SUMMARY
Bloodstream infections (BSIs) are a major cause of morbidity and mortality. Although population-based studies have been proposed as an optimal means to define their epidemiology, the merit of these designs has not been well documented. This report investigated the potential value of using population-based designs in defining the epidemiology of BSIs. Population-based BSI surveillance was conducted in Calgary, Canada (population 1·24 million) and illustrative comparisons were made between the overall and selected subgroup cohorts within five main themes. The value of population denominator data, and age and gender standardization for calculation and comparison of incidence rates were demonstrated. In addition, a number of biases including those related to differential admission rates, selected hospital admission, and referral bias were highlighted in non-population-based cohorts. Due to their comprehensive nature and intrinsic minimization of bias, population-based designs should be considered the gold standard means of defining the epidemiology of an infectious disease.
Key words: Bloodstream infections, epidemiology, surveillance
INTRODUCTION
Bloodstream infections (BSIs) are a major cause of morbidity and mortality worldwide [1–8]. These infections may arise secondary to a focus of infection at a specific body site or may be classified as primary when no focus is evident. Although traditionally classified as either hospital-acquired (HA) or community-acquired (CA) infections, it is now widely accepted that a third category of community-onset healthcare-associated (HCA) BSI be recognized [9–11]. In any case, a diagnosis of BSI is a serious condition with associated overall case-fatality rates of about 15–20% [1–8].
Population-based studies have been proposed as the optimal means of defining the epidemiology of infectious diseases. In these designs, all cases of disease occurring in residents of a defined geographical area are ascertained and therefore selection bias is minimized [12]. In addition, when the population at risk is known, incidence rates can be determined that may be used to establish the burden of disease and facilitate comparison between different regions and time periods. However, despite these merits, studies investigating the epidemiology of BSIs have largely been reported from selected hospital-based cohorts [13].
The objective of this report was to investigate the potential value of using population-based designs in evaluating the epidemiology of BSIs. To achieve this goal, population-based BSI data from Calgary, Canada were utilized with illustrative comparisons made with selected subgroup cohorts.
METHODS
Population-based surveillance was conducted in the Calgary Zone of Alberta Health Services using the Electronic Surveillance System (ESS) during 2000–2008 [14]. The Calgary Zone administers all publicly funded healthcare to the 1·24 million population of the cities of Calgary and Airdrie and more than 20 surrounding smaller communities. The population is among the wealthiest, best educated, and youngest in Canada and is rapidly growing due to migration from other parts of the country and abroad. With the exception of acute heart, liver, and lung transplantation which is performed in Edmonton, all healthcare services from primary to tertiary care are provided within the Zone. Since 2000, the ESS has registered all incident episodes of BSIs occurring in residents of the Calgary Zone. Episodes of BSIs are identified by the regional laboratory system that includes all microbiology specimens including those submitted by community physicians, ambulatory clinics, emergency departments, and all healthcare institutions including the four major hospitals that are responsible for more than 95% of all admissions in the Calgary Zone. Clinically validated algorithms were applied in the ESS in order to exclude duplicate specimens and contaminants, and to further classify cases as HA, CA, or HCA [9, 14]. Residency was established using a regional flag identified through the unique provincial personal healthcare identifier. Homeless individuals are considered to be residents as long as they have a personal health number and the specimen is submitted from a location within the Calgary Zone.
Five main areas were explored in order to evaluate the potential benefit of population-based designs. In the first two cases the importance of denominator data was explored as this relates to incidence rate determinations and age and gender standardization. In the latter three cases, the value of population-based data in minimizing bias in comparison to selected cohorts is detailed.
RESULTS
Population denominator data
A key feature of population-based studies that sets them aside from other designs is that the entire population at risk is known. While it is possible for hospital-based studies to have a known population at risk in the case of HA infections (i.e. all patients admitted to that hospital for ⩾2 days), this is not the case for community-onset disease. This is because patients in the community setting may or may not present to a given hospital under surveillance. Population-based studies include all cases occurring in a defined region (inclusive of all patients in the community, long-term care facilities, and hospitals), the number of cases that may develop community-onset disease may then be defined. Such an establishment of a population at risk has two major advantages including the ability to determine incidence rates and age and gender standardization.
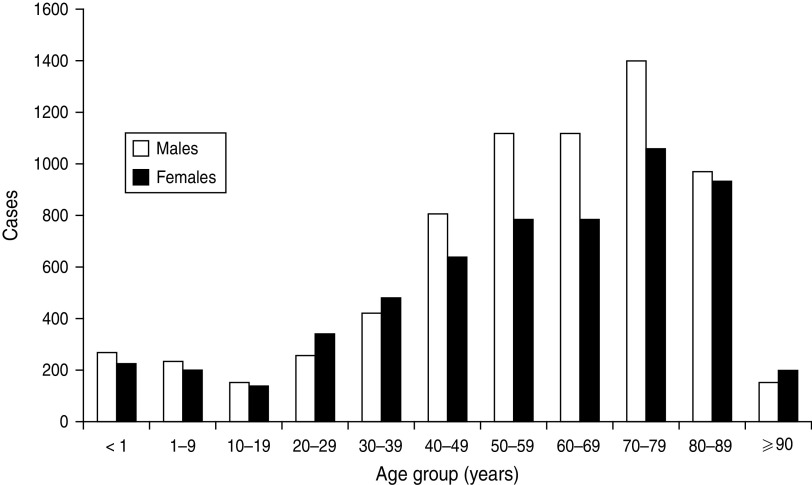
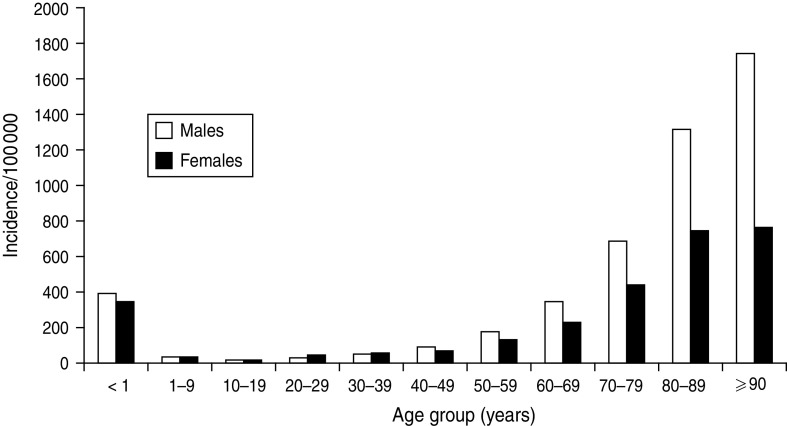
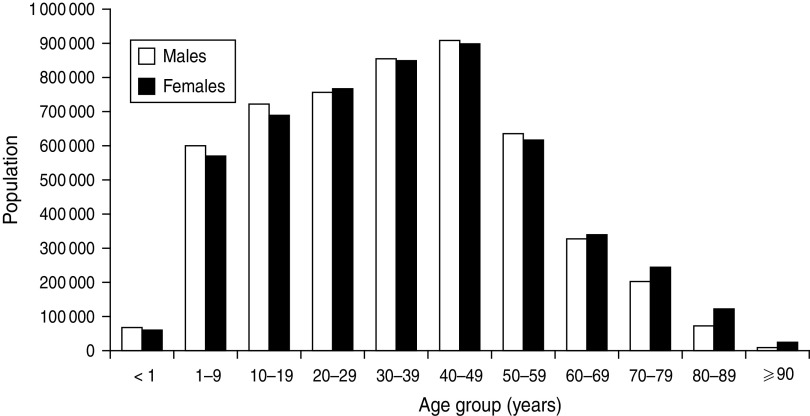
The value of determining incidence rates is illustrated by the data shown in Figures 1–3. Figure 1 displays the occurrence of all incident episodes of BSI in Calgary area residents during a 9-year period. By evaluating Figure 1, it may reasonably be concluded that there is an increase in occurrence of BSIs starting in young adulthood that continues up to age 79 years at which point the occurrence decreases with only rare cases observed in the very old. Furthermore, the data in Figure 1 suggest that males have a modestly higher occurrence of disease, but that this is limited to the middle-age ranges. However, in the absence of appropriate denominator data these data are misleading. Figure 2 shows the age and gender distribution of the Calgary area population at risk during 2000–2008. When incidence rates are calculated a very different interpretation becomes evident as shown in Figure 3. Notably, the risk for acquiring a BSI is clearly highest in the very young and the very old. Furthermore, males are at significantly higher risk throughout late adulthood with this excess risk increasing progressively with advancing age. Elderly males represent the highest risk group.
Fig. 1.
Incident cases of bloodstream infection occurring in the Calgary area, 2000–2008.
Fig. 3.
Population-based incidence of bloodstream infections occurring in the Calgary area, 2000–2008.
Fig. 2.
Population demographics of the Calgary area, 2000–2008. (Source: Alberta Health Registry, Alberta Health Services.)
Age and gender standardization
A second major advantage of having a definable population at risk is that incidence rates may be standardized by age and gender to facilitate comparison among populations over time or among different regions. As previously noted, Figure 3 demonstrates that advancing age and male gender is associated with increased risk for BSI. Therefore, it can be expected that populations that have a high proportion of elderly individuals, particularly elderly males, will have higher overall incidences of BSIs. On the other hand, young populations or those with a greater proportion of women will be expected to have overall lower rates of BSIs. It is therefore important when comparing different regions, or looking at the same region over time, that age and gender standardization be performed against a reference population such that the rates may be compared in a like fashion.
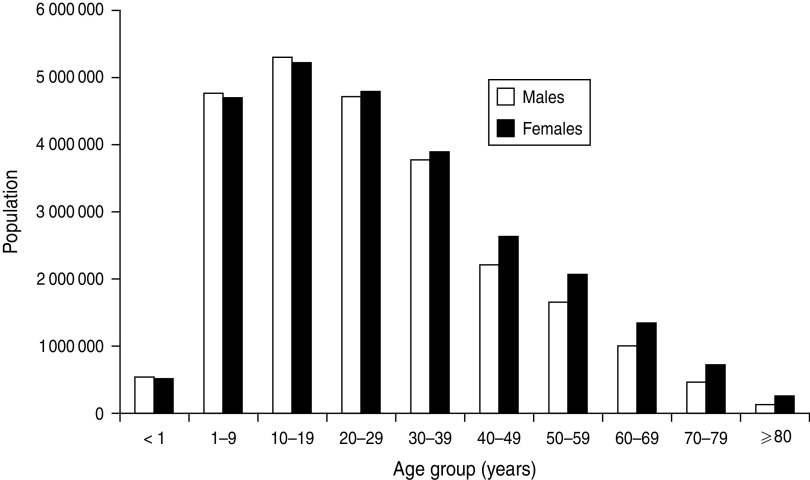
To illustrate this point, during 2000–2008, the overall annualized incidence of BSIs in the Calgary area was 122 episodes/100 000 population. If these Calgary data are standardized for age and gender, e.g. to the South African population (Fig. 4), a much different rate of 85/100 000 is observed [15]. This is because the age structure of South Africa is characterized by a much younger population with a high proportion of young adults and relatively few very elderly citizens. This is important, because even if two studies found that the epidemiology of BSIs were identical in these two countries, failure to standardize the results would lead to falsely concluding that the incidences are some 30% different.
Fig. 4.
Population demographics of South Africa, 2011. (Source: Statistics South Africa.)
Subpopulation shifts
It is frequently of interest for investigators to evaluate BSIs that are acquired in a certain setting such as HA or community-onset (i.e. HCA and/or CA) infections. Indeed, these represent distinct entities as far as risk factors and microbiology are concerned, and the use of such data for future preventive efforts may have clearly different objectives. However, if the objective of an investigation is to establish the occurrence of disease as a measure of success for preventative programmes, there is risk to limiting evaluation to certain acquisition types when shifts in healthcare delivery may be occurring.
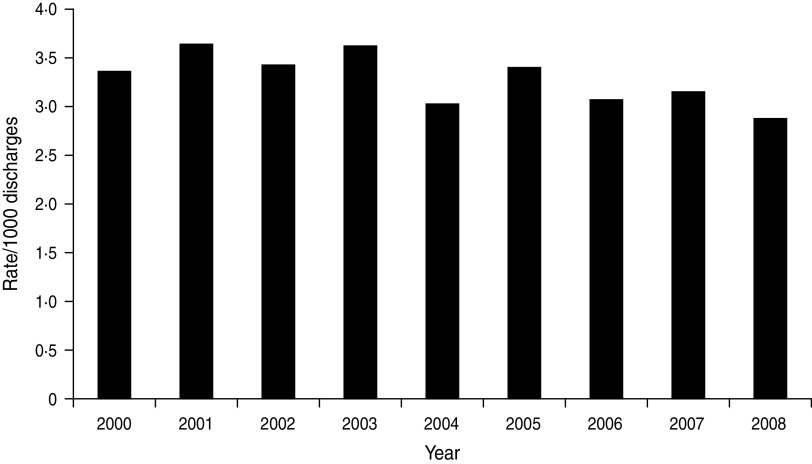
Figure 5 shows the annual rate of HA BSI expressed as an annual rate/1000 discharges in the Calgary area during a 9-year period. While some typical year-to-year variability is observed, an overall decreasing rate of HA infection is evident (Fig. 5) with the rate during 2005–2008 significantly lower than that observed during 2000–2004 [3·1 vs. 3·4/1000 discharges, incidence rate ratio (IRR) 0·91, 95% confidence interval (CI) 0·85–0·98, P = 0·009]. Based on this observation alone, it may be concluded that efforts for infection prevention and control have been successful. However, studying this selected sub-population may provide misleading conclusions. This is because there has been substantial changes in healthcare delivery in recent years with a much greater degree of care conducted in the community. It therefore follows that the population of inpatients at risk for HA infections has been changing.
Fig. 5.
Hospital-acquired bloodstream infections/1000 discharges, Calgary, 2000–2008.
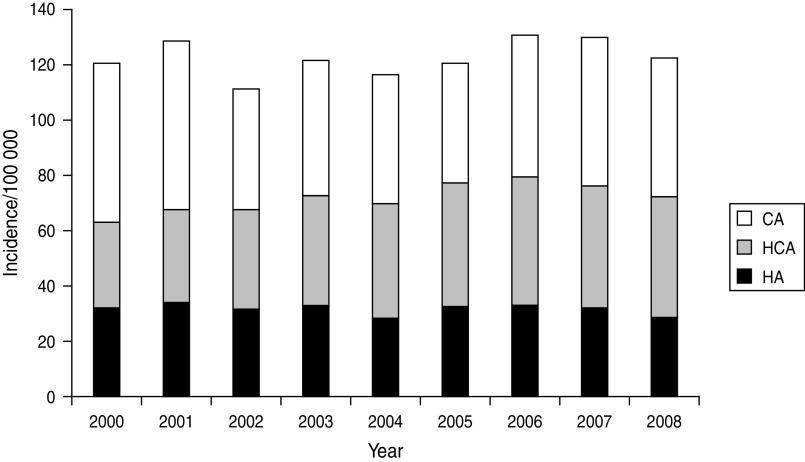
Figure 6 displays the population-based incidence of all incident episodes of BSI per category (HA, HCA, CA) of acquisition. There is a modest increase in overall burden of BSIs when the periods of 2005–2008 and 2000–2004 are compared (125·8 vs. 119·5/100 000, IRR 1·05, 95% CI 1·02–1·09, P = 0·004) but no differences in either the population incidence of HA (31·5 vs. 31·7/100 000, IRR 0·99, 95% CI 0·93–1·06, P = 0·853) or CA (49·6 vs. 51·3/100 000, IRR 0·97, 95% CI 0·92–1·02, P = 0·233) BSIs. On the other hand, during 2005–2008 compared to 2000–2004, a significantly higher rate of HCA (44·7 vs. 36·5/100000, IRR 1·22, 95% CI 1·15–1·30, P < 0·0001) disease was observed. Thus, despite evidence for a decreasing rate of HA BSI/1000 discharges, evaluating population-based data reveals that the true overall burden of HA disease has not changed in the population at large. In contrast, there has been an actual overall increase in the burden of BSIs associated with exposure to healthcare as evidenced by the combined increasing incidence of HA and HCA BSIs (Fig. 6).
Fig. 6.
Population-based incidence of bloodstream infections by acquisition category, Calgary area, 2000–2008. CA, Community acquired; HCA, healthcare associated; HA, hospital acquired.
Hospital admission bias
Patients who are admitted to hospital represent an easily assessable cohort of patients for study and accordingly most of the published literature on BSI epidemiology is obtained from investigations conducted in the hospital-based setting. However, there are a number of issues surrounding the use of such data. In particular differential rates of admission and study of selected hospitals may have an important effect on defining the determinants of an infectious disease. These are minimized by inclusion of all cases in population-based studies.
In the first case regarding differential rates of admission, if not all patients with BSIs are admitted to hospital, then inclusion of only hospitalized patients may lead to significant bias. Table 1 displays a number of characteristics of patients with incident episodes of BSI who are treated as outpatients compared to those who are admitted to hospital for management of community-onset BSI (by definition all HA BSIs are admitted to hospital). Admitted patients are older, more likely to have a HCA BSI, and are at nearly twice the risk for death compared to non-admitted patients (Table 1).
Table 1.
Comparison of patients admitted to hospital, or not, for management of incident community-onset bloodstream infection episodes, Calgary 2000–2008
| Factor | Admitted (n = 7731) | Not-admitted (n = 1670) | P value |
|---|---|---|---|
| Male | 4089 (53%) | 849 (51%) | 0·137 |
| Median age (interquartile range) | 63 (45–77) | 54 (34–72) | <0·0001 |
| 30-day mortality | 974 (13%) | 112 (7%) | <0·0001 |
| Healthcare associated | 3592 (46%) | 585 (35%) | <0·0001 |
| Isolate* | <0·001 | ||
| Staphylococcus aureus | 1104 (14%) | 173 (10%) | |
| Escherichia coli | 2050 (27%) | 440 (26%) | |
| Klebsiella pneumoniae | 385 (5%) | 53 (3%) | |
| Streptococcus pneumoniae | 867 (11%) | 166 (10%) | |
| Coagulase-negative staphylococci | 326 (4%) | 255 (15%) | |
| Other mono-microbial | 2503 (32%) | 507 (31%) | |
| Polymicrobial | 496 (6%) | 76 (5%) |
Too many individual isolates to list; the five most common species are displayed for illustrative purposes.
In the second case, study of selected hospital(s) may similarly potentially also lead to false attribution of determinants of BSIs. As shown in Table 2, the three adult hospitals serving the Calgary area population differ significantly in the types of patients admitted with community-onset BSIs. Study of only one selected hospital provides a biased assessment of the true overall epidemiology in the admitted population.
Table 2.
Comparison of patients (aged ⩾18 years) admitted to each of the three adult major acute-care hospitals with community-onset bloodstream infection episodes, Calgary area, 2000–2008
| Factor | Hospital A (n = 2972) | Hospital B (n = 2192) | Hospital C (n = 1916) | P value |
|---|---|---|---|---|
| Male | 1578 (53) | 1125 (51) | 1042 (54) | 0·140 |
| Median age (interquartile range) | 64 (49–77) | 64 (48–77) | 69 (52–80) | <0·001 |
| 30-day mortality | 455 (15%) | 273 (12%) | 233 (12%) | 0·001 |
| Healthcare associated | 1619 (54%) | 917 (42%) | 877 (46%) | <0·001 |
| Isolate* | 0·001 | |||
| Staphylococcus aureus | 478 (16%) | 284 (13%) | 241 (13%) | |
| Escherichia coli | 725 (26%) | 609 (28%) | 596 (31%) | |
| Klebsiella pneumoniae | 167 (6%) | 96 (4%) | 113 (6%) | |
| Streptococcus pneumoniae | 268 (9%) | 315 (14%) | 157 (8%) | |
| Coagulase-negative staphylococci | 146 (5%) | 91 (4%) | 74 (4%) | |
| Other monomicrobial | 975 (33) | 654 (30%) | 611 (32%) | |
| Polymicrobial | 213 (7%) | 143 (7%) | 124 (6%) |
Too many individual isolates to list; the five most common species are displayed for illustrative purposes.
Referral bias
For a number of potential reasons not limited to proximity to experienced research personnel, availability of research funding, academic interest, reputation, and larger patient volumes, there is a tendency for university-affiliated, tertiary-care referral hospitals to successfully report their results on BSIs in the published literature. However, when studies are performed that include patients referred from elsewhere ‘referral bias’ may arise [16]. In population-based studies, non-resident patients that are external to the base population are excluded such that this risk is minimized. In order to explore the potential effect of referral bias on the epidemiology of BSIs in the Calgary area, a cohort of incident BSI patients identified in the Calgary area who were non-resident (i.e. referred) was assembled and compared to the true ‘resident’ population-based cohort. As shown in Table 3, failure to exclude these non-resident or referred patients leads to an overestimate of the occurrence of BSIs, and biases the assessment of age, acquisition location, and microbiology.
Table 3.
Comparison of residents and non-residents (referral patients) with incident bloodstream infection episodes admitted to hospitals in the Calgary area, 2000–2008.
| Factor | Residents (n = 11 002) | Non-residents (n = 1527) | P value |
|---|---|---|---|
| Male | 6043 (55%) | 859 (56%) | 0·328 |
| Median age (interquartile range) | 63 (45–77) | 57 (39–69) | <0·0001 |
| Acquisition | <0·001 | ||
| Hospital acquired | 3271 (30%) | 1034 (68%) | |
| Healthcare associated | 3592 (33%) | 156 (10%) | |
| Community acquired | 4139 (38%) | 337 (22%) | |
| Isolate* | <0·001 | ||
| Staphylococcus aureus | 1834 (17%) | 279 (18%) | |
| Escherichia coli | 2472 (22%) | 228 (15%) | |
| Klebsiella pneumoniae | 542 (5%) | 54 (4%) | |
| Streptococcus pneumoniae | 910 (8%) | 74 (5%) | |
| Coagulase-negative staphylococci | 693 (6%) | 196 (13%) | |
| Other monomicrobial | 3792 (34%) | 578 (38%) | |
| Polymicrobial | 759 (7%) | 118 (8%) |
Too many individual isolates to list; the five most common species are displayed for illustrative purposes.
DISCUSSION AND CONCLUSIONS
This report is important and novel because it highlights the potential biases that may arise in studies that attempt to define the epidemiology of infectious diseases using selected cohorts where the population at risk is not known. Based on these data presented from the Calgary area, it is evident that failure to use population denominator data may lead to false attribution of risk. In addition, failure to standardize incidence rates for age and gender may lead to false comparisons of incidence rates where base populations differ significantly in demographic composition. Furthermore, the risk of following a selected subpopulation of BSIs in the setting of shifts in healthcare delivery is highlighted. Finally, is it is demonstrated that study of selected populations such as those only admitted to hospital or to a given hospital(s) and failure to recognize referral bias may lead to false conclusions about infectious disease epidemiology.
Despite the notable merits of population-based designs, they are infrequently reported in the published literature. To the author's knowledge, the first population-based study investigating all incident BSIs occurring in a defined population at risk was only relatively recently reported in 1986 [7]. While there is a modest and growing body of literature on population-based studies investigating selected pathogens or population subgroups, few studies have included all cases of BSIs occurring in large well-defined populations [4, 6–8].
There may be a number of potential reasons for the relative paucity of population-based studies reported in the literature. Issues surrounding methodological quality of surveillance data, and in particular the merits of population-based studies in minimizing bias have rarely been topics of published reports or conference proceedings [17]. Despite their numerous limitations, it remains commonplace for hospital-based studies and others conducted in selected cohorts to be published in high-impact journals. Another major obstacle to conducting population-based studies is that they are generally much more labour-intensive to perform than the study of selected cohorts. It may be a major challenge to include all community and hospital laboratories in the study particularly in areas where there are many different service providers or blends of private and public service delivery.
While there are a number of key strengths to the population-based design, there are some limitations that merit discussion. When a laboratory parameter such as a positive culture is used to identify a case, differences in culture practices may influence results [18]. For example, if clinicians fail to draw blood cultures or administer antimicrobial agents prior to drawing blood culture then cases of BSI will fail to be recognized and the occurrence of true disease will be underestimated. However, this limitation is universal to all observational study designs of BSIs. A second consideration with population-based studies is that the population under study must be relatively captive for surveillance. If a significant number of residents seek healthcare services external to the surveillance region then failure of case ascertainment will occur. This may arise if resident populations are transient, if there are large adjacent populations where healthcare facilities are readily available, or if patients are regularly referred out of the region for healthcare services. Ideally, surveillance regions should be geographically isolated and provide a comprehensive range of healthcare services. A third consideration is that the denominator data must be accurate and that cases must have arisen out of that denominator population. In the present study this was assured by requiring cases to have Alberta personal healthcare numbers, and the denominator data were obtained from a registry of all Albertans with personal healthcare numbers within the Calgary Zone.
In summary, this report highlights a number of strengths of the population-based design and exposes several potential biases that may arise from conducting studies within selected cohorts. Population-based studies should be considered the gold standard design for defining the epidemiology of an infectious disease.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Diekema DJ, et al. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. Journal of Clinical Microbiology 2003; 41: 3655–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearman GM, Wenzel RP. Bacteremias: a leading cause of death. Archives of Medical Research 2005; 36: 646–659. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen G, Schonheyder HC, Sorensen HT. Source of infection and other factors associated with case fatality in community-acquired bacteremia – a Danish population-based cohort study from 1992 to 1997. Clinical Microbiology and Infection 2003; 9: 793–802. [DOI] [PubMed] [Google Scholar]
- 4.Madsen KM, et al. Secular trends in incidence and mortality of bacteraemia in a Danish county 1981–1994. Acta Pathologica, Microbiologica et Immunologica Scandinavica 1999; 107: 346–352. [DOI] [PubMed] [Google Scholar]
- 5.Laupland KB, et al. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiology and Infection 2007; 135: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uslan DZ, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Archives of Internal Medicine 2007; 167: 834–839. [DOI] [PubMed] [Google Scholar]
- 7.Filice GA, et al. Bacteremia in Charleston County, South Carolina. American Journal of Epidemiology 1986; 123: 128–136. [DOI] [PubMed] [Google Scholar]
- 8.Skogberg K, et al. Increase in bloodstream infections in Finland, 1995–2002. Epidemiology and Infection 2008; 136: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman ND, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Annals of Internal Medicine 2002; 137: 791–797. [DOI] [PubMed] [Google Scholar]
- 10.Lenz R, et al. The distinct category of healthcare associated bloodstream infections. BMC Infectious Diseases 2012; 12: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Bano J, et al. Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals. Clinical Microbiology and Infection 2010; 16: 1408–1413. [DOI] [PubMed] [Google Scholar]
- 12.Laupland KB. Population-based epidemiology of intensive care: critical importance of ascertainment of residency status. Critical Care 2004; 8: R431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rempel OR, Laupland KB. Surveillance for antimicrobial resistant organisms: potential sources and magnitude of bias. Epidemiology and Infection 2009; 137: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 14.Leal J, et al. Development of a novel electronic surveillance system for monitoring of bloodstream infections. Infection Control and Hospital Epidemiology 2010; 31: 740–747. [DOI] [PubMed] [Google Scholar]
- 15.Statistics South Africa. Mid-year population estimates 2011. (http://www.statssa.gov.za/publications/P0302/P03022011.pdf). Accessed 24 April 2012.
- 16.Steckelberg JM, et al. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. American Journal of Medicine 1990; 88: 582–588. [DOI] [PubMed] [Google Scholar]
- 17.Bax R, et al. Surveillance of antimicrobial resistance – what, how and whither? Clinical Microbiology and Infection 2001; 7: 316–325. [DOI] [PubMed] [Google Scholar]
- 18.Klemets P, et al. Trends and geographical variation in invasive pneumococcal infections in Finland. Scandinavian Journal of Infectious Diseases 2008; 40: 621–628. [DOI] [PubMed] [Google Scholar]