SUMMARY
We describe trends in incidence rates of methicillin-resistant Staphylococcus aureus (MRSA) in HIV-infected and HIV-uninfected patients enrolled in a large northern California Health Plan, and the ratio of MRSA to methicillin-susceptible S. aureus (MSSA) case counts. Between 1995 and 2010, 1549 MRSA infections were diagnosed in 14060 HIV-infected patients (11·0%) compared to 89546 MRSA infections in 6597396 HIV-uninfected patients (1·4%) (P = 0·00). A steady rise in MRSA infection rates began in 1995 in HIV-uninfected patients, peaking at 396·5 infections/100000 person-years in 2007. A more rapid rise in MRSA infection rates occurred in the HIV-infected group after 2000, peaking at 3592·8 infections/100000 in 2005. A declining trend in MRSA rates may have begun in 2008–2009. Comparing the ratio of MRSA to MSSA case counts, we observed that HIV-infected patients shouldered a greater burden of MRSA infection during most years of study follow-up compared to HIV-uninfected patients.
Key words: Epidemiology, HIV/AIDS, incidence, methicillin-resistant S, aureus (MRSA)
INTRODUCTION
Staphylococcus aureus can be both a part of normal human flora and a human pathogen causing skin and soft tissue infections (SSTIs), as well as invasive infections acquired by both healthy individuals and hospital patients [1]. S. aureus strains exhibiting resistance to methicillin (MRSA) were first observed in hospitals in Europe in the early 1960s and later in the USA [2]. In succeeding decades, MRSA have emerged in patients lacking contact with a hospital/healthcare setting, and are termed ‘community-onset’ MRSA. Outbreaks of MRSA infection have arisen in selected populations [3–6] including HIV-infected patients with generally more severe outcomes in immunocompromised individuals, and are associated with increased mortality [7–9]. However, most studies of the epidemiology of MRSA in HIV-infected individuals have been conducted mainly in the hospitalized setting or involved small clinic patient populations, and few have compared incidence rates in HIV-uninfected individuals from the same setting [9–22]. Therefore, MRSA incidence rate estimates from previous studies may be less generalizable and likely to have under-ascertained the incidence of community-onset MRSA infections [17, 23, 24].
In the current study we examined time trends in MRSA infection rates, comparing known HIV-infected patients to the general HIV-uninfected patient population who were members of the Kaiser Permanente Northern California (KPNC) health plan during the same 16 years of study follow-up. We ascertained laboratory-confirmed MRSA infection from patients receiving ambulatory care as well as from hospitalized patients, and examined the time trends in the ratio of MRSA to methicillin-susceptible S. aureus (MSSA) infection case counts.
METHODS
Study population
The KPNC is a fully integrated healthcare delivery system with a current membership of about 3·3 million individuals (>6·5 million cumulative members since 1995), representing 30% of the medically insured population in Northern California. KPNC health plan members are seen at medical centres throughout the 17-county catchment region. The membership is representative of the Northern California population with respect to race/ethnicity, gender, and socioeconomic status, except for some under-representation of both extremes of the economic spectrum [25].
A retrospective cohort study was conducted between 1995 and 2010 in all members of the KPNC health plan. During that period 14 060 patients were ascertained to be HIV-infected. Since 1988, KPNC's Division of Research (DOR) has maintained a surveillance system of patients who are HIV-1 seropositive, ascertained through monitoring electronic inpatient, outpatient, laboratory testing, and pharmacy dispensing databases for sentinel indicators of probable HIV infection. HIV-1 seropositivity is then confirmed through review of patient's medical records. Ascertainment of HIV-infected patients has been shown to be at least 95% complete.
Date of entry into the study cohort was 1 January 1995 if a patient was a member of the KPNC health plan on that date; otherwise, date of entry was defined as the date of first health plan membership between January 1995 and December 2010. Study follow-up ended at the first occurrence of one of the following events: (1) termination of health plan membership, (2) death from any cause, or (3) administrative end of the study (31 December 2010). A study patient who left the health plan could re-enter the study if he/she rejoined the health plan at a later date. During a gap in health plan membership the study patient would neither contribute to MRSA events nor to person-time denominators. HIV infection status was time-dependent, which allowed for a patient to contribute person-time and events to the HIV-uninfected group at the start of follow-up, but switch to the HIV-infected group if HIV infection was subsequently confirmed.
The study design was approved by the KPNC Institutional Review Board (under guidelines from the Kaiser Permanente Human Research Protection Programme), which provided a waiver of patient informed consent.
Case ascertainment
Data on all MRSA infection diagnoses were identified between 1 January 1995 and 31 December 2010. Ascertainment of MRSA infections was based upon laboratory culture of S. aureus isolates and determination of antibiotic susceptibility. Isolates from all human sources, except faeces/stool, nares, throat, genital or catheter tip culture, were included. For multiple isolates from the same person, we included the first isolate per patient occurring in the calendar year; if a second isolate was cultured >90 days after the first isolate date the second isolate was assumed to be a new infection. A MRSA infection diagnosis that occurred ⩾48 h after admission to hospital was classified as a hospital-onset case and as healthcare-acquired if: (1) a prior hospitalization occurred within the 30 days preceding the MRSA diagnosis date, or (2) a patient was transferred from another medical facility, or (3) a patient had been having ongoing chemotherapy or was undergoing haemodialysis at the time of MRSA diagnosis. All other MRSA infections were classified as community-acquired [26–28].
Data sources
Microbiology data sources
All S. aureus isolates were identified in KPNC's microbiology laboratory, a single laboratory that also performed all antibiotic susceptibility tests using the Microscan Microbiology System (Dade Behring, USA). Minimum inhibitory concentrations (MICs) of antibiotics were interpreted according to National Committee for Clinical Laboratory Standards (NCCLS) recommendations. The sources of isolates were classified as either ‘sterile’ (blood, bone, CSF) or ‘other’ (urine, tissue, miscellaneous).
Other patient-level data
KPNC also maintains electronic databases on patient demographics, hospital admission/discharge/transfer data, outpatient visits, pharmacy dispensing, and laboratory test results. Mortality information including date and cause of death were obtained from California death certificates and Social Security Administration databases.
Statistical analysis
For all study years annual age-adjusted MRSA infection rates (per 100000 person-years) and associated 95% confidence intervals based on the Poisson distribution were calculated using the direct method with the 2000 U.S. census population as the standard [29], and stratified by HIV infection status. Standard errors for all rates were adjusted for over-dispersion due to possible multiple events per patient per year. A χ2 test for trend in incidence rates across year was performed in the context of a Poisson regression analysis, with adjustment for age. Additionally, age-specific incidence rates of MRSA infection were estimated for 1995 and 2010, stratified by HIV infection status. We chose those two years to demonstrate the change in the rates by age distribution at the beginning and end of our study follow-up. To ascertain the magnitude of MRSA incidence in relation to all S. aureus infections, we calculated the ratio (and 95% confidence intervals) of the number of MRSA to MSSA infections over time. The change in the proportion of infections defined as hospital onset across years was also tested via the Cochran–Armitage trend test. All statistical analyses were completed using SAS software, version 9.1·3 (SAS Institute Inc., USA).
RESULTS
The study sample included 14 060 HIV-infected patients and 6 597 396 HIV-uninfected patients who were members of the KPNC health plan at some time between 1 January 1995 and 31 December 2010. Table 1 indicates that the age distribution for the HIV-uninfected patients is dissimilar to the HIV-infected patients (P < 0·0001). About 1% of all HIV-infected patients were aged ⩽19 years at study entry, compared to 31·8% of HIV-uninfected patients. The majority (71·1%) of HIV-infected patients was in the 30–49 years age group; 31·5% of HIV-uninfected patients were in the 30–49 years age group. The gender distribution was also dissimilar between the two groups (P < 0·0001).
Table 1.
Distribution of demographic characteristics of KPNC HIV-infected and HIV-uninfected patients during the study period 1995–2010
| Characteristic | HIV-infected | HIV-uninfected | ||
|---|---|---|---|---|
| N | % | N | % | |
| Age at entry into study (yr) | ||||
| <10 | 33 | 0·23 | 1 241 570 | 18·82 |
| 10–19 | 111 | 0·79 | 857 995 | 13·01 |
| 20–29 | 1751 | 12·45 | 1 243 101 | 18·84 |
| 30–39 | 5501 | 39·13 | 1 154 883 | 17·51 |
| 40–49 | 4499 | 32·00 | 922 618 | 13·98 |
| 50–59 | 1718 | 12·22 | 587 500 | 8·91 |
| ⩾60 | 447 | 3·18 | 589 729 | 8·94 |
| Gender | ||||
| Female | 1332 | 9·47 | 3 313 058 | 50·22 |
| Male | 12 728 | 90·53 | 3 284 338 | 49·78 |
| Total | 14 060 | 100 | 6 597 396 | 100 |
KPNC, Kaiser Permanente Northern California.
Between 1995 and 2010, a total of 1549 (11·0%) MRSA infections were diagnosed in 14060 HIV-infected patients, and 89546 (1·4%) MRSA infections in 6597396 HIV-uninfected patients (P = 0·00, Z test for difference in percentages). The proportion of hospital-onset vs. community-associated vs. healthcare-associated MRSA infections changed over time and differed by HIV infection status. In 1995, 23% of all diagnosed MRSA infections (stratified by HIV infection status: 23% of MRSA infections diagnosed in HIV-uninfected patients vs. 27% of MRSA infections diagnosed in HIV-infected patients, P = 0·74) were considered to be hospital-onset. In the same year, 22% of all diagnosed MRSA infections (22% diagnosed in HIV-uninfected patients vs. 18% in diagnosed HIV-infected patients, P = 0·78) were classified as healthcare-associated. The remaining 55% of diagnosed MRSA infections in 1995 (55% diagnosed in HIV-uninfected patients, 55% diagnosed in HIV-infected patients, P = 0·96) were considered community-associated. In 2010, 5% of all diagnosed MRSA infections (stratified by HIV infection status: 5% diagnosed in HIV-uninfected patients vs. 2% diagnosed in HIV-infected patients) were considered to be hospital-onset (P < 0·001 test for trend across all years 1995–2010). In the same year 6% of all diagnosed MRSA infections (6% diagnosed in HIV-uninfected patients vs. 4% diagnosed in HIV-infected patients) were considered healthcare-associated (P < 0·001 test for trend across all years 1995–2010), while the remaining 89% (89% diagnosed in HIV-uninfected patients vs. 95% diagnosed in HIV-infected patients) of all diagnosed MRSA infections were community-associated (P < 0·001 test for trend across all years 1995–2010). Clinical syndromes associated with MRSA diagnoses varied in proportion over the 16 years of study follow-up. In the early years (1995–2001) of our study follow-up, 46% of MRSA cases were associated with SSTIs; during 2006–2010, 82% of MRSA diagnoses were linked to SSTIs. Pneumonia was associated with 19% of MRSA diagnoses from 1995 to 2001; during 2006–2010, only 4% of MRSA cases were linked to pneumonia. Urinary tract infections were associated with 21% of MRSA diagnoses between 1995 and 2001; that proportion fell to 6% during 2006–2010.
The annual age-adjusted incidence rates of MRSA infection are presented in Table 2. Between 1995 and 1999, incidence rates fluctuated from a high of 245·9 MRSA infections/100 000 in 1996 and a low of 118·6 MRSA infections in 1999 in HIV-infected patients. For the same years, there was a rise in incidence from 15·5 to 40·6 MRSA infections/100000 in HIV-uninfected patients. The number of MRSA infections began to rise markedly in 2000 in HIV-infected patients; the rates peaked in 2005 (3592·8 MRSA infections/100 000). An elevated rate was also observed in 2008 (4345·5 MRSA infections) in the HIV-infected group. However, that estimate is likely unstable because the youngest age groups (<10, 10–19 years) had few MRSA events and a small amount of person-time in that year, while in all other years there were no MRSA events in the youngest age groups. Generally an increasing trend was observed from 1995 to 2005 (P < 0·001) in the HIV-infected group. MRSA infection rates continued to rise in the HIV-uninfected group to a high of 396·5 MRSA infections/100000 in 2007, with a significantly increasing trend between 1995 and 2007 (P < 0·001). In 2009 and 2010, there appeared to be a fall in age-adjusted incidence rates in HIV-infected patients. A less steep decline was observed in HIV-uninfected patients in 2008–2010.
Table 2.
Incidence of MRSA infections for diagnosis years 1995–2010 in HIV-infected and HIV-uninfected patients
| Year | HIV-infected patients | HIV-uninfected patients | ||||
|---|---|---|---|---|---|---|
| No. of MRSA | Person-years | Age-adjusted rate (95% CI)* | No. of MRSA | Person-years | Age-adjusted rate (95% CI)* | |
| 1995 | 7 | 4048·4 | 181·5 (0·0–410·6) | 357 | 2 386 462 | 15·1 (13·5–16·7) |
| 1996 | 11 | 3911·4 | 245·9 (18·8–473·1) | 516 | 2 462 349 | 20·9 (18·9–22·9) |
| 1997 | 4 | 4018·8 | 126·6 (0·0–306·3) | 690 | 2 609 994 | 26·3 (24·2–28·4) |
| 1998 | 6 | 4244 | 123·8 (0·0–276·0) | 934 | 2 748 975 | 33·9 (31·5–36·2) |
| 1999 | 6 | 4430·2 | 118·6 (0·0–251·7) | 1173 | 2 834 890 | 40·6 (38·1–43·1) |
| 2000 | 22 | 4518·9 | 337·7 (140·2–535·2) | 1400 | 2 886 699 | 46·5 (43·9–49·2) |
| 2001 | 31 | 4644·2 | 439·9 (148·5–731·2) | 1897 | 2 988 653 | 60·4 (57·4–63·3) |
| 2002 | 62 | 4786·7 | 863·3 (507·2–1219·4) | 2599 | 3 053 511 | 80·0 (76·6–83·3) |
| 2003 | 167 | 4974·2 | 2037·8 (1564·7–2510·9) | 4014 | 3 061 940 | 123·1 (119·0–127·2) |
| 2004 | 209 | 5113·1 | 3218·5 (2580·3–3856·8) | 6307 | 3 046 451 | 198·9 (193·6–204·1) |
| 2005 | 245 | 5363·1 | 3592·8 (2831·8–4353·8) | 10289 | 3 099 700 | 324·9 (318·1–331·6) |
| 2006 | 176 | 5583·1 | 2496·0 (1921·2–3070·8) | 12596 | 3 149 244 | 391·3 (383·9–398·6) |
| 2007 | 172 | 5793·4 | 1882·6 (1420·7–2344·5) | 12808 | 3 171 326 | 396·5 (389·2–403·9) |
| 2008 | 160 | 5947·9 | 4072·1 (1181·0–6963·2) | 12738 | 3 188 597 | 392·4 (385·1–399·8) |
| 2009 | 140 | 6102·4 | 1592·8 (1219·9–1965·6) | 11117 | 3 145 796 | 343·8 (337·0–350·7) |
| 2010 | 131 | 6248·8 | 1655·3 (1266·1–2044·5) | 10111 | 3 156 461 | 309·8 (303·3–316·2) |
MRSA, Methicillin-resistant Staphylococcus aureus.
Age-adjusted rates and 95% confidence intervals (CI) per 100 000 person-years.
Examining age-specific MRSA incidence rates, in 1995 we observed a sparse number of MRSA infections in HIV-infected patients, occurring mostly in the 30–49 years age group (see Table 3). In the same year MRSA infections were diagnosed in all age groups in the HIV-uninfected patients. In 2010 elevated MRSA incidence rates were found in the 20–29 years to ⩾60 years age groups in HIV-infected patients compared to 1995. Incidence rates in 2010 were elevated in all age groups in HIV-uninfected patients compared to rates in 1995.
Table 3.
Age-specific incidence of MRSA infections for diagnosis years 1995 and 2010 in HIV-infected and HIV-uninfected patients
| Year | Age (yr) | HIV-infected patients | HIV-uninfected patients | ||||
|---|---|---|---|---|---|---|---|
| No. of MRSA | Person-years | Age-specific rate (95% CI)* | No. of MRSA | Person-years | Age-specific rate (95% CI)* | ||
| 1995 | <10 | 0 | 9·2 | 0·0 (0·0–0·0) | 12 | 306 330 | 3·9 (1·7–6·1) |
| 10–19 | 0 | 11 | 0·0 (0·0–0·0) | 13 | 350 675 | 3·7 (1·7–5·7) | |
| 20–29 | 0 | 314·5 | 0·0 (0·0–0·0) | 12 | 269 837 | 4·4 (1·7–7·2) | |
| 30–39 | 2 | 1668·6 | 119·9 (0·0–291·5) | 25 | 388 140 | 6·4 (3·8–9·1) | |
| 40–49 | 4 | 1403 | 285·1 (18·3–551·9) | 35 | 410 017 | 8·5 (5·1–12·0) | |
| 50–59 | 0 | 506·4 | 0·0 (0·0–0·0) | 41 | 288 284 | 14·2 (9·7–18·8) | |
| ⩾60 | 1 | 135·6 | 737·3 (0·0–2113·7) | 219 | 373 179 | 58·7 (50·7–66·7) | |
| 2010 | <10 | 0 | 4 | 0·0 (0·0–0·0) | 967 | 365 542 | 264·5 (247·2–281·8) |
| 10–19 | 0 | 24·5 | 0·0 (0·0–0·0) | 1020 | 441 798 | 230·9 (216·2–245·6) | |
| 20–29 | 8 | 234·3 | 3413·8 (1204·3–5623·3) | 1077 | 347 264 | 310·1 (290·7–329·6) | |
| 30–39 | 20 | 818·2 | 2444·3 (1382·6–3506·0) | 1113 | 416 892 | 267·0 (250·6–283·3) | |
| 40–49 | 50 | 2255·7 | 2216·6 (1543·6–2889·6) | 1263 | 463 097 | 272·7 (256·6–288·9) | |
| 50–59 | 37 | 1948·6 | 1898·8 (1253·5–2544·0) | 1465 | 471 475 | 310·7 (293·7–327·7) | |
| ⩾60 | 16 | 963·4 | 1660·7 (817·7–2503·8) | 3206 | 650 393 | 492·9 (474·8–511·1) | |
MRSA, Methicillin-resistant Staphylococcus aureus.
Age-specific rates and 95% confidence intervals (CI) per 100000 person-years.
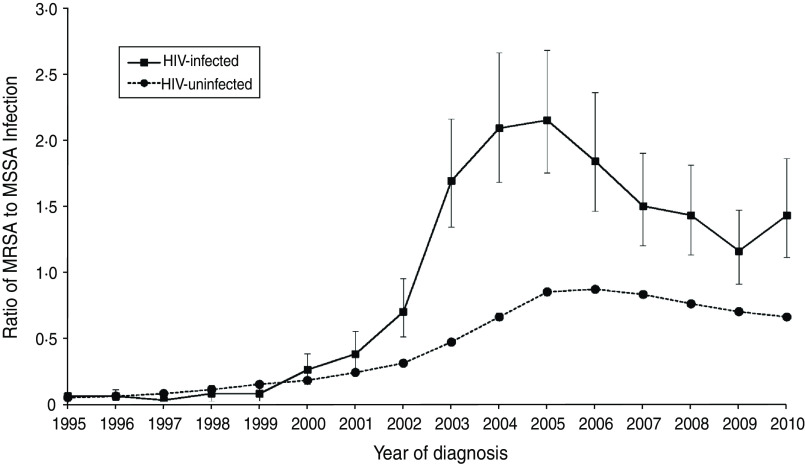
Figure 1 presents the ratio of the number of MRSA to MSSA infections by year. The ratio increased over time in HIV-uninfected patients until 2005 when it began to level off. Between 1999 and 2005, the ratio increased in the HIV-infected group, then declined until 2009. The ratio in HIV-uninfected patients never exceeded 0·87 throughout the 16 years of follow-up, while the ratio did exceed 1·0 in HIV-infected patients in years 2003–2010.
Fig. 1.
Ratio (and 95% confidence intervals) of number of MRSA to MSSA infection case counts by year of diagnosis in HIV-infected and HIV-uninfected patients.
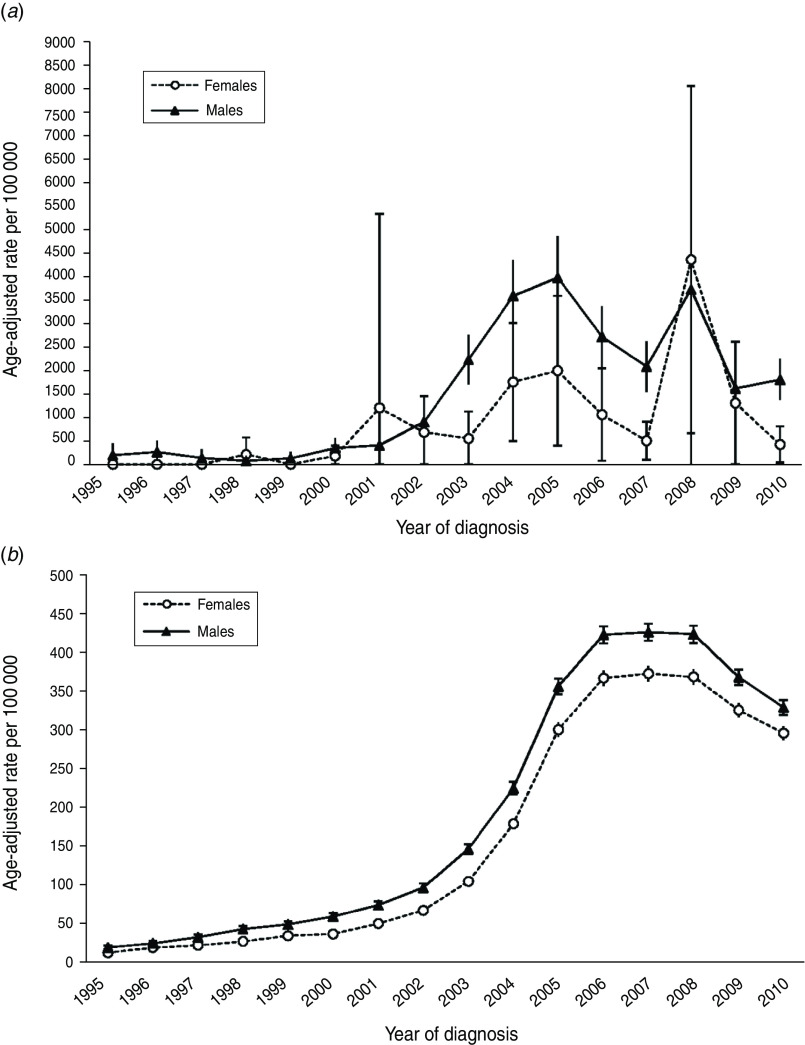
The annual age-adjusted incidence rates by gender are plotted in Figure 2(a, b) for HIV-infected and HIV-uninfected patients, respectively (Fig. 2b) [note: y-axis scales are different in panels (a) and (b) in order to highlight gender differences in incidence rates within each patient population rather than between populations]. From 1995 to 1997 and in 1999, there were no cases of MRSA infection in female HIV-infected patients. In the succeeding years female rates rose and declined, reaching a high of 4356·8 MRSA infections/100 000 in 2008. Beginning in 1995, annual infection rates in HIV-infected males increased and decreased to 2010 showing a bimodal distribution with rate peaks in 2005 and 2008. For HIV-uninfected patients, incidence rates in both females and males rose slowly between 1995 and 2002, then began an accelerated climb until peaking in 2007; rates slowly declined thereafter. From 2000 to 2010 annual incidence rates in HIV-uninfected males were higher than incidence rates in HIV-uninfected females, as evidenced by non-overlapping 95% confidence intervals.
Fig. 2.
Annual age-adjusted incidence rates (and 95% confidence intervals) of MRSA infection by sex in (a) HIV-infected patients and (b) HIV-uninfected patients.
Some patients experienced re-infection with MRSA in the same calendar year. In the HIV-infected, only four patients had multiple infections during years 1995–2001. Between 2002 and 2010 the proportion of HIV-infected patients with multiple annual infections increased, reaching a high of 14% of all infections in 2003. In the HIV-uninfected group, the proportion of patients with multiple annual infections averaged 8% during 1995–2002, and 6% during 2003–2010. The foregoing results demonstrate the greater burden of MRSA infection in HIV-infected patients compared to HIV-uninfected patients. Other studies have highlighted some clinical characteristics that appear to make some HIV-infected individuals more vulnerable to MRSA infection. In Table 4 we present the distribution of demographic and clinical characteristics in HIV-infected patients who were or were not diagnosed with MRSA infection during our study follow-up. The proportion of HIV-infected females diagnosed with MRSA infection was somewhat lower than the proportion of MRSA in HIV-infected males. Whites showed a higher proportion of MRSA infection compared to other race groups. HIV-infected patients who reported their HIV risk transmission as men who have sex with men (MSM) or MSM with injecting drug use had proportionately higher numbers of MRSA infection than HIV-infected patients who reported heterosexual contact. Patients who were receiving antiretroviral medication had proportionately higher numbers of MRSA infection compared to antiretroviral-naive patients. A similar pattern was observed in patients who had entered the study with an existing AIDS diagnosis compared to those who entered AIDS free.
Table 4.
Distribution of demographic and clinical characteristics by MRSA infection diagnosis status in KPNC HIV-infected patients during the study period, 1995–2010
| Characteristic | MRSA infected | No MRSA infection | ||
|---|---|---|---|---|
| N | % | N | % | |
| Age at entry into study (yr) | ||||
| <10 | 1 | 0·09 | 32 | 0·25 |
| 10–19 | 9 | 0·81 | 102 | 0·79 |
| 20–29 | 125 | 11·18 | 1626 | 12·56 |
| 30–39 | 483 | 43·20 | 5018 | 38·77 |
| 40–49 | 335 | 29·96 | 4164 | 32·17 |
| 50–59 | 131 | 11·72 | 1587 | 12·26 |
| ⩾60 | 34 | 3·04 | 413 | 3·19 |
| Gender | ||||
| Female | 55 | 4·92 | 1277 | 9·87 |
| Male | 1063 | 95·08 | 11 665 | 90·13 |
| Race | ||||
| African-American | 189 | 16·91 | 2230 | 17·23 |
| Asian/Pacific Islander | 35 | 3·13 | 577 | 4·46 |
| Hispanic | 140 | 12·52 | 1696 | 13·10 |
| White | 709 | 63·42 | 7126 | 55·06 |
| Other/unknown | 45 | 4·03 | 1313 | 10·15 |
| Risk group | ||||
| Heterosexual | 97 | 8·68 | 1690 | 13·06 |
| IDU (no MSM) | 44 | 3·94 | 495 | 3·82 |
| IDU (MSM) | 71 | 6·35 | 505 | 3·90 |
| MSM | 790 | 70·66 | 8241 | 63·68 |
| Other | 7 | 0·63 | 219 | 1·69 |
| Unknown | 109 | 9·75 | 1792 | 13·85 |
| Antiretroviral use | ||||
| Ever | 965 | 86·31 | 10 018 | 77·41 |
| Never | 153 | 13·69 | 2924 | 22·59 |
| AIDS diagnosis at baseline | ||||
| Yes | 235 | 21·02 | 3701 | 28·60 |
| No | 883 | 78·98 | 9241 | 71·40 |
| CD4 counts at baseline | ||||
| <100 | 131 | 11·72 | 2216 | 17·12 |
| 100–200 | 110 | 9·84 | 1433 | 11·07 |
| 201–349 | 226 | 20·21 | 2387 | 18·44 |
| 350–499 | 224 | 20·04 | 2367 | 18·29 |
| ⩾500 | 307 | 27·46 | 3136 | 24·23 |
| Missing | 120 | 10·73 | 1403 | 10·84 |
| Hepatitis B diagnosis at baseline | ||||
| Yes | 13 | 1·16 | 113 | 0·87 |
| No | 1105 | 98·84 | 12 829 | 99·13 |
| Hepatitis C diagnosis at baseline | ||||
| Yes | 31 | 2·77 | 282 | 2·18 |
| No | 1087 | 97·23 | 12 660 | 97·82 |
| Total | 1118 | 100 | 12 942 | 100 |
KPNC, Kaiser Permanente Northern California; IDU, injecting drug user; MSM, men who have sex with men.
DISCUSSION
In the current study, we have described the incidence of MRSA infections in a clinical population who were members of Northern California's largest fully integrated health plan. Patient-level data that included laboratory cultures and inpatient/outpatient/pharmacy records were used to identify MRSA and MSSA infections. The study results indicate a steadily increasing trend in MRSA infection rates from 1995 to 2007 in HIV-uninfected patients, with some evidence of a decline in 2008. National surveillance of MRSA infections conducted by the Centers for Disease Control and Prevention in nine US metropolitan areas found a 9·4% decrease in hospital-onset MRSA invasive infections and a 5·7% decrease in community-onset healthcare-associated MRSA infections between 2005 and 2008 [30]. Female HIV-uninfected patients had lower annual rates of MRSA infection compared to males. This dichotomy was also observed in a German hospital study [31], whose authors concluded that male patients had more MRSA risk factors (e.g. diabetes-related vascular disease or renal failure). MRSA infection rates increased rapidly between 2000 and 2008 in the HIV-infected group. It is of note that the MRSA infection rate in the HIV-infected group increased almost fivefold from 2001 to 2003, which coincides with the emergence of the USA300 MRSA clone that has driven increases in community-associated MRSA infections over the past decade [32]; however, the lack of molecular data on specific MRSA strains in our study cohort does not allow us to support that hypothesis. HIV-infected patients were more burdened with MRSA infections compared to HIV-uninfected patients, as evidenced by a higher (>1·0) ratio of MRSA to MSSA infections in the HIV-infected group. There is probably some confounding of these results due to differences in the number of laboratory tests ordered over time. However, there was no evidence that testing patterns differed between HIV-infected and HIV-uninfected patients. High rates of MRSA colonization in HIV-infected patients have been reported [18, 19, 33, 34]. Reasons for these high MRSA carriage rates have been attributed to high-risk sexual activities [35], hospital stays [36], and possible immunological defects [37].
In HIV-infected patients receiving care at the Owen Clinic (San Diego, CA, USA), a 6·2-fold increase in the semi-annual incidence rates of MRSA infections was observed between early 2000 and late 2003 [17] which does not differ markedly from the 5·7-fold increase in MRSA incidence rates in 2000–2003 found here. Researchers examining MRSA incidence rates in the Cook County Health and Hospital System (HHS), Chicago, Illinois, USA, found that the ratio of MRSA SSTI incidence in years 2000–2003 compared to 2004–2007 was 3·6 in HIV-infected patients [24]. Although we included MRSA incidence from all sites, i.e. not restricting to SSTIs, the corresponding ratio of 3·3 in our study was similar to the Cook County HHS estimate. In a study of HIV-infected patients receiving care at the Naval Medical Centre (San Diego, CA, USA), researchers ascertained a MRSA incidence rate of 4030 infections/100 000 during 2005 [38]; our study observed an incidence rate of 3837 infections/100 000 in the same year.
In HIV-uninfected patients receiving care at Cook County HHS, unadjusted MRSA incidence rates increased from 61 infections/100000 during the period 2000–2003 to 253 infections/100 000 during 2004–2007 [24]. For the same time periods unadjusted MRSA incidence rates were 87/100 000 and 357/100 000, respectively in the KPNC HIV-uninfected. Liu et al. [39] conducted a survey of MRSA incidence (combined HIV-infected and HIV-infected patients), ascertaining diagnoses from medical centres in San Francisco, California, USA, for the period February–September 2004 and continuing for 12 months; they reported unadjusted incidence rates of MRSA infection in residents of San Francisco of 316 cases/100 000 population during the study period. For a comparable time interval (2004–2005) the average unadjusted MRSA incidence rate was 290 cases/100 000 in our KPNC patient population, suggesting that the rates are similar but potentially more concentrated in San Francisco than the entire Northern California area.
Our study contributes new information to the reports of MRSA incidence and has several strengths. It provides MRSA infection rates in a clinical population receiving care from the largest fully integrated health plan in California, which includes one of the largest clinical cohorts of HIV-infected patients in the USA. Our study population received standardized health care; thus results are not biased by differential access to care (e.g. limits of coverage from different insurance plans, or the inability of the uninsured to pay for all medical services needed) or extent and type of therapy received. A limitation of the study is that information on the molecular types of S. aureus isolates was not available and therefore we can only speculate that most MRSA infections diagnosed after 2002 were likely to be due to the USA300 strain. Moreover the molecular type of MRSA may vary by geographical location, and there may also have been differences in laboratory culturing protocols in institutions across the USA. Thus our results may be less generalizable to other regions of the USA. Second, the denominator used in the calculation of our rates for HIV-uninfected patients could have included some patients who had undiagnosed HIV infection; however, the number of misclassified patients in the HIV-uninfected denominator would need to be very large (which is unlikely) to have any effect on the MRSA rate estimates.
In conclusion, the MRSA epidemics in both the HIV-infected and HIV-uninfected KPNC patient communities saw increases in annual incidence after 2000. However, the acceleration of MRSA incidence rates began earlier in HIV-infected patients, and that population appears to have shouldered the greater burden of MRSA infection compared to the HIV-uninfected group. A possible declining trend in infection rates may have begun in 2008–2009. Given the community-onset nature of the MRSA epidemic in recent years, the observed decline may be partly associated with increased public health awareness of MRSA and its potential consequences through media dissemination and education efforts by healthcare providers [40]. However, our study results still indicate the need for much greater efforts to significantly reduce the MRSA epidemic in the general population, and for specific interventions that will greatly reduce this public health threat in the HIV-infected population, beyond nosocomial decolonization practices [41].
ACKNOWLEDGEMENTS
This work was supported by a grant from the Garfield Memorial Fund (G.D., M.S., A.T., and C.Q., grant number GMF BSC#0391).
DECLARATION OF INTEREST
R. Baxter has received funding from Merck and GlaxoSmithKline who have an interest in developing vaccines for S. aureus infection. Neither company provided any funding for this study.
REFERENCES
- 1.National Nosocomial Infections Surveillance (NNIS). System report, data summary from January 1992 to June 2002, issued August 2002. American Journal of Infection Control 2002; 30: 458–475. [DOI] [PubMed] [Google Scholar]
- 2.Barrett FF, McGehee RF Jr., Finland M. Methicillin-resistant Staphylococcus aureus at Boston City Hospital. Bacteriologic and epidemiologic observations. New England Journal of Medicine 1968; 279: 441–448. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Methicillin-resistant Staphylococcus aureus infections in correctional facilities – Georgia, California, and Texas, 2001–2003. Morbidity and Mortality Weekly Report 2003; 52: 992–996. [PubMed] [Google Scholar]
- 4.CDC. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants – Colorado, Indiana, Pennsylvania, and Los Angeles County, 2000–2003. Morbidity and Mortality Weekly Report 2003; 52: 793–795. [PubMed] [Google Scholar]
- 5.CDC. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections – Los Angeles County, California, 2002–2003. Morbidity and Mortality Weekly Report 2003; 52: 88. [PubMed] [Google Scholar]
- 6.Diekema DJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clinical Infectious Diseases 2001; 32 (Suppl. 2): S114–S132. [DOI] [PubMed] [Google Scholar]
- 7.Fichtenbaum CJ, Dunagan WC, Powderly WG. Bacteremia in hospitalized patients infected with the human immunodeficiency virus: a case-control study of risk factors and outcome. Journal of the Acquired Immune Deficiency Syndrome and Human Retrovirology 1995; 8: 51–57. [PubMed] [Google Scholar]
- 8.Northfelt DW, Polsky B. Bacteremia in persons with HIV infection. AIDS Clinical Reviews 1991; 1: 59–79. [PubMed] [Google Scholar]
- 9.Senthilkumar A, Kumar S, Sheagren JN. Increased incidence of Staphylococcus aureus bacteremia in hospitalized patients with acquired immunodeficiency syndrome. Clinical Infectious Diseases 2001; 33: 1412–1416. [DOI] [PubMed] [Google Scholar]
- 10.Alonso R, et al. Outbreak among HIV-infected patients of Staphylococcus aureus resistant to cotrimoxazole and methicillin. Infection Control and Hospital Epidemiology 1997; 18: 617–621. [DOI] [PubMed] [Google Scholar]
- 11.Anderson EJ, et al. A series of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in HIV-infected patients. Journal of Acquired Immune Deficiency Syndrome 2006; 41: 125–127. [DOI] [PubMed] [Google Scholar]
- 12.Bar A, et al. Spectrum of bacterial isolates in HIV-positive patients with skin and soft tissue infections: emergence of methicillin-resistant Staphylococci. AIDS 2003; 17: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 13.Eulalia VM, et al. Nosocomial infection with methicillin resistant Staphylococcus aureus in 14 human immunodeficiency virus infected patients. Medicina Clínica (Barcelona) 1997; 109: 261–263. [PubMed] [Google Scholar]
- 14.Gordon RJ, et al. A molecular epidemiological analysis of 2 Staphylococcus aureus clonal types colonizing and infecting patients with AIDS. Clinical Infectious Diseases 2005; 40: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 15.Klein PA, et al. Prevalence of methicillin-resistant Staphylococcus aureus in outpatients with psoriasis, atopic dermatitis, or HIV infection. Archives of Dermatology 1997; 133: 1463–1465. [DOI] [PubMed] [Google Scholar]
- 16.Lee NE, et al. Risk factors for community-associated methicillin-resistant Staphylococcus aureus skin infections among HIV-positive men who have sex with men. Clinical Infectious Diseases 2005; 40: 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews WC, et al. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. Journal of Acquired Immune Deficiency Syndrome 2005; 40: 155–160. [DOI] [PubMed] [Google Scholar]
- 18.McDonald LC, et al. Colonization of HIV-infected outpatients in Taiwan with methicillin-resistant and methicillin-susceptible Staphylococcus aureus. International Journal of STD & AIDS 2003; 14: 473–477. [DOI] [PubMed] [Google Scholar]
- 19.Shet A, et al. Colonization and subsequent skin and soft tissue infection due to methicillin-resistant Staphylococcus aureus in a cohort of otherwise healthy adults infected with HIV type 1. Journal of Infectious Diseases 2009; 200: 88–93. [DOI] [PubMed] [Google Scholar]
- 20.Sztramko R, et al. Community-associated methicillin-resistant Staphylococcus aureus infections in men who have sex with men: a case series. Canadian Journal of Infectious Disease and Medical Microbiology 2007; 18: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skiest DJ, et al. Prospective comparison of methicillin-susceptible and methicillin-resistant community-associated Staphylococcus aureus infections in hospitalized patients. Journal of Infection 2007; 54: 427–434. [DOI] [PubMed] [Google Scholar]
- 22.Skiest D, et al. Community-onset methicillin-resistant Staphylococcus aureus in an urban HIV clinic. HIV Medicine 2006; 7: 361–368. [DOI] [PubMed] [Google Scholar]
- 23.Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Journal of the American Medical Association 2007; 298: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 24.Popovich KJ, et al. Community-associated methicillin-resistant Staphylococcus aureus and HIV: intersecting epidemics. Clinical Infectious Diseases 2010; 50: 979–987. [DOI] [PubMed] [Google Scholar]
- 25.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American Journal of Public Health 1992; 82: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosario-Rosado RV, Rene AA, Jones B. Descriptive analysis of patients with community-onset and hospital-onset methicillin-resistant Staphylococcus aureus infections. Infection Control and Hospital Epidemiology 2004; 25: 171–173. [DOI] [PubMed] [Google Scholar]
- 27.Kempker RR, et al. Association of methicillin-resistant Staphylococcus aureus (MRSA) USA300 genotype with mortality in MRSA bacteremia. Journal of Infection 2010; 61: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis SL, et al. Characteristics of patients with healthcare-associated infection due to SCCmec type IV methicillin-resistant Staphylococcus aureus. Infection Control and Hospital Epidemiology 2006; 27: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 29.Teutsch SM, Churchill RE. Principles and Practice of Public Health Surveillance, 2nd edn. Oxford University Press, 2000. [Google Scholar]
- 30.Kallen AJ, et al. Health care-associated invasive MRSA infections, 2005–2008. Journal of the American Medical Association 2010; 304: 641–648. [DOI] [PubMed] [Google Scholar]
- 31.Kupfer M, et al. MRSA in a large German University Hospital: male gender is a significant risk factor for MRSA acquisition. GMS Krankenhaushygiene Interdisziplinär 2010; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King MD, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Annals of Internal Medicine 2006; 144: 309–317. [DOI] [PubMed] [Google Scholar]
- 33.Padoveze MC, et al. Nasal MRSA colonization of AIDS Patients cared for in a Brazilian university hospital. Infection Control and Hospital Epidemiology 2001; 22: 783–785. [DOI] [PubMed] [Google Scholar]
- 34.Peters PJ, et al. Methicillin-resistant Staphylococcus aureus colonization in HIV-infected outpatients is common and detection is enhanced by groin culture. Epidemiology and Infection 2011; 139: 998–1008. [DOI] [PubMed] [Google Scholar]
- 35.Crum-Cianflone NF, et al. Association of methicillin-resistant Staphylococcus aureus (MRSA) colonization with high-risk sexual behaviors in persons infected with Human Immunodeficiency Virus (HIV). Medicine (Baltimore) 2011; 90: 379–389. [DOI] [PubMed] [Google Scholar]
- 36.Chacko J, Kuruvila M, Bhat GK. Factors affecting the nasal carriage of methicillin-resistant Staphylococcus aureus in human immunodeficiency virus-infected patients. Indian Journal of Medical Microbiology 2009; 27: 146–148. [DOI] [PubMed] [Google Scholar]
- 37.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews 1997; 10: 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crum-Cianflone NF, Burgi AA, Hale BR. Increasing rates of community-acquired methicillin-resistant Staphylococcus aureus infections among HIV-infected persons. International Journal of STD & AIDS 2007; 18: 521–526. [DOI] [PubMed] [Google Scholar]
- 39.Liu C, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clinical Infectious Diseases 2008; 46: 1637–1646. [DOI] [PubMed] [Google Scholar]
- 40.Office of the Associate Director for Communication. CDC steps up efforts to fight drug-resistant germ. Centers for Disease Control and Prevention, Press release, 8 September 2008 (http://www.cdc.gov/media/pressrel/2008/r080908.htm). [Google Scholar]
- 41.Herwaldt LA. Control of methicillin-resistant Staphylococcus aureus in the hospital setting. American Journal of Medicine 1999; 106 (Suppl. 1): S8–S11. [DOI] [PubMed] [Google Scholar]