers [24]. The asymptomatic nature of HEV infection in pigs, which requires laboratory analyses for its detection, and the absence of effective vaccination, means that spread of HEV within the pig population can only be prevented by applying prophylactic measures. Several studies have been conducted to investigate the dynamics of infection at individual and collective levels, but knowledge of farm-level risk factors for HEV infection and propagation remains scarce.
The objectives of the present study were therefore to identify farm-level factors associated with (i) the probability of HEV being present in pig livers at slaughter, and (ii) the spread of HEV infection in swine farms based on within-farm seroprevalence data at slaughter.
MATERIALS AND METHODS
Study design and sample description
This retrospective study was based on HEV serological and virological results obtained from samples collected during a previous prevalence study conducted between 1 May 2008 and 30 November 2009, involving 186 pig farms in France [24]. Briefly, the herds to be sampled in this study were determined by random selection from a list of slaughter dates and times from a database table. This database was constituted by compiling all possible slaughter dates and times from 1 May 2008 to 30 November 2009 for the 35 selected slaughterhouses (which represented more than 95% of national production according to a preliminary census survey in French slaughterhouses). The number of herds to be sampled per slaughterhouse was determined from the number of pigs slaughtered/year and by considering a minimum of four herds/slaughterhouse. The list of dates and times to sample the herd, depending on the number required per slaughterhouse and stratification according to season, was randomly defined using the surveyselect procedure in SAS v. 9.1 (SAS Institute Inc., USA). Twenty to 40 sera per farm were obtained depending on the number of pigs to be slaughtered per farm. Whatever the herd size, 20 livers per farm were analysed (matched with sera samples) for HEV detection. For economic and practical reasons, the retrospective study was focused on the subsample of 115 farms located in Western France (i.e. Brittany, Normandy, and Pays de la Loire regions which account for more than 70% of the national pig production), irrespective of their HEV status. The list of farms was shared with the related farm organizations so that they could present the study to the farmers beforehand and optimize the participation rate. Ninety of the 115 farms in this subsample agreed to participate and the farmers were then contacted to arrange an appointment for the farm visit and to administer the questionnaire.
Herd data collection
On-farm visits were organized between April and August 2010 to collect information by questionnaire about farm structure, biosecurity, hygiene and rearing practices since serological and virological statuses had been determined previously [18]. Two trained investigators collected the information from all farms blind to the HEV status of the farms, as recommended by Dohoo et al. [29]. A two-part questionnaire was used which included a general questionnaire to be completed with the farmer (face-to-face interview), and a second part based on measurements taken by the investigator on the premises (available upon request). Nine hundred questions (mostly close-ended type) were completed as follows: location and neighbourhood description, structure of premises, replacement stock policy, internal and external biosecurity, hygiene practices, feed and water origin, slurry management, rearing practices and housing conditions in the different compartments associated with breeding stock and growing pigs. The contents and interpretation of the questionnaires had already been pre-tested and assessed on three herds.
After an adjustment period designed to harmonize the way the questionnaires were completed by the investigators (first 10 farms visited jointly), each investigator remained autonomous throughout the survey.
Biological samples
Blood and liver samples had been collected and analysed during the previous prevalence study [18]. Briefly, samples were obtained from selected herds at the slaughterhouse taking special care not to mingle the sampled pigs with pigs from other herds. Blood samples were collected at the bleeding post in individual tubes without any additives immediately after exsanguination and covered immediately to avoid cross-contamination. Each sampled pig was identified by a numbered ear tag to ensure that the livers were collected from the corresponding carcasses. After liver collection, small 2 cm x 1 cm x 1 cm sections were sampled in the area just above the gall-bladder (left medial lobe) with new gloves and sterile scalpel blades to minimize cross-contamination between livers. These samples were kept at 4°C, then frozen and stored at −80°C until analysis.
The methods used for serological analysis and molecular detection of HEV in samples have been described in detail in our previous HEV prevalence study [24].
Statistical analyses
Definition of outcome variables
Two outcome variables were considered in this study: (1) the HEV virological status of the herd based on RT–PCR results from liver analyses and (2) the HEV within-herd seroprevalence. For virological status, the unit of observation was the herd and a farm was considered as positive if at least one of the 20 sampled livers was positive for HEV RNA. For HEV serological data, it was essential to check the non-independence of serum samples taken from the same farm. To meet this requirement and further identify the explanatory variables associated with within-herd seroprevalence, the serological results were analysed at the individual level and by taking into account correlations between serological results from a similar farm (farm cluster effect). Finally, the virological and serological outcome variables were dichotomous and defined at the herd and individual levels, respectively.
Data analysis
Reading, management and analysis of variables were facilitated by entering the questionnaires into a Microsoft Access database and tables corresponding to the different domains were prepared. Beforehand, each quantitative variable (continuous) was converted into categorical variables using a discretization method which consisted of aggregating the values into groups of nominal intervals. Given the sample size, the number of categories created for each quantitative variable in this study was limited to three. At the end of this step, the categories for which the frequency was too low (less than 10% of the sample size) were merged with others of the same variable in order to ensure sufficient statistical reliability [30]. A univariate analysis (independently conducted for each dependent variable) was performed to assess the statistical link between each explanatory variable and the outcome. Logistic regression (GLM function, R [31]) was used to relate the herd's HEV virological status to each explanatory variable. For the serological outcome, a generalized estimating equation (GEE) logistic regression was performed using the GEEPACK R package with ‘herd’ effect being included as a repeated statement with an exchangeable correlation matrix. Only those factors associated with outcome (likelihood ratio χ2 test for logistic regression and Wald χ2 test for GEE models, P < 0·20) were included in the multivariate model [30].
The selected variables were then subjected to bivariate analysis. The objective was to identify strong correlations between each explanatory variable to prevent multicollinearity. For relationships between variables showing strong structural collinearity (P < 0·05), one of the two variables of interest (the one we believed to be most associated with the outcome variable) was chosen. The last step involved a multiple logistic regression model which included all factors that passed the first screening. The contribution of each factor to the model was tested using a likelihood ratio χ2 test (simple logistic regression) or Wald χ2 test (GEE model). The variable with the highest P value was removed and the logistic regression was rerun. This process was continued until a model was obtained in which all factors were significant at P < 0·05 (two-tailed).
Because the proportion of herds with HEV-positive livers (30%) could not be considered as low, the odds ratios (ORs) were converted into relative risks (RRs) to circumvent overestimation of ORs in the case of a frequent disease [32].
RESULTS
Description of sampled farms
The economic and technical results from our selected farms were compared to those from a reference group consisting of a population of Brittany herds, taking 2009 as the reference year because of the sampling periods [33]. The characteristics of the investigated farms were similar to those of the reference population for the majority of variables. However, significant differences were found concerning herd size and some technical-economic performances such as prolificacy and growing parameters which suggested that the sampling procedure at the slaughterhouse tended to select larger farms with slightly better performances, on average, than farms belonging to the reference group (Table 1).
Table 1.
Comparison of technical-economic results from sampled herds with reference population.
| Criteria | N* | Sample mean value | s.d. | Population mean value† | P value‡ |
|---|---|---|---|---|---|
| Number of sows present | 90 | 321 | 227 | 230 | <0·001 |
| Post-weaning daily weight gain (g) | 49 | 493 | 58·59 | 473 | 0·01 |
| Fattening daily weight gain (g) | 53 | 763 | 148·5 | 784 | 0·85 |
| Post-weaning technical feed conversion ratio (kg/kg) | 48 | 1·7 | 0·3 | 1·69 | 0·27 |
| Fattening technical feed conversion ratio (kg/kg) | 56 | 2·9 | 0·3 | 2·83 | 0·20 |
| Post-weaning mortality (%) | 55 | 2 | 1·4 | 2·1 | 0·51 |
| Fattening mortality (%) | 60 | 4 | 4·2 | 3·6 | 0·21 |
| Nursery final age (days) | 57 | 67 | 16·8 | 75 | 1·00 |
| Fattening final age (days) | 60 | 174 | 25·0 | 184 | 1·00 |
| Weight at nursery entrance (kg) | 57 | 7 | 2·9 | 7·1 | 0·26 |
| Weight at fattening entrance (kg) | 57 | 35 | 27·0 | 31·4 | 0·18 |
| Fattening final weight (kg) | 58 | 118 | 11·6 | 115·7 | 0·05 |
| Number of slaughter pigs produced/year | 63 | 6863 | 13 918 | 4665 | 0·10 |
| Number of piglets born alive/litter | 64 | 13·3 | 0·7 | 13·1 | 0·02 |
| Number of piglets weaned/litter | 63 | 11·4 | 25·3 | 11·4 | 0·55 |
| Replacement rate (%) | 60 | 41·5 | 9·3 | 40 | 0·11 |
Number of sampled farms considered for each variable.
Results for 2009 Brittany population [33].
Test of comparison between an observed and a theoretical mean (95% Z test).
Outcome description
The distribution of the number of positive pig livers per farm was almost dichotomous and revealed the existence of three groups of herds with the main group numbering 70% (63/90) of total swine farms which had no HEV-positive liver. A second cluster of 26 farms had between one and seven HEV-positive livers, and on one farm about 75% of the livers were HEV positive (Fig. 1).
Fig. 1.
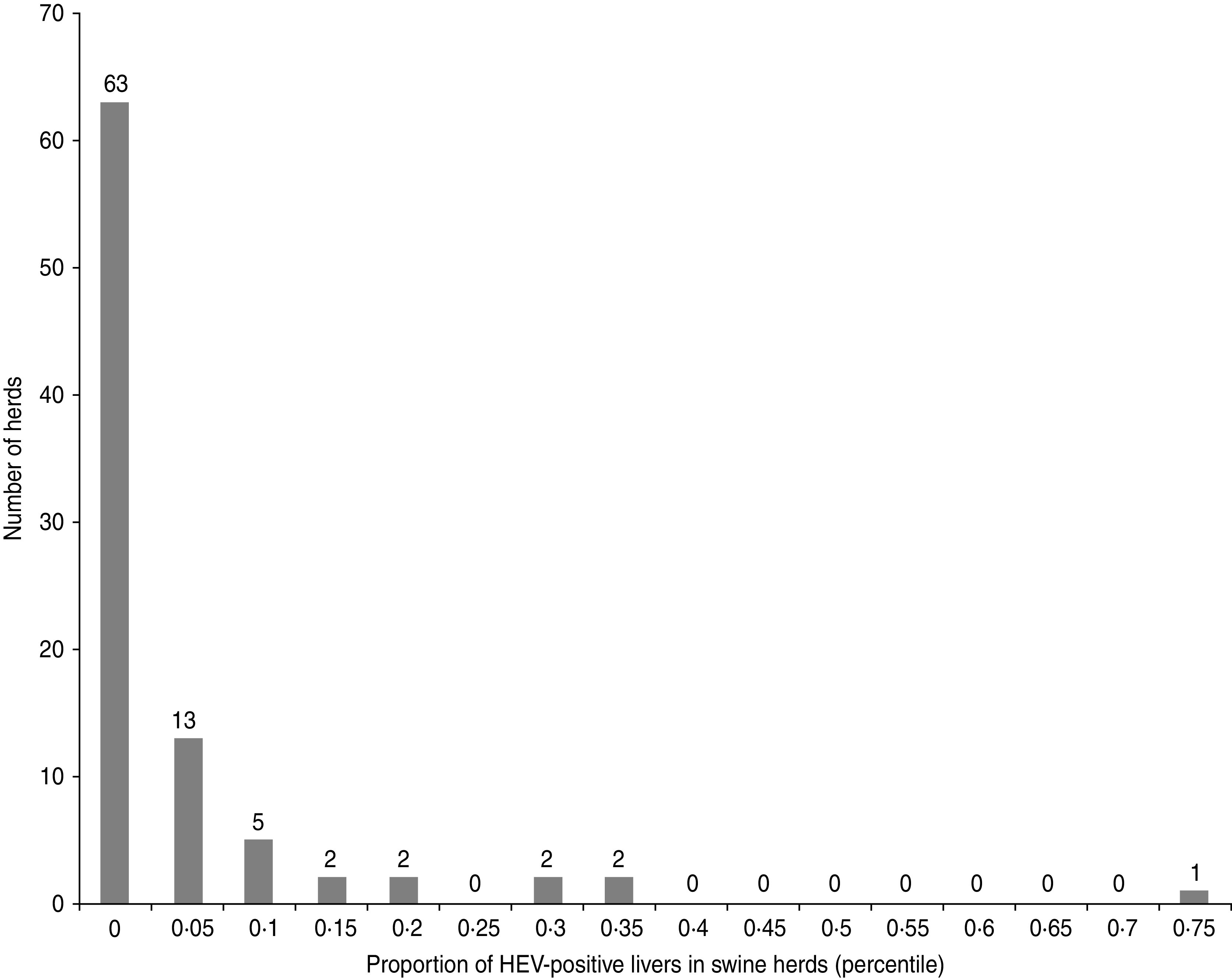
Distribution of the farms according to the proportion of HEV-positive pig livers in sampled herds (90 pig farms, Western France, 2010).
The second outcome, associated with the presence of anti-HEV antibodies in blood samples of pigs collected at slaughterhouse, exhibited a larger variability (Fig. 2). The first group of 36 farms, including 23 seronegative farms, had between 0 and 2 seropositive pigs. A second more diffuse group of 44 herds had a higher proportion of seropositive pigs, i.e. between 10% and 35%, while more than 40% of the pigs were seropositive in a third cluster of 10 farms.
Fig. 2.
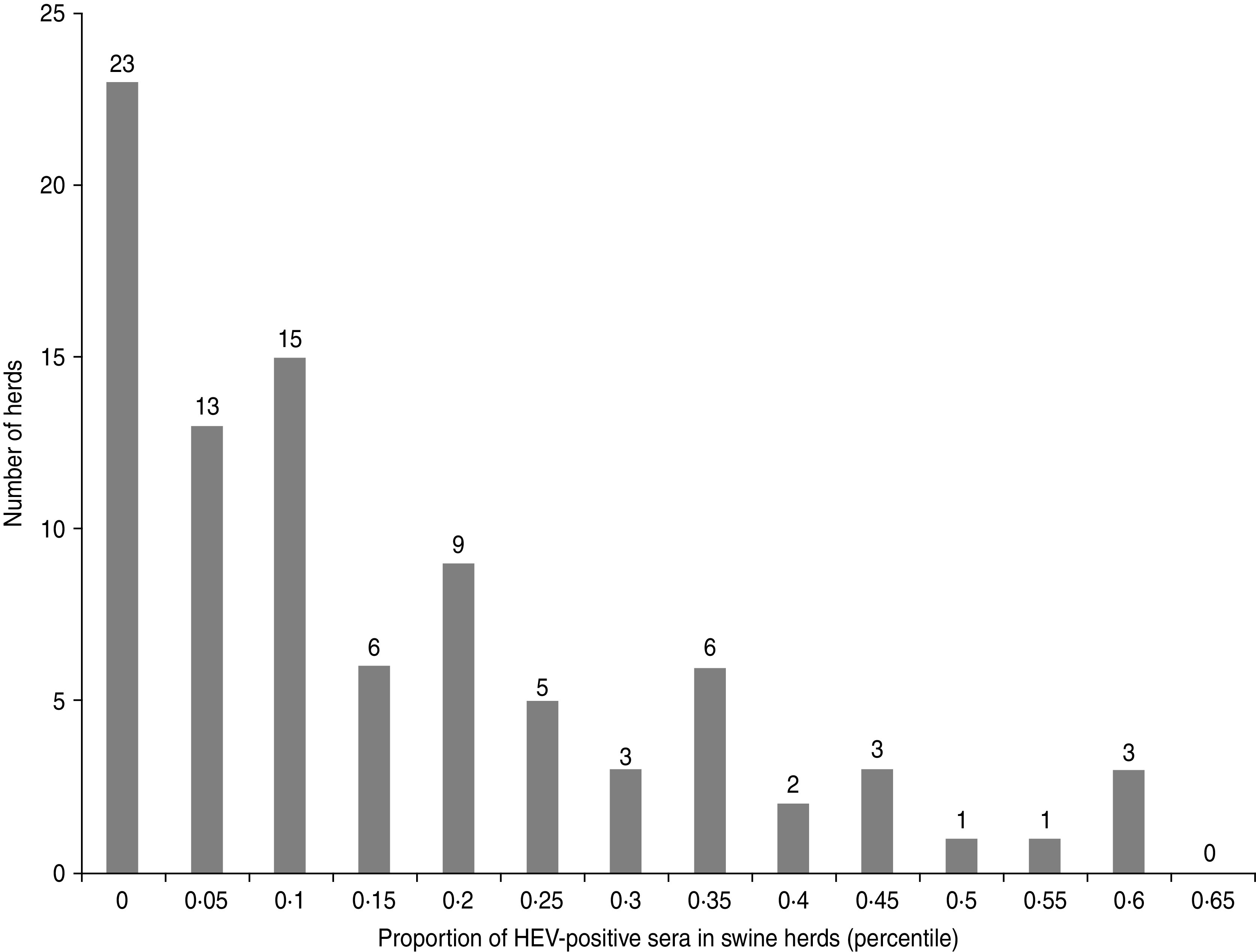
Distribution of the farms according to the proportion of HEV-seropositive sera in sampled herds (90 pig farms, Western France, 2010).
Risk factors associated with the presence of HEV in livers of slaughter-age pigs
At the end of the univariate analysis, 120 variables linked to the HEV status of the farms, based on the presence of HEV in livers, were retained. Only 15 of these variables, which showed no correlation with each other, were kept at the end of the bivariate stage and included in the multivariate logistic regression model. Five variables remained in the final model (Table 2). When the age gap between the first and last shipments of slaughter pigs from the same batch exceeded 20 days, the risk of the presence of HEV in livers of pigs at slaughter was increased [RR 6·0, 95% confidence interval (CI) 1·3–66·0]. A cross-fostering rate higher than 25% was also associated with an increased risk of the presence of HEV in livers of pigs at slaughter (RR 2·7, 95% CI 1·2–5·5). Biosecurity deficiencies featured by the absence of use of specific boots for swine activities, were also associated with an increased risk of detecting the virus in livers at the slaughterhouse (RR 6·2, 95% CI 1·6–45·9). In addition, the genetic background of sows was found to be associated with the HEV status of the farm. Sino-European and Landrace × Duroc × Large White genetic types were associated with a greater risk of HEV infection (RR 4·5, 95% CI 1·6–11·5 and RR 2·9, 95% CI 1·2–6·7, respectively) than Landrace × Large White breeds. The origin of drinking water was associated with the HEV status of farms, the risk being increased when the water sources were superficial (drilling <50 m or spring), rather than from deep drilling or the public water supply (RR 2·9, 95% CI 1·9–2·7).
Table 2.
Multivariate logistic regression model of risk factors associated with the presence of HEV in livers of slaughter-age pigs (n = 90 pig farms, Western France, 2010)
| Variables | % HEV positive* | RR | 95% CI | P value |
|---|---|---|---|---|
| Age gap between first and last slaughter pig shipment to the slaughterhouse for a given batch | ||||
| >20 days | 37·3 | 6·0 | (1·3–66·0) | 0·02 |
| ⩽20 days | 10·0 | – | – | |
| Cross-fostering rate at farrowing | ||||
| >25% | 50·0 | 2·7 | (1·2–5·5) | 0·02 |
| ⩽25% | 23·5 | – | – | |
| Boot-wearing policy | ||||
| Specific to swine production | 11·1 | – | – | 0·01 |
| Non specific | 38·1 | 6·2 | (1·6–45·9) | |
| Origin of drinking water supply | ||||
| >50 m drilling or public network | 19·5 | – | – | 0·047 |
| ⩽50 m (drilling or spring) | 38·8 | 2·9 | (1·2–9·7) | |
| Maternal genetic background | ||||
| Large White × Landrace | 18·2 | – | – | 0·006 |
| Large White × Landrace × Duroc | 55·6 | 2·9 | (1·2–6·7) | |
| Sino-European | 42·9 | 4·5 | (1·6–11·5) |
RR, Relative risk; CI, confidence interval.
Percentage of HEV-positive herds/variable category.
Risk factors associated with HEV seroprevalence
Fourteen of the 123 variables selected at the end of the univariate step were included in the multivariate regression model. Five variables were significantly associated with HEV seropositivity of slaughter-age pigs after application of the backward stepwise procedure (Table 3). Increased within-herd HEV seroprevalence was associated with a down period of <4 days in the nursery (OR 1·7, 95% CI 1·0–2·9) and with having large pens in the nursery, i.e. >26 pigs/pen (OR 2·4, 95% CI 1·2–4·7). Similarly, the within-farm HEV seroprevalence was strongly associated with the nature of piglet management between farrowing and post-weaning. Mingling piglets from different rooms during an intermediate stage between farrowing and nursery led to a higher within-herd HEV seroprevalence at slaughter age (OR 1·8, 95% CI 1·1–2·9). Some factors, associated with management of the breeding herd, were also identified. Hence, the distribution of faeces and placenta among breeding gilts during the acclimatization phase was associated with a decrease in HEV seroprevalence (OR 0·3, 95% CI 0·2–0·6). Finally, HEV seroprevalence was increased if the gap between the pit manure and the slatted floor in fattening rooms was small (OR 1·9, 95% CI 1·1–3·5).
Table 3.
Multivariate generalized estimating equation logistic regression model of risk factors associated with HEV seroprevalence in slaughter-age pigs (3341 sera, 90 pig farms, Western France, 2010)
| Variables and categories | OR | 95% CI | P value |
|---|---|---|---|
| Duration of the down period in nursery (days) | |||
| ⩽4 | 1·7 | (1·04–2·9) | 0·04 |
| >4 | – | – | |
| Distance between pit manure and slatted floor in fattening premises (m) | |||
| ⩽0·80 | 1·9 | (1·1–3·5) | 0·02 |
| >0·80 | – | – | |
| Mingling of pigs from different premises between farrowing and nursery stages | |||
| Yes | 1·8 | (1·1–2·9) | 0·02 |
| No | – | – | |
| Gilts' acclimatization: distribution of placenta and faeces from sows | |||
| Yes | 0·3 | (0·2–0·6) | 0·002 |
| No | – | – | |
| Pen size in nursery rooms (pigs/pen) | |||
| ⩽16 | – | – | |
| 16–26 | 1·7 | (0·9–3·3) | 0·07 |
| >26 | 2·4 | (1·2–4·8) |
OR, Odds ratio; CI, confidence interval.
DISCUSSION
Over the past few years, numerous investigations involving experimental and observational studies have been performed to determine the dynamics of HEV infection at individual and collective levels [14–16, 22, 25, 28, 34]. However, none of these was designed to identify the circumstances associated with HEV infection. The present study is the first to identify certain farm characteristics and husbandry practices that are associated with within-herd HEV spread (HEV seroprevalence at slaughter) and the presence of HEV in slaughter-age pigs (HEV status of pig livers). The two outcomes were defined from analyses of a point sample taken from one batch per farm at a specific time, and some variability between batches could therefore be expected. However, repeated sampling of farms (three successive batches, livers and sera) from eight farms at slaughterhouse indicated that HEV status did not change radically over time (data not shown). The farms to be sampled in this study were not selected on the basis of their HEV status. The proportion of HEV-positive herds was similar to the previously estimated national prevalence [24]. In addition, the variations in frequencies of within-farm virological prevalence (from 5% to 75%) and serological prevalence (from 3% to 60%) were also very similar [22, 24].
The previous national prevalence study conducted on 186 farms (including our selected farms), revealed that although HEV-positive livers were found more frequently in seropositive animals, liver samples from seronegative pigs (2·6%) could also contain HEV, which suggests that the two outcomes in our study are associated with different circumstances. Slaughter-age pigs can be HEV infected and seronegative in the case of recent infection [35, 36]. In fact, although all swine production stages from farrowing to finishing-aged pigs were concerned, different risk factors for our two outcomes were identified.
The probability that pig livers would be HEV infected was strongly influenced by biosecurity and husbandry practices. When the age gap between the first and the last shipments of slaughter pigs from the same batch exceeded 20 days, the risk of HEV being present in the livers was increased. This result was linked to the daily weight gain and the age at the end of fattening, which were both strongly correlated with this interval. Thus fattening pigs with a high daily weight gain were more likely to be slaughtered sooner than slower-growing pigs. Previous observations suggest that HEV shedding tends to decrease from the age of 3 months onwards [16, 37]. It is therefore possible that pigs slaughtered earlier could still be shedding HEV at the time of slaughter. HEV shedding is also strongly associated with infection time, which could not be determined in this study. Another factor identified as increasing the risk of HEV being present in the liver was a cross-fostering rate higher than 25%. Undoubtedly, this indirect association suggests the existence of intermediate events combining not only maternal immunity transmitted by the natural and the foster sows, but also viral transmission from sow to piglets at the farrowing stage. When a sow is HEV seropositive, she delivers antibodies to her piglets via the colostrum, which can delay HEV shedding in piglets [14, 16, 34, 36, 38–41]. Moreover, Casas et al. found that some sows could shed HEV pre- (17%) and post- (16%) farrowing which means that transmission is possible not only between sows and piglets but also between sows raised in group housing [38]. Hence, the multiplication of cross-fostering may amplify virus spread at the batch scale, thereby increasing the proportion of HEV-infected piglets which are shedding the virus after the decay of maternal antibodies.
The drinking-water source was shown to be strongly associated with the presence of HEV in slaughter-age pigs. The connection between drinking water originating from surface sources and HEV infection suggests that such water can be contaminated when infected manure is spread close to the water points, and a positive correlation between the presence of HEV in pigs and in manure pits has been demonstrated [39]. This statistically significant association with water origin is also consistent with the epidemiology of human HEV contamination in endemic areas [42] and with factors associated with human contamination in non-endemic areas [12, 43].
Biosecurity is also pivotal to the prevention of HEV spread. Poor hygiene measures, featured by the non-restricted use of boots used for the swine farm, increased the risk of HEV spread between rooms and pens, which is supported by the excretion route of the HEV [35] and its high environmental persistence [43].
To the best of our knowledge, the association between the maternal genetic background and HEV infection has not been reported previously. Certain immunological characteristics are known to be inherited and genetic resistance to swine diseases such as Porcine Reproductive and Respiratory Syndrome has been described [44]. It is therefore likely that certain swine genetic lines may be more susceptible to HEV infection. However, at this stage, further studies need to be conducted to fully understand whether the observed genetic differences in susceptibility are due to specific mechanisms, such as different immune responses, or to higher HEV prevalence in some genetic lines.
Several factors associated with a high seroprevalence rate in slaughter-age pigs highlight the pivotal role of rearing conditions in facilitating HEV spread within pig populations. Because of the ability of HEV to spread within a population [37], practices such as mingling piglets at the pre-nursery stage and rearing piglets in large pens in nursery (identified as a risk factor in this study) can easily explain the high seroprevalence rate observed at slaughter age. The assumption that massive exposure to HEV at the nursery stage led to a high seroprevalence rate was confirmed by the strong association found between short duration of the down period in nursery and the presence of antibodies against HEV at slaughter.
This correlation is indicative of the considerable turnover between batches and the difficulty of finding time to apply adequate cleaning and disinfection procedures. Although these factors are known to be associated with a high proportion of seropositive pigs, they were not found to be associated with the presence of HEV in the livers of slaughtered pigs. This suggests that they facilitate early spreading of the virus, and the pigs are no longer shedding virus at slaughter time. However, in some ways this seems to contradict the association between HEV detection in livers and cross-fostering, reinforcing the assumption that this latter factor would increase the homogeneity of passive immunity at the batch level and postpone shedding to a later stage.
The distance between the pit manure and the slatted floor, although corresponding to a late exposure period in pig life, was found to be associated with seroprevalence but not with the presence of HEV in livers. Pig manure in an HEV-contaminated farm constitutes an important virus reservoir which can constantly expose the pigs to infected droplets and aerosols. However, because of the high infectious dose required by the oral route for HEV shedding (at least 104 times higher than by intravenous inoculation) [45], this permanent exposure might result in some infection with seroconversion but not enough for further long-time virus shedding. Recently, the probability and duration of HEV shedding was shown to be strongly linked to the quantity of HEV particles ingested and pigs were reported to require at least 106 genome equivalents to ensure successful oral infection and subsequent shedding [46]. The distribution of faeces and placenta to young gilts is a frequent practice aimed at general immunization during the acclimatization phase. In an HEV-contaminated farm, this practice is likely to expose susceptible gilts to the virus occasionally shed by older sows, as reported previously [38, 40]. These studies showed that the prevalence of shedding sows was higher after farrowing, suggesting a link between the sow's physiological status and the probability of shedding. To the best of our knowledge, there is no published evidence of long-term immunity preventing re-infection in adult animals and, as described in humans [3], chronicity cannot be excluded either. Hence the HEV serological status of the reproductive herd should become increasingly homogeneous as a result of this acclimatization practice, which in turn might lead to an increased colostral immunity in young piglets. The seropositivity level of sows and the age at which they became HEV shedders could not be assessed due to the retrospective design of this study. Further studies such as longitudinal surveys are therefore required to check the above assumptions.
Different factors were found to be independently associated with the two outcomes even if biosecurity measures influenced both the presence of HEV in the liver and seroprevalence at the batch level. The risk factors associated with HEV presence in livers at slaughter are probably circumstances facilitating late or long-term shedding of the virus or possibly re-infection. However, this does not preclude a preliminary massive spread of the virus at the population scale. Hence, all risk factors have to be considered to determine efficient preventive strategies. These factors concern different crucial stages in piglet production, and mainly involve interactions between rearing conditions, loss of passive immunity and hygiene. Further work will be necessary, especially at the individual level, to identify which specific factors govern long-term shedding, re-infection and infections occurring close to slaughter time.
ACKNOWLEDGEMENTS
This study was supported by a national grant from Agence Nationale de la Recherche (PNRA07-008 HEVEZOONEPI). The authors are grateful to the farmers and the slaughterhouse organizations for their contribution. They also thank INAPORC, COOP de France and UGPVB for facilitating access to the farms.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Emerson SU, Purcell RH. Hepatitis E virus. Reviews in Medical Virology 2003; 13: 145–154. [DOI] [PubMed] [Google Scholar]
- 2.Smith JL. A review of hepatitis E virus. Journal of Food Protection 2001; 64: 572–586. [DOI] [PubMed] [Google Scholar]
- 3.Bihl F, Negro F. Chronic hepatitis E in the immunosuppressed: a new source of trouble? Journal of Hepatology 2009; 50: 435–437. [DOI] [PubMed] [Google Scholar]
- 4.Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. New England Journal of Medicine 2008; 358: 859–860. [DOI] [PubMed] [Google Scholar]
- 5.Kamar N, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. New England Journal of Medicine 2008; 358: 811–817. [DOI] [PubMed] [Google Scholar]
- 6.Khuroo MS. Discovery of hepatitis E: the epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Research 2011; 161: 3–14. [DOI] [PubMed] [Google Scholar]
- 7.Chandra V, et al. Molecular biology and pathogenesis of hepatitis E virus. Journal of Biosciences 2008; 33: 451–464. [DOI] [PubMed] [Google Scholar]
- 8.Buti M, et al. Sporadic cases of acute autochthonous hepatitis E in Spain. Journal of Hepatology 2004; 41: 126–131. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, et al. Present state of hepatitis E virus epidemiology in Tokyo, Japan. Hepatology Research 2003; 27: 169–173. [DOI] [PubMed] [Google Scholar]
- 10.Kwo PY, et al. Acute hepatitis E by a new isolate acquired in the United States. Mayo Clinic Proceedings 1997; 72: 1133–1136. [DOI] [PubMed] [Google Scholar]
- 11.Péron J-M, et al. Hepatitis E is an autochthonous disease in industrialized countries: Analysis of 23 patients in South-West France over a 13-month period and comparison with hepatitis A. Gastroenterologie Clinique et Biologique 2006; 30: 757–762. [DOI] [PubMed] [Google Scholar]
- 12.Nicand E, Delaune D, Tessé S. Surveillance data on human cases of hepatitis E in France, 2006–2009. Feuillets de Biologie 2011; 52: 19–24. [Google Scholar]
- 13.Roque-Afonso AM. Annual activity report from the National Reference Centre for enteric hepatitis A and E, 2011. [in French], 36 pp. [Google Scholar]
- 14.Meng X-J, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proceedings of the National Academy of Sciences USA 1997; 94: 9860–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper K, et al. Identification of genotype 3 hepatitis E virus (HEV) in serum and fecal samples from pigs in Thailand and Mexico, where genotype 1 and 2 HEV strains are prevalent in the respective human populations. Journal of Clinical Microbiology 2005; 43: 1684–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Deus N, et al. Hepatitis E virus infection dynamics and organic distribution in naturally infected pigs in a farrow-to-finish farm. Veterinary Microbiology 2008; 132: 19–28. [DOI] [PubMed] [Google Scholar]
- 17.Pavio N, et al. Hepatitis E as a zoonosis. Virologie 2006; 10: 341–351. [DOI] [PubMed] [Google Scholar]
- 18.Tei S, et al. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003; 362: 371–373. [DOI] [PubMed] [Google Scholar]
- 19.Tamada Y, et al. Consumption of wild boar linked to cases of hepatitis E. Journal of Hepatology 2004; 40: 869–870. [DOI] [PubMed] [Google Scholar]
- 20.Colson P, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. Journal of Infectious Diseases 2010; 202: 825–834. [DOI] [PubMed] [Google Scholar]
- 21.Bouquet J, et al. Close similarity between sequences of hepatitis E virus recovered from Human and Swine in France between 2008 and 2009. Emerging Infectious Diseases 2011; 17: 2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Bartolo I, et al. Widespread diffusion of genotype 3 hepatitis E virus among farming swine in Northern Italy. Veterinary Microbiology 2008; 132: 47–55. [DOI] [PubMed] [Google Scholar]
- 23.Peralta B, et al. Anti-HEV antibodies in domestic animal species and rodents from Spain using a genotype 3-based ELISA. Veterinary Microbiology 2009; 137: 66–73. [DOI] [PubMed] [Google Scholar]
- 24.Rose N, et al. High prevalence of hepatitis E virus in French domestic pigs. Comparative Immunology, Microbiology and Infectious Diseases 2011; 34: 419–427. [DOI] [PubMed] [Google Scholar]
- 25.Seminati C, et al. Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. Veterinary Journal 2008; 175: 130–132. [DOI] [PubMed] [Google Scholar]
- 26.Bouwknegt M, et al. Hepatitis E virus RNA in commercial porcine livers in The Netherlands. Journal of Food Protection 2007; 70: 2889–2895. [DOI] [PubMed] [Google Scholar]
- 27.Feagins AR, et al. Detection and characterization of infectious hepatitis E virus from commmercial pig livers sold in local grocery in the USA. Journal of General Virology 2007; 88: 912–917. [DOI] [PubMed] [Google Scholar]
- 28.Jung K, et al. Prevalence and genotyping of hepatitis E virus in swine population in Korea between 1995 and 2004: A retrospective study. Veterinary Journal 2007; 173: 683–687. [DOI] [PubMed] [Google Scholar]
- 29.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island, Canada: AVC Inc., 2003, pp. 706. [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley, 1989, pp. 307. [Google Scholar]
- 31.Ihaka R, Gentleman R. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics 1996; 5: 299–314. [Google Scholar]
- 32.Beaudeau F, Fourichon C. Estimating relative risk of disease from outputs of logistic regression when the disease is not rare. Preventive Veterinary Medicine 1998; 36: 243–256. [DOI] [PubMed] [Google Scholar]
- 33.Badouard B, Pellois H. Technical-economic results for pigs reared in Brittany 2009 [in French]. Bulletin des Chambres d'Agriculture de Bretagne 2010, 6 pp. [Google Scholar]
- 34.dos Santos DRL, et al. Serological and molecular evidence of hepatitis E virus in swine in Brazil. Veterinary Journal 2009; 182: 474–480. [DOI] [PubMed] [Google Scholar]
- 35.Bouwknegt M, et al. The course of hepatitis E virus infection in pigs after contact-infection and intravenous inoculation. BMC Veterinary Research 2009; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanai Y, et al. Long-term shedding of hepatitis E virus in the feces of pigs infected naturally, born to sows with and without maternal antibodies. Journal of Medical Virology 2011; 82: 69–76. [DOI] [PubMed] [Google Scholar]
- 37.Bouwknegt M, et al. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Veterinary Research 2008; 39: 1–11. [DOI] [PubMed] [Google Scholar]
- 38.Casas M, et al. Longitudinal study of hepatitis E virus infection in Spanish farrow-to-finish swine herds. Veterinary Microbiology 2011; 148: 27–34. [DOI] [PubMed] [Google Scholar]
- 39.Feng R, et al. Infection dynamics of hepatitis E virus in naturally infected pigs in a Chinese farrow-to-finish farm. Infection, Genetics and Evolution 2011; 11: 1727–1731. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Barredo S, et al. Detection of hepatitis E virus shedding in feces of pigs at different stages of production using reverse transcription-polymerase chain reaction. Journal of Veterinary Diagnostic Investigation 2006; 18: 462–465. [DOI] [PubMed] [Google Scholar]
- 41.Kasorndorkbua C, et al. Experimental infection of pregnant gilts with swine hepatitis E virus. Canadian Journal of Veterinary Research 2003; 67: 303–306. [PMC free article] [PubMed] [Google Scholar]
- 42.Clemente-Casares P, et al. Hepatitis E virus epidemiology in industrialized countries. Emerging Infectious Diseases 2003; 9: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalton HR, et al. Hepatitis E: an emerging infection in developed countries. Lancet Infectious Diseases 2008; 8: 698–709. [DOI] [PubMed] [Google Scholar]
- 44.Lunney JK, Chen H. Genetic control of host resistance to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Virus Research 2010; 154: 161–169. [DOI] [PubMed] [Google Scholar]
- 45.Bouwknegt M, et al. Estimation of the likelihood of fecal-oral hev transmission among pigs. Risk Analysis 2011; 31: 940–950. [DOI] [PubMed] [Google Scholar]
- 46.Andraud M, et al. Direct contact and environmental contaminations are responsible for HEV transmission in pigs. Veterinary Research 2013; 44: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]


