SUMMARY
Since their discovery, four species of human bocavirus (HBoV) have been described in patients with respiratory and gastrointestinal diseases. However, a clear causal association between HBoV-1 and gastroenteritis has not been demonstrated. In this study, we describe the detection and quantification of HBoV-1 in stools from children with acute non-bacterial gastroenteritis using quantitative polymerase chain reaction. HBoV-1 genome was detected in 10·6% of stools with frequent association with rotavirus and norovirus. The median of HBoV-1 viral load was 1·88 × 104 genome/ml, lower than previously shown in secretions of patients with respiratory infections, without any obvious association between high viral load and presence of HBoV as single agent. Thus, although HBoV-1 was frequently detected in these patients, there is no clear causal association of this agent with diarrhoea. Indeed, HBoV-1 DNA in stools of patients with gastroenteritis without respiratory symptoms may be a remnant of previous infections or associated with prolonged shedding of virus in the respiratory or digestive tracts.
Key words: Gastroenteritis, HBoV, norovirus, rotavirus, viral co-infections
INTRODUCTION
Human bocavirus (HBoV) was discovered during a meta-genomic study of pooled specimens of respiratory secretions from Swedish children with acute respiratory symptoms [1]. HBoV was classified in the family Parvoviridae, subfamily Parvovirinae, genus Bocavirus. It is a non-enveloped, icosahedral virus, with a 5·3 kb single-stranded DNA genome with three open reading frames [1]. Recent studies have revealed that there are four species of HBoV, numbered sequentially HBoV-1 to HBoV-4 [2–4].
HBoV has been detected in samples from patients with symptoms of acute respiratory infection, gastroenteritis and tonsillitis in several regions of world [5–8]. However, the association of HBoV with human respiratory and gastrointestinal infections is unclear. Similar to other viruses of the family Parvoviridae, HBoV may persist [9, 10], leading to prolonged detection of its genome in secretions. Therefore, it is unsurprising that HBoV is frequently detected simultaneously with genomes of other agents, including respiratory and enteric viruses [10, 11]. Detection of direct or surrogate viral replication markers in association with acute symptoms could partially help to circumvent the non-fulfilment of Koch's postulates by the lack of an experimental animal model. Recent studies have shown that only a small portion of patients with HBoV-1 and respiratory diseases have an acute infection by this virus, characterized by the presence of replicative markers, high viral load and absence of viral co-infections in nasopharyngeal secretions, in addition to the frequent simultaneous presence of diarrhoea [10]. In Latin America, few studies have been conducted on the role of HBoV and gastroenteritis in children [12, 13]. This report describes a study of HBoV-1 viral loads, as an indicator of acute infection, in stool samples from patients with non-bacterial acute diarrhoea with or without the simultaneous detection of other enteric viruses.
METHODS
Samples
In this retrospective, cross-sectional study, 349 stool samples from children (52·5% boys) were screened by quantitative polymerase chain reaction (qPCR) for HBoV DNA. Samples were collected between January and December 2004 from children aged <5 years with acute gastroenteritis but negative for common enterobacteria by microbiological assays (Salmonella spp., Shigella spp., and enteropathogenic and enteroinvasive Escherichia coli), and without respiratory symptoms, admitted to one of the main medical centres (Sanatorio San Roque) in Paraguay [14, 15]. Of the tested samples, 253 (72·3%) were from children aged <2 years. All samples had been previously tested for rotavirus (RV) and norovirus (NoV) [14, 15]. All stools samples were stored at −20°C until DNA extraction. Only one clinical specimen was tested for each patient included in this study.
Ethical statement
The study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the Ethics Review Committee of Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción (IICS-UNA), Number P45/07, and informed consent was obtained from all parents and guardians prior to enrolment.
qPCR
DNA was extracted from 300 μl of a stool suspension prepared in PBS and centrifuged at 3500 g, using a commercial kit (Wizard Genomic DNA Purification kit; Promega, USA). The qPCR assay for HBoV detection and quantification, targeted to the viral NP1 gene, was based on previously published procedures [16]. Amplification of serial decimal dilutions of plasmid pGEM-FULL4HBoV containing a 1 kb insert from the HBoV NP1 gene (2270–3280 nt) was included in each batch to calculate viral loads [10]. The assay detection limit was 4·6 copies of HBoV plasmid, and the slope and correlation coefficient of the standard curve were −3·386 and 0·994, respectively. All applicable measures were taken to prevent cross-contamination of samples, including the use of separate rooms for DNA extraction and PCR mix preparation. Negative and positives control samples were included in all batches. All samples were tested by qPCR for β-actin as an endogenous control of sample integrity, as described previously [10].
Phylogenetic analysis
All HBoV-positive samples were tested by PCR to obtain a 500 bp product from the HBoV VP1/VP2 region (nt 3640–4140) using primers VP1-F (5′-ACAATTCAGAATGGTCACCTCTACA-3′) and VP2-R (5′-TTGGTGGTCACTGTTTTCAAG-3′). All obtained products were excised from 1·0% agarose gels, purified with QIAquick Gel Extraction kit (Qiagen, Germany) and sequenced with BigDye Terminator v. 3.1 Cycle Sequencing (Applied Biosystems, USA) using the ABI Prism 3500 Genetic Analyzer. Both DNA strands were sequenced using VP1-F and VP2-R primers. A representative Paraguayan isolate, Py380SR04, was deposited in the GenBank database (accession no. JN701022). The 37 sequences obtained were manually edited using Bioedit v. 7.0.9 [17] and phylogenetic analysis was performed with an additional 45 sequences of HBoV (1–4) VP1/VP2 genes retrieved from GenBank, aligned with Clustal X 5.2 [18], using MEGA v. 5.0 [19], with Kimura-2 as the substitution model for nucleotide, and statistically supported by the bootstrap method using 1000 replicates.
RESULTS
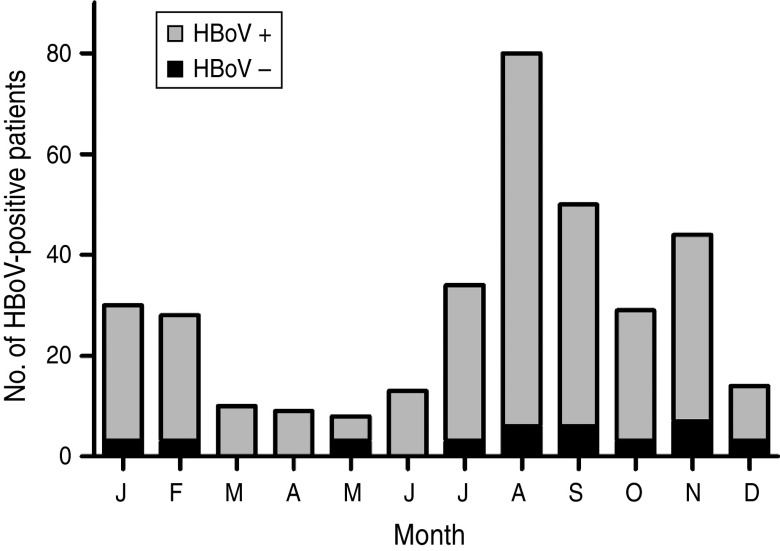
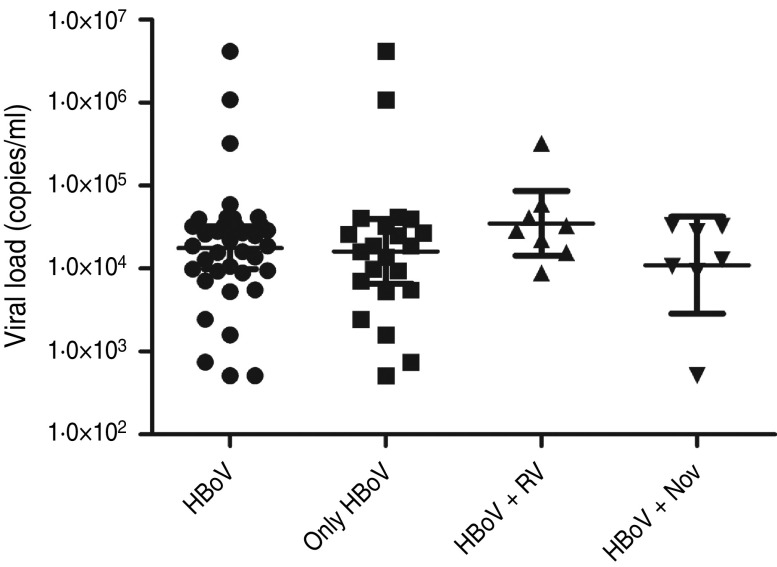
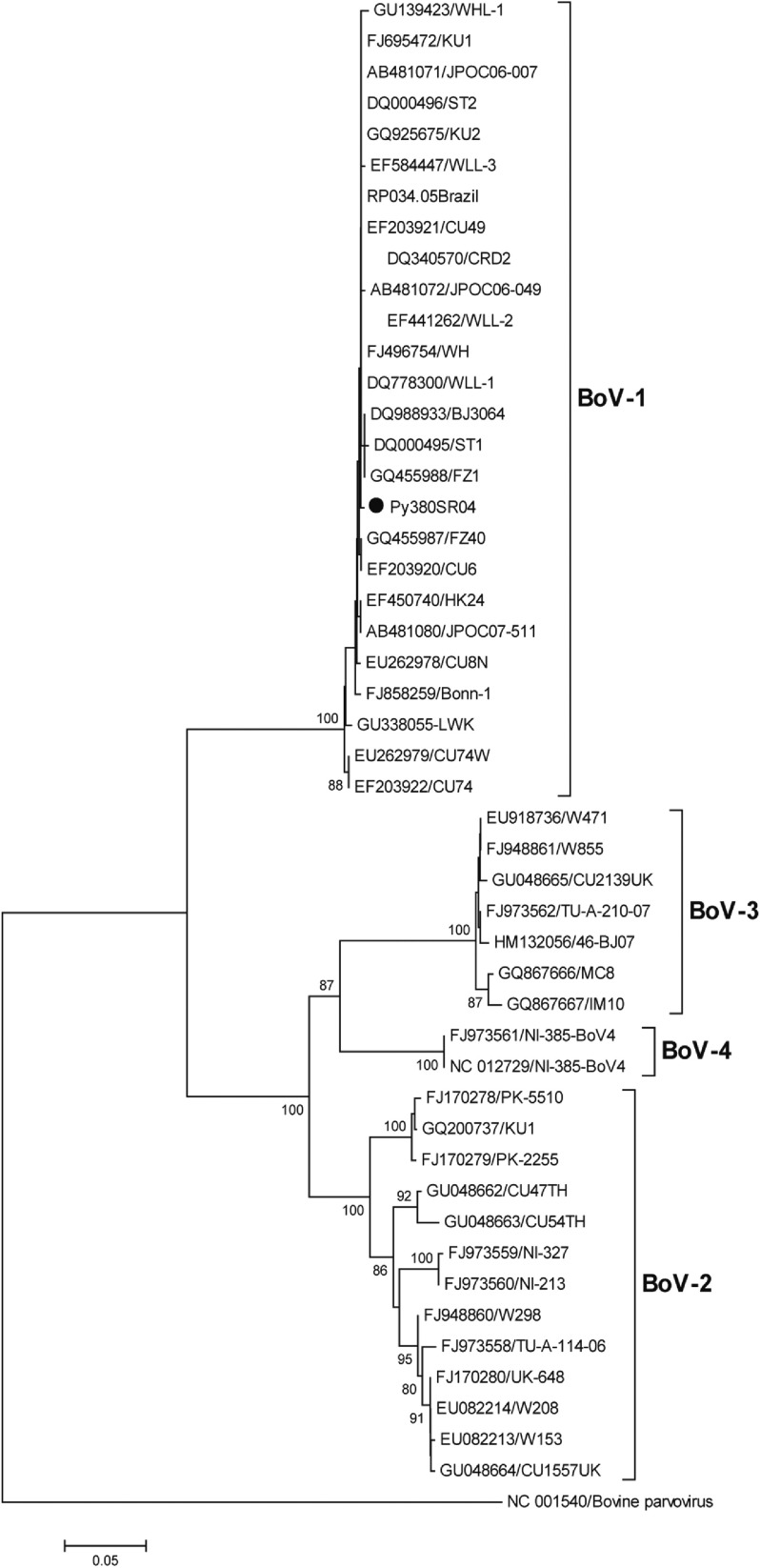
HBoV-1 genome was detected by qPCR in 37 (10·6%) samples, the majority (67·6%) of which were from children aged <2 years (Table 1). No significant association was noted between the presence of HBoV-1 and child age or gender. Monthly distribution showed no clear seasonality (Fig. 1). Co-infection with RV or NoV was detected in 15 (40·5%) of the 37 HBoV-positive samples. RV was detected in eight (21·6%) and NoV in seven (18·9%), and co-detection of RV and NoV was not found (Table 1). Absolute quantification of HBoV DNA by qPCR revealed broad variation in samples, with a median 1·88 × 104 (mean ± s.d.: 1·69 × 105 ± 6·9 × 105) copies/ml (Fig. 2). The median viral load in stools in which only HBoV-1 was detected was 1·74 × 104 (2·54 × 10 ± 9·02 × 105) copies/ml, whereas in samples from patients with HBoV-1 and RV co-infection it was 3·07 × 104 (6·64 × 104 ± 1·05 × 105) copies/ml, and in patients with HBoV-1 and NoV co-infection it was 1·27 × 104 (1·81 × 104 ± 1·29 × 104) copies/ml (Fig. 2). There was no association of high viral load with any pattern of viral co-infections. The sequencing of 500 bp in the VP1/VP2 region of HBoV genomes confirmed they were all HBoV-1 (Fig. 3), and revealed ∼99% sequence similarity (data not shown).
Table 1.
Age and gender distribution of positive samples for HBoV-1 alone or in association with other viruses
| Age (months)and gender | SingleHBoV+ | HBoV+ | HBoV– | Total | |
|---|---|---|---|---|---|
| RV+ | NoV+ | ||||
| 0–24 | 15 | 7 | 3 | 228 | 253 |
| 25–60 | 7 | 1 | 4 | 84 | 96 |
| Male | 6 | 6 | 4 | 167 | 183 |
| Female | 16 | 2 | 3 | 145 | 166 |
| Total | 22 | 8 | 7 | 312 | 349 |
RV, Rotavirus; NoV, norovirus.
Fig. 1.
Monthly distribution of HBoV-positive stool samples from children with acute gastroenteritis in Paraguay, 2004.
Fig. 2.
Viral loads of HBoV-1 (copies of genome/ml) determined by quantitative polymerase chain reaction in stool samples from patients with HBoV-1 as single agent, and with simultaneous detection of rotavirus (RV) and norovirus (NoV).
Fig. 3.
Phylogenetic tree constructed with nucleotide sequences of 46 HBoVs in a region of the VP1/VP2 gene (500 bp). Because of the high sequence similarity of all 37 Paraguayan strains (>99%), only one representative strain (Py380SR04) is shown in the tree (solid black circle). The outgroup sequence corresponds to a strain of bovine parvovirus. Bootstrap values <70% are not shown.
DISCUSSION
HBoV-1 has been detected in studies conducted around the world, usually in association with respiratory infections [20]. HBoV detection in stool samples from patients with acute gastroenteritis has been reported to range from 2% to 20% [21–24]. In the present study, about 10% of the tested samples were positive for HBoV-1 DNA by qPCR, which was confirmed by an independent amplification of another genomic region with sequence analysis of the VP1 gene. In a study recently conducted in Brazil [12], a frequency of 2% of HBoV was reported in children aged <15 years with acute diarrhoea between 2003 and 2005. The difference in frequencies between these two studies probably reflects variations in yearly rates of HBoV circulation, and the ages of patients. In fact, studies have shown that HBoV can circulate with different rates in consecutive years [25], and is more prevalent in younger patients [26]. Moreover, exclusion of patients with a positive stool sample for common enterobacteria may have contributed to the higher HBoV frequency observed.
The role of HBoV-1 as causative agent of gastroenteritis in humans is still unclear and it is reasonable to assume that HBoV-1 is in fact a virus of the respiratory tract, which is detected in stools as a remnant of its passage through the gastrointestinal tract. Despite the fact that in ∼60% of the HBoV-1-positive samples this agent was the only virus detected, this is not sufficient to support its role in the aetiology of gastroenteritis. Therefore, determination of HBoV-1 viral load was attempted to further substantiate this issue, bearing in mind previous results obtained with HBoV-2, a predominantly enteric HBoV that may reach up to 109 DNA copies/ml of stools [27]. The present analysis showed that viral loads varied broadly, with no correlation with detection of HBoV-1 as the only detectable virus in stools. This is in contrast with a previous study conducted in patients with respiratory diseases, which showed that HBoV viral loads can be as high as 1012 copies of viral genome/ml of nasopharyngeal secretions, and that higher viral loads correlated with lack of simultaneous detection of other respiratory viruses [10].
The present results support the hypothesis that HBoV-1 replicates primarily in the respiratory tract, and that swallowed nasopharyngeal secretions might serve as a source of virus contamination of the stools. This hypothesis is not contradicted by the fact that the stools tested in the present study were from children with diarrhoea without respiratory illnesses, as asymptomatic prolonged shedding of respiratory HBoV is frequent [9]. In addition, there is evidence of HBoV persistence in respiratory and gastrointestinal tracts. HBoV-1 is highly frequent in adenoids and palatine tonsils of patients with adenotonsillar chronic diseases without respiratory symptoms [28] and the episomal genomes of HBoV-1, -2 and -3 were detected in clinical samples from humans [29–31].
Given their propensity for prolonged asymptomatic shedding, definitive association between HBoVs and gastroenteritis awaits fulfilment of classic or alternative Koch's postulates. Before that becomes achievable by in vivo experimentation in animal models, prospective studies of clinical samples, with simultaneous sampling of respiratory secretions, stools and blood, and detection of HBoV replication markers are still needed. Such studies should ideally be supported by in situ methods to demonstrate HBoV replication in gastrointestinal mucosa either in vivo or ex vivo.
Although multiple viruses and bacteria have been associated with gastroenteritis, consistently high proportions of diarrhoeal diseases remain without identification of a pathogen. Therefore, the continuous discovery of new agents of diarrhoeal diseases, especially based on meta-genomic approaches, will hopefully close this gap and eventually help the development of effective interventions to lessen the burden of this disease.
ACKNOWLEDGEMENTS
This research was partially funded by Sustainable Science Institute (G.I.P.), FAPESP and CNPq (J.L.P.M, V.H.A, E.A.). The funders had no role in design, data collection, analysis, or interpretation of the data.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Allander T, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proceedings of the National Academy of Sciences USA 2005; 102: 12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur JL, et al. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathogens 2009; 5: e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor A, et al. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. Journal of Infectious Diseases 2010; 201: 1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor A, et al. A newly identified bocavirus species in human stool. Journal of Infectious Diseases 2009; 199: 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry AM, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. Journal of Infectious Diseases 2007; 195: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastien N, et al. Human Bocavirus infection, Canada. Emerging Infectious Diseases 2006; 12: 848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagliardi TB, et al. Human bocavirus respiratory infections in children. Epidemiology and Infection 2009; 137: 1032–1036. [DOI] [PubMed] [Google Scholar]
- 8.Vicente D, et al. Human bocavirus, a respiratory and enteric virus. Emerging Infectious Diseases 2007; 13: 636–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin ET, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. Journal of Infectious Diseases 2010; 201: 1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proenca-Modena JL, et al. Detection of human bocavirus mRNA in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS One 2011; 6: e21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonzel L, et al. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatric Infectious Disease Journal 2008; 27: 589–594. [DOI] [PubMed] [Google Scholar]
- 12.Albuquerque MC, et al. Human bocavirus infection in children with gastroenteritis, Brazil. Emerging Infectious Diseases 2007; 13: 1756–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos N, et al. Human bocavirus species 2 and 3 in Brazil. Journal of Clinical Virology 2010; 48: 127–130. [DOI] [PubMed] [Google Scholar]
- 14.Parra GI, et al. Diversity of group A rotavirus strains circulating in Paraguay from 2002 to 2005: detection of an atypical G1 in South America. Journal of Clinical Virology 2007; 40: 135–141. [DOI] [PubMed] [Google Scholar]
- 15.Amarilla A, et al. Rotavirus infection in the Paraguayan population from 2004 to 2005: high incidence of rotavirus strains with short electropherotype in children and adults. Medical Science Monitor 2007; 13: CR333–337. [PubMed] [Google Scholar]
- 16.Neske F, et al. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. Journal of Clinical Microbiology 2007; 45: 2116–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 1999; 41: 95–98. [Google Scholar]
- 18.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007; 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 19.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 2011; 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allander T. Human bocavirus. Journal of Clinical Virology 2008; 41: 29–33. [DOI] [PubMed] [Google Scholar]
- 21.Cheng W, et al. Phylogenetic and recombination analysis of human bocavirus 2. BMC Infectious Diseases 2011; 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, et al. Detection of human bocavirus 3 in China. European Journal of Clinical Microbiology and Infectious Diseases 2011; 30: 799–805. [DOI] [PubMed] [Google Scholar]
- 23.Pham NT, et al. Human bocavirus infection in children with acute gastroenteritis in Japan and Thailand. Journal of Medical Virology 2011; 83: 286–290. [DOI] [PubMed] [Google Scholar]
- 24.Nadji SA, et al. Phylogenetic analysis of human bocavirus isolated from children with acute respiratory illnesses and gastroenteritis in Iran. Scandinavian Journal of Infectious Diseases 2010; 42: 598–603. [DOI] [PubMed] [Google Scholar]
- 25.Maggi F, et al. Human bocavirus in Italian patients with respiratory diseases. Journal of Clinical Virology 2007; 38: 321–325. [DOI] [PubMed] [Google Scholar]
- 26.Karalar L, et al. Prevalence and clinical aspects of human bocavirus infection in children. Clinical Microbiology and Infection 2010; 16: 633–639. [DOI] [PubMed] [Google Scholar]
- 27.Kantola K, et al. Real-time quantitative PCR detection of four human bocaviruses. Journal of Clinical Microbiology 2010; 48: 4044–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proenca-Modena JL, et al. High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease. PLoS One 2012; 7: e42136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lusebrink J, et al. Detection of head-to-tail DNA sequences of human bocavirus in clinical samples. PLoS One 2011; 6: e19457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapoor A, et al. Bocavirus episome in infected human tissue contains non-identical termini. PLoS One 2011; 6: e21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, et al. Detection of a bocavirus circular genome in fecal specimens from children with acute diarrhea in Beijing, China. PLoS One 2012; 7: e48980. [DOI] [PMC free article] [PubMed] [Google Scholar]