SUMMARY
A retrospective cohort study was conducted to identify risk factors for bovine tuberculosis (bTB) recurrence in New Zealand cattle and deer herds identified as bTB-infected from 1 June 2006 to 1 November 2010. A Cox proportional hazards model identified a positive relationship between the daily hazard of bTB recurrence and: (1) the number of prior bTB episodes for two episodes [hazard ratio (HR) 3·22, 95% confidence interval (CI) 1·21–8·60], and for five episodes (HR 89·5, 95% CI 13·8–580), (2) more than one positive bTB case animal at the index episode (HR 2·25, 95% CI 1·19–4·25) and (3) the presence of cleared test-positives at the final test of the index episode. The proportional hazards assumption was violated for the latter variable so a time-dependent covariate was introduced. Up to 2 years post-clearance, the daily hazard of bTB recurrence was greater in herds with test-positives at the final test (HR 2·59, 95% CI 1·30–5·13), but this effect was not observed more than 2 years' post-clearance (HR 1·05, 95% CI 0·28–3·91). We conclude that unresolved infection contributes to further bTB episodes in the first 2 years after herd clearance.
Key words: Infectious disease control, mycobacteria, statistics, veterinary epidemiology
INTRODUCTION
Bovine tuberculosis (bTB) is a disease of international significance and zoonotic potential. In New Zealand, bTB has been present in domestic cattle since the 1800s. The primary wildlife reservoir of bTB in New Zealand is the introduced brushtail possum (Trichosurus vulpecula) [1].
Under the management of the Animal Health Board (now TBfree New Zealand) and using a combination of vector control in geographical areas of endemic feral animal infection (Vector Risk Areas; VRAs), test and slaughter, and movement restrictions, New Zealand has seen a reduction in the number of herds infected with bTB. In the mid-1990s there were about 1700 infected herds; by 2013 this number had reduced to fewer than 100. Surveillance involves 1- to 3-yearly testing of eligible cattle and deer, and abattoir monitoring of all animals slaughtered for human consumption.
With the progressive decline in the prevalence of bTB, recurrence of infection at the herd level has emerged as a potential threat to eradication in the immediate future. Of the herds identified as infected in New Zealand between 2005 and 2011, 59% had experienced a prior episode of infection (J. Sinclair, unpublished data). Recurrence of bTB in a previously infected herd is a multifactorial problem [2]. Repeated exposure to infected wildlife and movement risk are well recognized as causes of repeated episodes of bTB infection in New Zealand, but the role of recrudescence of within-herd infection is less well understood. The problems presented by recrudescent infections are threefold: financial costs to herd managers and to TBfree New Zealand, emotional impacts on herd managers including loss of confidence in testing programmes, and outward spread of infection from herds released from movement restrictions where undetected infection may remain. Elucidation of causal factors involved in recurrence of bTB should allow appropriate policies to be put in place to: (1) test herds more intensively post-clearance where risk factors exist, and (2) track at-risk outward movements from these herds.
Herds that had experienced at least one bTB episode within a defined study period were the population of interest for the analyses presented in this paper. Our aim was to assess the influence of herd-level characteristics, previous bTB history and details of management on the time taken for a herd to be detected as having a further bTB episode, if one occurred. Identification of characteristics of influence over the risk of herds having further bTB episodes provides useful information for the design of strategies to manage newly identified bTB-positive herds. A secondary aim of this study was to provide a means for identifying herds most at risk of recurrent bTB episodes, allowing tiered, pre-emptive controls to be applied.
MATERIALS AND METHODS
This was a retrospective cohort study of cattle and deer herds monitored by TBfree New Zealand's bTB surveillance programme. The source population was comprised of all cattle and deer herds over the four Disease Control Areas (DCAs) (Northern and Southern North Island, and Northwestern and Southern South Island) that received at least one bTB test event by TBfree New Zealand during the study recruitment period from 1 June 2006 to 1 November 2010. If more than one further bTB episode occurred following clearance in the study period, only the first episode was considered. The eligible population comprised those members of the source population that: (1) had a culture-positive case of bTB identified on or after 1 June 2006 (the index bTB episode); (2) had their herd status designated as cleared on the basis of testing performed before 1 November 2010; and (3) had their bTB clear status achieved using methods that did not include whole herd destocking. All herds meeting the eligibility criteria were included in the study population. All study herds were followed until the end of the follow-up period, which occurred on 5 May 2011.
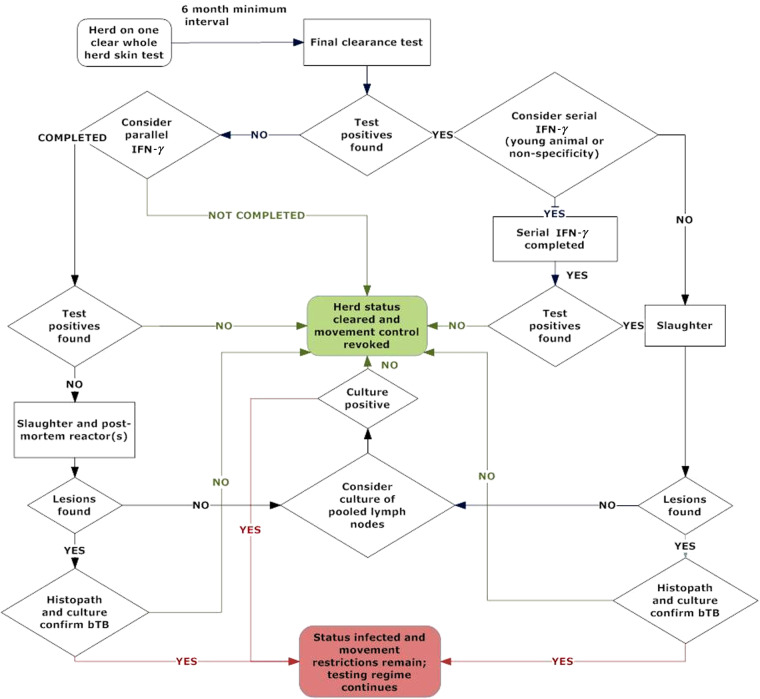
Start and end dates of the index bTB episode, defined as the date of issue and revocation of a Restricted Place Notice, were recorded for each herd in the study population. Under the Biosecurity Act 1993, a Restricted Place Notice must be issued within five working days of determining a herd as infected based on positive culture, and prevents uncontrolled outward movements from a herd. In New Zealand, herds remain under movement restrictions and have the status I (Infected) until two consecutive whole-herd tuberculin tests at least 6 months apart are clear (Fig. 1). The duration of the index episode was defined as the number of days from the date of bTB diagnosis to the date on which movement restrictions for the herd were lifted. The outcome of interest for this study was the number of days between the date on which movement restrictions were lifted and the date on which a further bTB episode began, as defined by the issue of a further Restricted Place Notice. We designate this time period ‘bTB recurrence interval’ in the remainder of this paper. Herds were treated as censored observations if they did not experience a further bTB episode by the end of the follow-up period on 5 May 2011. If more than one further episode occurred following clearance in the study period, only the first episode was considered.
Fig. 1.
[colour online]. Flowchart of bovine tuberculosis (bTB) management decision processes for final clearance testing in New Zealand at the time of the study, 2006–2010.
Explanatory variables thought to influence the bTB recurrence interval were in three broad categories: herd-level variables, episode-specific variables and bTB management variables (Table 1). Explanatory variables were tested for their relationship with the bTB recurrence interval using univariable Cox proportional hazards regression models and stratified Kaplan–Meier curves. Variables were selected for inclusion in a multivariable Cox proportional hazards model if the P value from the univariable Cox model was P < 0·3.
Table 1.
Herd-level, episode- specific and bovine tuberculosis (bTB) management variables considered for inclusion in a Cox proportional hazards regression model of factors influencing bTB recurrence interval
| Variable category | Variable name | Details |
|---|---|---|
| Herd-level variables | Disease Control Area (DCA) | Four geographical DCAs within New Zealand; 1 = northern North Island, 2 = southern North Island, 3 = northwestern South Island, 4 = southern South Island |
| Vector Risk Area (VRA) | Location of herd with regard to VRAs; 1 = vector risk area, 0 = vector-free area | |
| Herd type | Herd type; 1 = dairy, 2 = dairy dry, 3 = beef breeding, 4 = beef dry, 5 = deer breeding | |
| Herd size | Herd size, defined as the number of animals in the index whole-herd test after detection of infection; includes all animals aged ⩾6 weeks present on the property | |
| Prior episodes | The number of infected episodes prior to the index episode in the study period | |
| Episode-specific variables | Whole herd test | The number of skin test-positive animals at the first whole-herd test at or following the detection of the index bTB episode |
| Skin test positive | The total number of skin test-positive reactions during the index episode | |
| Slaughter test-positives | The total number of animals slaughtered as test-positives during the index episode | |
| Lesions | The total number of animals detected with lesions during the index episode (includes test-positive post-mortem and meat inspection of non-test-positives) | |
| bTB management variables | Parallel testing | Whether a parallel blood (IFN-γ) test was used at any time during the index episode (1 = yes, 0 = no) |
| Clear | The combination of tests used at the final clearance test of the index episode (1 = skin test only all clear; 2 = skin test-positive animal(s) cleared on negative serial IFN-γ; 3 = skin or IFN-γ positive animal(s) cleared on slaughter with no visible lesions | |
| rxyn | Whether any test-positive animals were found by skin or IFN-γ testing at the final clearance test of the index episode (1 = yes, 0 = no) |
Detection of any remaining infection before removing movement restrictions was considered important to reduce the risk of bTB recurrence, although imperfect sensitivity of available combinations of tests is a challenge [3]. The final tuberculin test of all animals on the property before revocation of the Restricted Place Notice is referred to as the ‘final clearance test’, and is often followed by a parallel interferon-gamma (IFN-γ) test of breeding animals or the risk cohort. Disease control decision-making processes in place at the time of the study are shown in Figure 1.
A multivariable Cox proportional hazards model was developed to quantify factors influencing the bTB recurrence interval. The model was built using a backwards stepwise selection procedure. Explanatory variables were removed from the model one at a time in order of decreasing statistical significance and re-fitting the model. The likelihood ratio test and Akaike's Information Criterion (AIC) were used to evaluate the fit of successive nested models.
The effect of possible confounders was analysed by comparing models with and without the explanatory variable of interest. An explanatory variable was included in the model if its inclusion altered the regression coefficients of one or more of the other explanatory variables by more than 20%.
Analyses were performed to identify the presence of biologically plausible interactions between each of the explanatory variables included in the final model. Interaction terms were included in the final multivariable model if they were statistically significant and they significantly altered model fit as measured by the likelihood ratio test. Analyses were conducted using the survival package implemented in R [3, 4].
The assumption of proportionality of hazards was examined both globally and for each explanatory variable using the weighted residual method proposed by Grambsch & Therneau [5]. The proportional hazards assumption was further investigated for each explanatory variable by plotting the scaled Schoenfeld residuals for each herd as a function of bTB recurrence interval. The proportional assumption was considered to be violated if a line of gradient zero could not be drawn between the 95% confidence intervals (CIs) of the loess-smoothed line of best fit for each of these plots [6].
The presence of influential observations was assessed by calculating delta-beta values for each herd for each explanatory variable. Records for individual herds were considered for removal if their delta-beta values were >0·1 for more than one explanatory variable. A herd's record was considered influential if its removal resulted in a change of more than 25% in the value of at least one regression coefficient.
This study is reported in compliance with the STROBE statement [7].
RESULTS
The source population of cattle and deer herds in New Zealand screened at least triennially for bTB by routine tuberculin testing or by slaughter surveillance numbered 71 950 as at 30 June 2010. The eligible population consisted of 568 herds under infected movement control between 1 June 2006 and 1 May 2011. Two hundred and thirteen herds were excluded because their index episode did not begin after 1 June 2006 or finish by 1 May 2011, or because the herd status was cleared by destocking the herd, or because culture had not confirmed a true bTB infection. The median bTB recurrence interval was 315 days and the maximum was 1709 days (Table 2). The number of days between episodes for herds experiencing a further episode of bTB ranged from 48 days to 1617 days with a median of 547 days. Overall, 14% of herds experienced a further bTB episode, and the highest proportion of herds experiencing a further bTB episode was in the northwest of the South Island (27% of herds compared to 2–6% of herds in the other DCAs), in VRAs (16% of herds compared to 3% of herds in vector-free areas) and in dairy herds (26% of herds compared to 0–8% for herds of other types).
Table 2.
Descriptive statistics for each of the non-normally distributed continuous variables in this study
| Variable | Minimum | Q1 | Median | Q3 | Maximum |
|---|---|---|---|---|---|
| Herd size* | 6 | 135 | 296 | 547 | 4285 |
| Duration of index episode (days) | 102 | 251 | 315 | 463 | 1709 |
| Time between episodes (days) | 48 | 273 | 547 | 795 | 1617 |
| Number of skin test-positive animals during index episode | 0 | 1 | 2 | 7·86 | 145 |
| Proportion of herd skin-test positive | 0 | 0·002 | 0·009 | 0·024 | 0·500 |
| Number of animals slaughtered during index | 0 | 0 | 1 | 3 | 52 |
| Proportion of herd slaughtered | 0 | 0 | 0·004 | 0·013 | 0·679 |
| Number of positive bTB cases during index episode | 1 | 1 | 1 | 2 | 21 |
| Proportion of herd detected with bTB esions | 0·001 | 0·002 | 0·004 | 0·012 | 0·333 |
bTB, Bovine tuberculosis.
Number of animals tested at index test following breakdown.
In the univariable models, when skin test-positive animals had been found and serial IFN-γ testing had been used at a final clearance test, the daily hazard of detection of a further episode of bTB was 4·5 (95% CI 2·4–8·7) times that of herds where no test-positives had been found. Where animals had been slaughtered and there were no visible lesions present at the final whole-herd test, the hazard of detection of a further bTB episode was 3·3 (95% CI 1·5–7·0) times that of herds where no test-positives had been found. This variable (‘clear’, Table 1) was also highly significant in multivariable models, but was replaced by a dichotomous variable ‘rxyn’ (Table 1) representing the presence or absence of test-positives at the final clearance test in the final model, because ‘clear’ was considered to be too highly correlated with DCA; both ‘clear’ and DCA have components of geographical disease management differences.
In the main-effects multivariable model (Table 3), there was a positive association between bTB recurrence interval and:
-
(1)
the number of bTB episodes in a herd prior to the index episode [hazard ratio (HR) 3·19 (95% CI 1·19–8·52) for two prior episodes; HR 86·7 (95% CI 13·3–560) for five prior episodes];
-
(2)
the presence of more than one positive bTB case animal at the index bTB episode (HR 2·28, 95% CI 1·20–4·30);
-
(3)
the presence of one or more cleared test-positive animals in the final clearance test at the index episode (HR 2·10, 95% CI 1·16–3·83). Positive reactions to skin and/or IFN-γ testing are permitted in a final clearance test where, by ancillary testing, the bTB control manager is satisfied that the reactions were false positive and that the animal(s) were uninfected (Fig. 1). ‘Test-positives’ or ‘cleared test-positives’ denotes animals belonging to any of the following categories:
-
•
skin test-positive animals slaughtered with no visible lesions;
-
•
skin test-positive animals determined as negative by serial IFN-γ testing;
-
•
skin test-positive, serial IFN-γ-positive animals slaughtered with no visible lesions;
-
•
skin test-negative, parallel IFN-γ-positive animals slaughtered with no visible lesions.
-
•
Table 3.
Estimated regression coefficients (and their standard errors) from a Cox proportional hazards regression model of factors associated with bovine tuberculosis recurrence interval
| Explanatory variable | At risk | Events | Coefficient (s.e.) | P | HR | 95% CI |
|---|---|---|---|---|---|---|
| Number of prior bTB episodes | ||||||
| 0 | 147 | 7 | Reference | 1·00 | ||
| 1 | 85 | 9 | 0·4949 (0·5107) | 0·34 | 1·64 | 0·60–4·46 |
| 2 | 66 | 11 | 1·1706 (0·5007) | 0·02 | 3·22 | 1·21–8·60 |
| 3 | 38 | 10 | 1·6619 (0·5177) | <0·01 | 5·27 | 1·91–14·54 |
| 4 | 17 | 10 | 2·4491 (0·5128) | <0·01 | 11·6 | 4·24–31·60 |
| 5 | 3 | 2 | 4·4942 (0·9541) | <0·01 | 89·5 | 13·8–580 |
| Disease Control Area in which the herd was located | ||||||
| Northern North Island | 72 | 3 | −0·1585 (0·7142) | 0·83 | 0·85 | 0·21–3·46 |
| Southern North Island | 45 | 1 | −1·7293 (1·1291) | 0·13 | 0·18 | 0·02–1·62 |
| Northwest South Island | 144 | 39 | 1·3872 (0·4520) | 0·02 | 4·02 | 1·65–9·71 |
| Southern South Island | 95 | 6 | Reference | 1·00 | ||
| Duration of prior episode (6-month intervals) | −0·3775 (0·1562) | 0·02 | 0·69 | 0·51–0·94 | ||
| Number of positive bTB case animals at index episode | ||||||
| 1 | 249 | 31 | Reference | 1·00 | ||
| >1 | 107 | 18 | 0·8119 (0·3239) | 0·01 | 2·25 | 1·19–4·25 |
| Test-positives at final test (<730 days) | ||||||
| Absent | 280 | 26 | Reference | 1·00 | ||
| Present | 76 | 23 | 0·9508 (0·3494) | 0·01 | 2·59* | 1·30–5·13 |
| Test-positives at final test (>730 days) | ||||||
| Absent | 176 | 10 | Reference | 1·00 | ||
| Present | 39 | 3 | 0·0440 (0·6739) | 0·37 | 1·05† | 0·28–3·91 |
bTB, Bovine tuberculosis; s.e., standard error; HR, hazard ratio; CI, confidence interval.
Interpretation: For herds where test-positive animal(s) had been present in the final clearance test for clearance, the daily hazard of detection of a further bTB episode was increased by a factor of 2·59 (95% CI 1·30–5·13) in the index episode 730 days at risk, compared to herds in which no test-positives had been detected.
Interpretation: The daily hazard of detection of a further bTB episode was increased by a factor of 1·05 (95% CI 0·62–3·64) for risk periods >730 days, compared to herds in which no test-positives had been detected.
Six-monthly increases in duration of the index episode of infection decreased the daily hazard of detection of a further episode by a factor of 0·69 (95% CI 0·51–0·94). The duration of the index episode was included in the model as a confounder, because its inclusion changed the regression coefficients of two other covariates by more than 20%.
The DCA in which a herd was located was strongly correlated with the presence of test-positive animals at the final clearance test (χ2 = 18·14, P = 0·0004). DCA was therefore considered an important a priori explanatory variable and when excluded from the multivariable model it changed the estimated regression coefficients for the other explanatory variables by more than 20%. None of the interaction terms tested in the model had a significant effect, therefore none were included in the final model.
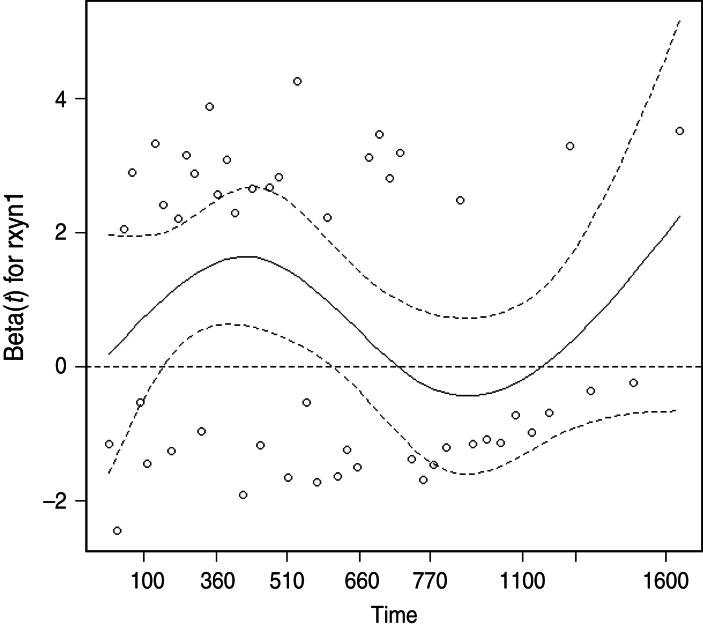
The proportional hazards assumption was violated for the presence of cleared test-positives at the final clearance test, as indicated by plots of the modified Schoenfeld residuals as a function of bTB recurrence interval (Fig. 2). The point of inflection of the loess line occurred between 510 and 770 days, therefore 2 years (730 days) was thought to be a reasonable cutpoint for inclusion of a time-dependent variable to account for the number of cleared test-positives. In the final model the monthly hazard of recurrence during the first 730 days after clearance was significantly increased in herds with one or more cleared test-positives at the final test (HR 2·59, 95% CI 1·30–5·13), but this effect was no longer significant more than 2 years after clearance (HR 1·05, 95% CI 0·28–3·91).
Fig. 2.
Scatterplot of scaled Schoenfeld residuals as a function of bovine tuberculosis recurrence interval for the explanatory variable quantifying the presence of test-positives at a final clearance test.
For the final model the Schemper & Stare R2 value was 0·233 indicating that 23% of the variation in bTB recurrence interval was explained by the predictors in the model. The likelihood ratio test comparing the final model with an intercept-only model returned a χ2 statistic of 94·55 on 6 d.f. (P < 0·01).
Overall model fit was evaluated by plotting the cumulative hazard function as a function of the modified Cox–Snell residuals (Fig. 3). The plot shows a straight-line relationship between the two variables, indicative of a model that provides an adequate fit to the data.
Fig. 3.
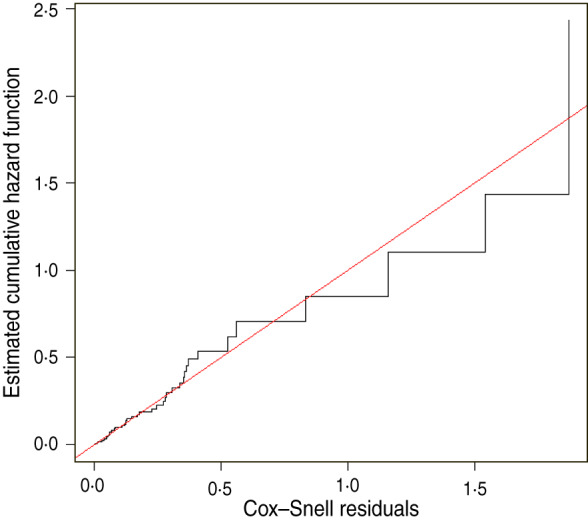
[colour online]. Plot of the estimated cumulative hazard function of the Cox–Snell residuals showing closeness of overall fit of model predictions.
Delta-beta values were plotted for all covariates in the final model. Twenty-eight herd records were excluded one by one from the dataset, based on their delta-beta value of >0·1 for more than one variable. The model was re-run using the remaining observations. A herd record was considered influential if its removal caused a change of more than 25% in the value of at least one regression coefficient. Using these criteria, there were no observations that could be considered influential, and all 356 herds were included in the final model.
DISCUSSION
To date New Zealand's bTB eradication scheme has been highly successful, evidenced by the period prevalence of herds under infected movement controls reducing from around 2% in 1994 to <0·2% in 2012 [8]. In the later stages of an infectious disease eradication programme it is important to identify all potential causes of herd-level failure and to address these by means of policy and auditing. One of the risks to bTB eradication in New Zealand is failure to detect residual infection in herds before removing movement restrictions. In Ireland, the odds of bTB detection was 1·91 times higher for animals sold from de-restricted herds compared to animals sold from ‘unexposed’ herds [9]. The same situation is likely to exist in New Zealand, and can potentially lead to between-herd spread. This study aimed to define and quantify factors associated with bTB recurrence interval in herds that had experienced one episode during the study recruitment period, from 1 June 2006 to 1 November 2010.
The final multivariable Cox model included herd-level explanatory variables (DCA and the number of previous infected episodes), episode-specific factors (one or >1 positive bTB case animal at the index episode; duration of index episode) and one bTB management factor (whether test-positives had been detected at the final clearance test of the index episode). Herd size, herd type and category (breeding vs. dry), VRA, the number and proportion of skin test-positives, slaughtered and positive bTB case animals (as a continuous variable), parallel IFN-γ testing and culture had no significant effect on bTB recurrence interval and were not included in the final multivariable model.
The four geographical DCAs of New Zealand differ with regard to the exposure of herds to infected wildlife, predominant herd type and bTB management. Each DCA is under a different bTB manager, and DCA bTB management plans apply. Herds located in the northwest South Island had four times the daily hazard of detection of a further episode compared to herds in the south of the South Island. The South Island's West Coast was the first DCA in the country where bTB was found in wildlife, and continues to have the highest herd prevalence and incidence in the country, with about 20% of herds in some localities under infected movement control at any one time [10]. Twenty-seven per cent of herds in the northwest of the South Island experienced repeat bTB episodes throughout the study period, compared to 2–6% of herds in other DCAs. This area also has a high rate of non-specific reactions to bTB testing, possibly due to climatic and soil conditions, which complicates the diagnosis of true infection and increases the cost of the eradication programme, for both herd managers and TBfree New Zealand. For this reason, serial IFN-γ testing of skin test-positive animals has often been used on the West Coast of the South Island (Fig. 1). The cause of the much higher rate of repeat episodes in the northwest of the South Island is likely to be multifactorial. A higher rate of exposure to infected wildlife, the difficulty of managing infection in the face of non-specificity and undefined regional factors such as climate and soil type, affecting the survivability of Mycobacterium bovis in the environment, may all have a role in increasing the hazard of repeat episodes in this DCA.
When there had been more than one bTB episode prior to the index, additional infected episodes exponentially increased the hazard of detection of a further episode of bTB, after adjusting for the effect of DCA in which the herd was located. Reactivity to skin testing and IFN-γ involves cell-mediated immunity, which in individual animals wanes over time and is replaced by humoral immunity and therefore a longer history of infection may increase the chance that non-responsive (‘anergic’) animals are present in a herd to act as reservoirs of infection [11, 12].
The number of animals with lesions, as a continuous variable, was not significantly associated with bTB recurrence interval. However, episode severity as a categorical variable (1 vs. >1 animal with lesions detected) was significantly associated with bTB recurrence interval. When the index episode involved more than one positive bTB case animal, the daily hazard of detection of a further bTB episode was doubled. This agrees with findings from Ireland, where the severity of exposure increased the hazard of a future episode [13–16]. Infection severity as defined by numbers of standard skin test-positives was a significant risk factor for movement of infection out of de-restricted herds in Ireland [17].
The effect of having test-positive animal(s) present at a final clearance test on the hazard of detection of a further bTB episode was found to vary over time, and for this reason a time-dependent covariate was developed, with a cut-off of 730 days (2 years). When herds had a test-positive animal(s) at a final whole-herd test, the hazard of detection of a further bTB episode was 2·59 (95% CI 1·30–5·13) times that of herds where no test-positives were found, when the follow-up period ranged from 0 to 730 days. After 730 days, the effect of having a test-positive animal(s) at the clearance test increased the daily hazard of bTB recurrence by a factor of 1·05 (95% CI 0·28–3·91), but this effect was not significant at the alpha level of 0·05. Test-positives at a final herd test may be indicative of on-going within-herd transmission and significantly increase the hazard of a further bTB episode within the first 2 years after clearance, but we found no evidence that this effect persists beyond 2 years. Recurrent infection after 2 years is more likely to be attributable to repeated exposure to infected wildlife, or inward movement of infected animals from other herds.
Herds having longer index-infected episodes had a slightly reduced hazard of bTB recurrence interval. Six-monthly increases in the duration of the index episode decreased the daily hazard of bTB recurrence by a factor of 0·69 (95% CI 0·51–0·94). More rigorous ancillary testing may have prolonged the period for achieving a clear status but reduced the risk of recurrence in these herds.
Methodological issues
Much of the variation between herds in time to detection of a repeat bTB episode will be accounted for by exposures that are either unmeasured using current information-recording systems, or are not measurable. Such factors may include the actual proximity of herds to infected wildlife, differences in farm management (e.g. stocking rates and nutritional status of herds) and herd manager's purchasing behaviour. The data available for the present study did not allow us to determine the relative importance of vector risk, movement risk and recrudescent within-herd infection as a cause of further bTB episodes. The inclusion of VRA as a predictor was recognized to have limitations because: (1) vector risk is not homogeneous either within or between individual VRAs, and (2) VRA was highly correlated with the DCA in which a herd was located. VRA was not significant as a predictor of bTB recurrence interval, and did not appear in the final model. Future studies of this type could include a more accurate assessment of risk from infected vectors as a predictor of recurrent bTB risk. The R2 value for the model was 23%, indicating that a relatively large proportion of the variation in bTB recurrence interval was not explained by the explanatory variables included in the model. R2 measures for the Cox model are not considered reliable as a single measure of model fit [18], and a dataset with many censored observations may cause an artificially low R2 value. Regardless, we believe the model presented in this study has gone some way towards defining and quantifying the effect of factors that are both measurable and easily recorded. In addition, and perhaps more importantly, some of the explanatory variables included in the model are readily amenable to changes in bTB management policy.
The recruitment period for this study was relatively short (1 June 2006 to 1 November 2010). All herds were followed until the end of the follow-up period on 5 May 2011. The reason for beginning the risk period on 1 June 2006 was that major database changes occurred in 2005, and some herd records were either missing or thought to be inaccurate before 2006. If herds had not experienced a further infected episode by the end date of the follow-up period (5 May 2011) they were treated as censored observations. We acknowledge that the follow-up period may not have been long enough and that a longer period of follow-up of herds in the dataset will show the true rate of further episodes and some risk factors may change in importance. The overall percentage of herds experiencing a subsequent bTB episode during the follow-up period was 14%, whereas 59% of these same herds had experienced at least one bTB episode prior to their index episode. It is likely that many of the herds in the study population are yet to experience a repeat episode. The median time to a further infected episode in this study was 547 days (~1½ years), whereas in an earlier unpublished study (J. A. Sinclair, TBfree New Zealand), using the same source list but looking back in time at episodes prior to the index episode, the median interval was 5½ years. Our study has identified the most influential predictive factors for recurrence of bTB detection in New Zealand herds, and extending the study period should: (1) increase the size of the dataset by making some herds eligible that were excluded from the study; (2) allow the detection of more subtle effects of predictors that were not significant in the model, but may nevertheless be important.
We recognized that a collinearity issue existed between some explanatory variables, in that bTB management decisions were often subjective and differed by DCA. A χ2 test of DCA vs. whether or not a test-positive had been detected at the final clearance test was highly significant (χ2 = 18·1, d.f. = 3, P = 0·0004). DCA was considered important to include in the model a priori because of recognized geographical differences in the prevalence and behaviour of the infection. The results of the model may need to be interpreted with caution, because the correlation between these two variables may have resulted in an underestimate of the standard errors in the model and a higher chance of a Type I error. There is little precedent in the literature for dealing with multicollinearity issues in survival analysis, although it is recognized as a problem [19], particularly where correlations might change over time because of non-proportionality of hazards [20]. One group of investigators sequentially introduced covariates in order of least correlation, examining the stability of parameter estimates at each stage of the process [20]. This approach would have merit for a larger study, but was considered beyond the scope of the present work.
We conclude that the presence of unresolved infection in a herd is a contributor to further bTB episodes in the first 2 years after clearance. These findings agree with the investigations in the UK and Ireland, which have shown repeatedly that bTB spreads from de-restricted herds to clear herds via the transfer of undetected infection after de-restriction [9, 21, 22]. The present study has shown that under New Zealand conditions also, failure to detect infection before clearance puts a herd at risk for future episodes of bTB detection, particularly in the first 2 years post-clearance. The findings from this study would be difficult to extrapolate to other countries, because of the differences in bTB management policies between countries. However, this study has added weight to the growing body of evidence to show that residual infection in herds poses a problem to bTB eradication schemes, and that the goal should be to maximize within-herd sensitivity in the management of this problematic infection.
A bTB eradication strategy needs to be constantly reviewed and updated [23], identifying and, where possible, removing constraints to progress [24, 25]. TBfree New Zealand is reviewing policies to increase the sensitivity of detecting residual infection before clearance and to intensify post-clearance testing and movement tracking in herds known to be at risk for recrudescence of bTB infection. These policies should be tailored to individual herds, and based on assessment of risk factors and the consequence of a repeat episode. Measures may include the requirement for parallel IFN-γ testing of breeding stock before clearance, an increased frequency of testing post-clearance, following up of stock moving out of previously infected herds and testing these animals more intensively, and post-clearance parallel testing. Innovations in diagnostic testing, such as the use of an ‘electronic nose’ [26] and ELISA testing [27] when used in conjunction with skin testing have the potential to improve individual animal sensitivity and their potential for use in removing residual infection from New Zealand herds should be investigated further.
ACKNOWLEDGEMENTS
The authors acknowledge the input of Dr Paul Livingstone, TB Eradication and Research Manager, OSPRI New Zealand. This research received no specific grant from any funding agency, commercial or not-for profit sectors.
DECLARATION OF INTEREST
K.L.D, J.A.S. and M.A.B. are employed by TBfree New Zealand, a programme of OSPRI New Zealand.
REFERENCES
- 1.Morris RS, Pfeiffer DU, Jackson R. The epidemiology of Mycobacterium bovis infections. Veterinary Microbiology 1994; 40: 153–177. [DOI] [PubMed] [Google Scholar]
- 2.Olea-Popelka FJ, et al. Risk factors for disclosure of additional tuberculous cattle in attested-clear herds that had one animal with a confirmed lesion of tuberculosis at slaughter during 2003 in Ireland. Preventive Veterinary Medicine 2008; 85: 81–91. [DOI] [PubMed] [Google Scholar]
- 3.Therneau TM. A package for survival analysis in S. R version 2.37–7 (http://CRAN.R-project.org/package=survival), 2013.
- 4.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. [Google Scholar]
- 5.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994: 81: 515–526. [Google Scholar]
- 6.Therneau TM, Grambsch PM. Modelling Survival Data: Extending the Cox Model. New York: Springer, 2000, 350 pp. [Google Scholar]
- 7.von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. British Medical Journal 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Animal Health Board (now TBfree New Zealand). Annual Report, 2012. Wellington, New Zealand. [Google Scholar]
- 9.Berrian AM, et al. Risk of bovine tuberculosis for cattle sold out from herds during 2005 in Ireland. Veterinary Record 2012; 170: 620. [DOI] [PubMed] [Google Scholar]
- 10.Animal Health Board (now TBfree New Zealand). National Operational Plan, 2011. Wellington, New Zealand. [Google Scholar]
- 11.Goodchild AV, Clifton-Hadley RS. Cattle-to-cattle transmission of Mycobacterium bovis. Tuberculosis 2001; 81: 23–41. [DOI] [PubMed] [Google Scholar]
- 12.de la Rua-Domenech R, et al. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Research in Veterinary Science 2006; 81: 190–210. [DOI] [PubMed] [Google Scholar]
- 13.Olea-Popelka FJ, et al. Breakdown severity during a bovine tuberculosis episode as a predictor of future herd breakdowns in Ireland. Preventive Veterinary Medicine 2004; 63; 163–172. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe DM, et al. From explanation to prediction: a model for recurrent bovine tuberculosis in Irish cattle herds. Preventive Veterinary Medicine 2010; 94: 170–177. [DOI] [PubMed] [Google Scholar]
- 15.Karolemeas K, et al. Recurrence of bovine tuberculosis breakdowns in Great Britain: risk factors and prediction. Preventive Veterinary Medicine 2011; 102: 22–29. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher MJ, et al. Comparison of bovine tuberculosis recurrence in Irish herds between 1998 and 2008. Preventive Veterinary Medicine 2013; 111: 237–244. [DOI] [PubMed] [Google Scholar]
- 17.Clegg TA, et al. Potential infection-control benefit for Ireland from pre-movement testing of cattle for tuberculosis. Preventive Veterinary Medicine 2008; 84: 94–111. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, et al. A comparison of goodness-of-fit tests for the logistic regression model. Statistics in Medicine 1997; 16: 965–980. [DOI] [PubMed] [Google Scholar]
- 19.Mitra D, Golder PN. Whose culture matters? Near-market knowledge and its impact on foreign market entry timing. Journal of Marketing Research 2002; 39: 350–365. [Google Scholar]
- 20.Van den Poel D, Larivière B. Customer attrition analysis for financial services using proportional hazard models. European Journal of Operational Research 2004; 157: 196–217. [Google Scholar]
- 21.clegg TA, et al. Longer-term risk of Mycobacterium bovis in Irish cattle following an inconclusive diagnosis to the single intradermal comparative tuberculin test. Preventive Veterinary Medicine 2011; 100: 147–154. [DOI] [PubMed] [Google Scholar]
- 22.Clegg TA, et al. Shorter-term risk of Mycobacterium bovis in Irish cattle following an inconclusive diagnosis to the single intradermal comparative tuberculin test. Preventive Veterinary Medicine 2011; 102: 255–264. [DOI] [PubMed] [Google Scholar]
- 23.Collins JD. Tuberculosis in cattle: strategic planning for the future. Veterinary Microbiology 2006; 112 (2–4 Special Issue): 369–381. [DOI] [PubMed] [Google Scholar]
- 24.Good M, Duignan A. An evaluation of the Irish single test-positive breakdown protocol for 2005–2008 inclusive and its potential application as a monitor of tuberculin test performance. Veterinary Microbiology 2011; 151: 85–90. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan M. Progress in tuberculosis eradication in Ireland. Veterinary Microbiology 2011; 151: 160–169. [DOI] [PubMed] [Google Scholar]
- 26.Fend R, et al. Use of an electronic nose to diagnose Mycobacterium bovis infection in badgers and cattle. Journal of Clinical Microbiology 2005; 43, 1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh MD, et al. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 2005; 114: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]