Abstract
Non-starter lactic acid bacteria were isolated from 14 premium-quality and 3 sensorially defective mature Irish Cheddar cheeses, obtained from six manufacturers. From countable plates of Lactobacillus-selective agar, 20 single isolated colonies were randomly picked per cheese. All 331 viable isolates were biochemically characterized as mesophilic (i.e., group II) Lactobacillus spp. Phenotypically, the isolates comprised 96.4% L. paracasei, 2.1% L. plantarum, 0.3% L. curvatus, 0.3% L. brevis, and 0.9% unidentified species. Randomly amplified polymorphic DNA (RAPD) analysis was used to rapidly identify the dominant strain groups in nine cheeses from three of the factories, and through clustering by the unweighted pair group method with arithmetic averages, an average of seven strains were found per cheese. In general, strains isolated from cheese produced at the same factory clustered together. The majority of isolates associated with premium-quality cheese grouped together and apart from clusters of strains from defective-quality cheese. No correlation was found between the isomer of lactate produced and RAPD profiles, although isolates which did not ferment ribose clustered together. The phenotypic and genotypic methods employed were validated with a selection of 31 type and reference strains of mesophilic Lactobacillus spp. commonly found in Cheddar cheese. RAPD analysis was found to be a useful and rapid method for identifying isolates to the species level. The low homology exhibited between RAPD banding profiles for cheese isolates and collection strains demonstrated the heterogeneity of the L. paracasei complex.
Non-starter lactic acid bacteria (NSLAB) is a term used to describe the adventitious bacterial flora capable of growth under the selective conditions (typically 32 to 39% moisture, 4 to 6% salt in moisture, pH 4.9 to 5.3, and 5 to 13°C) of ripening cheese (15). During the ripening of Cheddar cheese, the starter organisms autolyze, releasing growth substrates and enzymes; concomitantly, an increase in NSLAB levels occurs to ∼107 CFU · g−1 of cheese 6 to 8 weeks postmanufacture (22, 34). The NSLAB community is predominated by mesophilic (group II) lactobacilli, though pediococci and micrococci may also be found (1, 8, 16). A recent study of the NSLAB population in 8-week-old Irish Cheddar revealed that it was composed of 55% Lactobacillus paracasei, 28% Lactobacillus plantarum, and 14% Lactobacillus curvatus (23).
Although NSLAB can be isolated from cheese milk, most of them are inactivated by pasteurization. Their presence in cheese is probably due to (i) postpasteurization contamination by the airborne flora (36) or from strains on the cheese-making equipment or in ingredients (40) or (ii) thermoduric strains surviving pasteurization (33).
The role of NSLAB in the development of Cheddar flavor was reviewed in 1990 by Peterson and Marshall (37), who concluded that it was, at best, equivocal. Since then, there have been a number of studies on the effect of adjunct lactobacilli on Cheddar flavor development, with most authors reporting increased levels of free amino acids and enhanced flavor intensity in the ripened cheese. High-quality Cheddar, ripened for 1 year, was produced by certain strains of Lactobacillus casei subsp. casei and L. casei subsp. pseudoplantarum, while other strains of these species resulted in cheese with acidic and bitter flavor defects (28). The addition of homofermentative lactobacilli to Cheddar during production reduced the frequency of openness, swelling, and gas production caused by intentionally added or heterofermentative lactobacilli in a study by Laleye et al. (25). Addition of L. casei subsp. casei L2A as an adjunct was reported in several investigations (29, 39, 44–46) to accelerate ripening and consistently yielded well-aged Cheddar without bitterness. The production of “aseptic” cheese (32) and the use of antibiotics (47) or starter cultures producing bacteriocins (42) to control adventitious NSLAB have facilitated research on the effect of adjunct lactobacilli on flavor development.
Evaluation of the relationship between NSLAB and flavor production requires knowledge of the relative abundance of NSLAB species and strain heterogeneity in cheese, but very few data on these topics are available. NSLAB originating from good-quality mature cheese have been investigated as potential starter adjuncts (26, 32), since if they are beneficial to flavor development, such strains should be abundant in mature, premium-quality cheese. Determination of the diversity of the NSLAB population to strain level necessitates the use of a rapid method suitable for handling large numbers of isolates. Randomly amplified polymorphic DNA (RAPD) analysis is a PCR-based method that uses a single primer of arbitrary sequence under low-stringency conditions to amplify fragments of the test organism’s genome. The amplicons may be species or strain specific. This method has been used to estimate the diversity among lactobacillus strains of the Centre National de Recherches Zootechniques (CNRZ) collection (43), to type strains of L. plantarum (21), and to establish the correct nomenclature and classification of strains of L. casei (12).
In this study we report the development and application of the RAPD technique for the rapid identification of NSLAB to strain level and the study of this population in mature commercial Cheddar cheese.
MATERIALS AND METHODS
Cheese samples and compositional analyses.
Fourteen premium-quality, 20-kg blocks of mature Cheddar cheese and three defective-quality cheeses were obtained from six manufacturers. Standard procedures were used to analyze cheeses for fat (17), NaCl (18), and moisture (19). The pH was measured on a cheese slurry prepared from 20 g of cheese in 12 g of water (2).
Bacteriological analysis of cheese and NSLAB isolation.
Each cheese was aseptically sampled, emulsified in sterile 2% (wt/vol) trisodium citrate (pH 8.75), diluted, plated on Lactobacillus-selective agar (Becton Dickinson and Co., Cockeysville, Md.) (41), overlaid, and incubated at 30°C for 5 days. Twenty isolated colonies were randomly selected, from a countable Lactobacillus-selective agar plate, for each cheese. These isolates were propagated in MRS (10) broth (Difco Laboratories, Detroit, Mich.), and purified. All isolates were checked for catalase reaction and examined microscopically prior to being stocked. Cultures were maintained in the Dairy Products Research Centre collection (Teagasc, Moorepark, Fermoy, County Cork, Ireland) at −80°C in a 1:1 glycerol-MRS mixture. Isolates from stock were subcultured in MRS broth and streaked on MRS plates before use.
Type and reference strains.
A selection of 31 type and reference strains of mesophilic lactobacilli were obtained from the Laboratorium voor Microbiologie, Universiteit Gent (LMG) culture collection, Universiteit Gent, Ghent, Belgium. These strains were used to corroborate the genotypic and phenotypic methods employed to characterize cheese isolates. Details of the species and strain numbers for these cultures are given in Table 1.
TABLE 1.
Differential characteristics of the clusters shown in Fig. 3
| Cluster | No. of isolates | LMG collection strain(s) | Factory | Cheese(s)a | Lactobacillus species designation (RAPD analysis)b | Lactate isomer(s) producedc |
|---|---|---|---|---|---|---|
| 1 | 2 | 12009, 12007 | curvatus | dl | ||
| 2 | 2 | 9198T | II | O | curvatus | dl |
| 3 | 1 | 12008 | curvatus | dl | ||
| 4 | 1 | 11479 | curvatus | dl | ||
| 5 | 1 | 10755T | pentosus | d(l) | ||
| 6 | 2 | 6400Ta+bd | rhamnosus | l | ||
| 7 | 1 | 10769 | rhamnosus | l | ||
| 8 | 1 | 10775 | rhamnosus | l | ||
| 9 | 1 | 8153 | rhamnosus | l | ||
| 10 | 1 | 12166 | rhamnosus | l | ||
| 11 | 3 | I | A | paracasei | dl and l | |
| 12 | 1 | I | A | paracasei | dl | |
| 13 | 1 | I | A | paracasei | dl | |
| 14 | 1 | II | N | paracasei | dl | |
| 15 | 1 | II | N | paracasei | l | |
| 16 | 3 | II | N | paracasei | l | |
| 17 | 1 | II | O | paracaseie | dl | |
| 18 | 1 | II | N | paracaseie | l | |
| 19 | 11 | II | O | paracasei | dl and l | |
| 20 | 1 | II | O | paracasei | l | |
| 21 | 2 | II | N | paracasei | l | |
| 22 | 2 | II | O | paracasei | dl and l | |
| 23 | 1 | II | N | paracasei | l | |
| 24 | 1 | II | N | paracasei | dl | |
| 25 | 1 | I | 3 | paracaseie | dl | |
| 26 | 1 | I | 2 | paracaseie | dl | |
| 27 | 1 | II | O | paracasei | l | |
| 28 | 1 | II | O | paracasei | l | |
| 29 | 1 | 14766 | paracasei | l | ||
| 30 | 1 | 13087T | paracasei | l | ||
| 31 | 1 | I | 3 | paracasei | l | |
| 32 | 1 | I | B | paracasei | l | |
| 33 | 4 | I | B | paracasei | l | |
| 34 | 3 | I | B | paracasei | l | |
| 35 | 3 | I | 1 | paracasei | l | |
| 36 | 1 | I | 3 | paracasei | l | |
| 37 | 1 | I | A | paracasei | l | |
| 38 | 1 | I | A | paracasei | l | |
| 39 | 4 | I | B | paracasei | l | |
| 40 | 2 | I | 1 | paracasei | l | |
| 41 | 1 | I | B | paracasei | l | |
| 42 | 2 | I | A | paracasei | dl | |
| 43 | 4 | I | A | paracasei | dl and l | |
| 44 | 4 | I | 1 | paracasei | l | |
| 45 | 3 | I | B | paracasei | l | |
| 46 | 4 | I | A | paracasei | dl and l | |
| 47 | 1 | I | A | paracasei | dl | |
| 48 | 2 | I | A | paracasei | dl | |
| 49 | 7 | I | K | paracasei | dl and l | |
| 50 | 1 | I | K | paracasei | l | |
| 51 | 12 | I | K, 1, 2 | paracasei | l | |
| 52 | 2 | I | B | paracasei | l | |
| 53 | 3 | I | B, 1 | paracasei | l | |
| 54 | 2 | I | K | paracasei | l | |
| 55 | 1 | I | 1 | paracasei | l | |
| 56 | 1 | II | N | paracasei | l | |
| 57 | 1 | 12586 | paracasei | l | ||
| 58 | 27 | 13724a+b,d 13732, 13722, 13717, 11965 | I | 1, 2, 3 | paracasei | l |
| 59 | 10 | I | 1, 3 | paracasei | l | |
| 60 | 1 | I | 2 | paracasei | l | |
| 61 | 1 | I | 2 | paracasei | l | |
| 62 | 14 | III | H | paracasei | l | |
| 63 | 4 | III | H | paracasei | l | |
| 64 | 1 | III | H | paracasei | l | |
| 65 | 2 | 11473, 9193 | paracasei | l | ||
| 66 | 1 | 9192T | paracasei | dl | ||
| 67 | 8 | I | 2 | paracasei | l | |
| 68 | 2 | I | 2 | paracasei | l | |
| 69 | 1 | 6904T | casei | l | ||
| 70 | 1 | III | H | paracasei | l | |
| 71 | 2 | 9191Ta+bd | paracasei subsp. tolerans | l | ||
| 72 | 1 | II | O | brevis | dl | |
| 73 | 2 | II | N | plantarum | dl | |
| 74 | 1 | II | O | paraplantarum | dl | |
| 75 | 5 | 11475 | II | N | plantarum | dl |
| 76 | 2 | 14769a,d 11460 | plantarum | dl | ||
| 77 | 3 | 14769b,d 6907T, 14760 | plantarum | dl and d(l) | ||
| 78 | 1 | II | N | plantarum | dl | |
| 79 | 1 | II | N | plantarum | dl | |
| 80 | 1 | II | N | plantarum | dl | |
| 81 | 1 | 1284 | plantarum | dl | ||
| 82 | 1 | 9205 | plantarum | dl |
A to O, premium-quality cheeses; 1 to 3, defective-quality cheeses.
Unless otherwise indicated, “paracasei” means L. paracasei subsp. paracasei.
Based on the content of l-(+)-lactic acid as a percentage of total lactic acid produced, d(l) indicates 20 to 40% l-(+)-lactic acid, dl indicates 40 to 60% and l indicates 80 to 100%.
a+b denotes two different colony morphologies, a and b.
Ribose-negative isolate, i.e., does not ferment ribose.
Phenotypic characterization of isolates.
Lactobacillus species commonly found in Cheddar cheese include L. paracasei, L. plantarum (3), L. curvatus (23), and Lactobacillus brevis (40). These species may be distinguished on the basis of differential levels of fermentation of various sugars, growth responses in the absence and presence of Tween 80 (23), isomers of lactate produced, and levels of ethanol production (see Table 2), as reported by Kandler and Weiss (24). Fermentations of rhamnose, cellobiose, melibiose, mannitol, ribose, raffinose, mannose, and glucose in MRS broth (made up without Lab Lemco, glucose, or citrate) were determined. Glucose, which is metabolized by all these species, was included as a positive control. Sterile sugar solutions were aseptically added to broths to a final sugar concentration of 1% (wt/vol) in a total volume of 5 ml. Cultures were incubated at 30°C for 7 days; a pH differential of ≥0.7 U relative to the pH of a control (sterile water substituted for sugar solution) was regarded as fermentation. A comparison of the growth responses (pH differential) in MRS broth minus acetate, citrate, and Tween 80 and in MRS broth minus acetate and citrate after 2 days at 30°C was used to determine the requirement of Tween 80 for growth. Supernatants from the latter cultures were analyzed for the isomer of lactate enzymatically (Boehringer, Mannheim, Germany) and for ethanol production by high-performance liquid chromatography (Waters, Watford, Hertfordshire, United Kingdom). Similar levels of lactate and ethanol indicated heterofermentation of sugars. Twenty microliters of supernatant was injected onto an HPX87H column and guard column (Bio-Rad Laboratories, Hemel Hempstead, Hertfordshire, United Kingdom) held at 50°C. The mobile phase was 5 mM H2SO4, and the flow rate was 0.6 ml · min−1 (Waters model 510 pump). A refractive-index detector (Waters model 410) was used. Data were collected and analyzed with a Minichrom system (VG Data Systems, Cheshire, United Kingdom). Growth at 15 and 45°C was recorded as a pH differential (range as described above), relative to the pH of an uninoculated control, after 7 days of incubation. Species designations were based on the phenotypic characteristics detailed in Table 2 (24).
TABLE 2.
Differential physiological and biochemical characteristics of the facultatively and obligately heterofermentative species of the genus Lactobacillusa
| Lactobacillus sp. | Growth
at:
|
Fermentation of:
|
Lactate isomer produced | Homo- or heterofermentation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15°C | 45°C | Rhamnose | Cellobiose | Melibiose | Mannitol | Ribose | Raffinose | Mannose | Glucose | |||
| paracasei subsp. paracaseib | + | − | − | + | − | + | + | − | + | + | l or dl | Homo |
| paracasei subsp. tolerans | + | − | − | − | − | − | − | − | − | + | l | Homo |
| plantarum | + | − | − | + | + | + | + | + | + | + | dl | Homo |
| paraplantarumc | + | − | − | + | + | + | + | − | + | + | dl | Homo |
| curvatus | + | − | − | + | − | − | + | − | + | + | dl | Homo |
| rhamnosusd | + | + | + | + | − | + | + | − | + | + | l | Homo |
| brevis | + | − | − | − | + | − | + | d | − | + | dl | Hetero |
| fermentum | − | + | − | d | + | − | + | + | +w | + | dl | Hetero |
Data are modified from the work of Kandler and Weiss (24). +, ≥90% of strains were positive; +w, positive-to-weak reaction; −, ≥90% of strains were negative; d, 11 to 89% of strains were positive.
dl-Lactate producers were formerly known as L. casei subsp. pseudoplantarum (see reference 5).
See reference 7.
L. rhamnosus was formerly known as L. casei subsp. rhamnosus (5).
Genotypic characterization of isolates. (i) Rapid extraction of DNA from lactobacilli.
Genomic DNAs were extracted as described previously (4) from 1.5-ml samples of fresh overnight cultures (inocula used for phenotypic characterization) by harvesting by centrifugation for 5 min; 2 μl of each DNA in a subsequent 50-μl PCR mixture was sufficient to give reproducible results. Lambda DNA (Boehringer) standards were used to estimate the level of DNA extracted.
(ii) DNA concentration.
Tenfold serial dilutions from 100 to 10−6 of template DNA were used in PCR preparations to determine the influence of DNA concentration on RAPD profiles.
(iii) Random primers.
Eight primers, the sequences of which were chosen at random, were tested at final concentrations of 0.1, 0.5, or 1 μM and were as follows: P1 (5′ ACGCGCCCT 3′) (21), P2 (5′ ATGTAACGCC 3′) (20), P3 (5′ TAGACTAT 3′), P4 (5′ GTCAACTA 3′), P5 (5′ CAATCTGATC 3′), P6 (5′ AGCTATACTA 3′), P7 (5′ CTGCGGCAT 3′), and P8 (5′ CCGCAGCGTT 3′). P3 to P6 were designed with low G+C contents in order to maximize binding to the Lactobacillus chromosome (33 to 53% G+C). Primers were synthesized at the National Food Biotechnology Centre, University College, Cork, Ireland.
(iv) Amplification of Lactobacillus DNA by PCR.
DNA amplification was performed by the method of Coakley and Ross (4). Taq polymerase (1.25 U; Bioline, London, United Kingdom) was added during a 6-min hot start at 94°C, followed by 35 amplification cycles of 94°C for 1 min and the desired annealing temperature for 1 min and an extension step of 72°C for 1 min. Annealing temperatures ranged from 25 to 60°C, and for extended annealing, the following conditions were used: 25°C for 1 min, 30°C for 1 min, 36°C for 2 min, and 40°C for 1 min. In an attempt to increase the proportion of low-molecular-weight fragments, a combined annealing and extension temperature of 36°C for 1 min was used. PCR products (5 to 10 μl) were separated on a 1.5% (wt/vol) gel (Promega Corp., Madison, Wis.) that was run for 1.25 h at 130 V, and the DNA was detected by UV transillumination.
(v) Reading of patterns, numerical analysis, and reproducibility.
RAPD patterns on Polaroid photographs were digitized with a Scanjet 4P scanner and Deskscan II version 2.7 software (Hewlett-Packard Co., Dublin, Ireland), normalized, and further processed with the GelCompar version 4.0 program (Applied Maths, Kortrijk, Belgium). Strains were grouped by using the Pearson product moment correlation coefficient (r) and cluster analysis by the unweighted pair group method with arithmetic averages (38). Reproducibility of RAPD banding profiles was determined from triplicate loadings of independent, triplicate RAPD reaction mixtures from three LMG collection strains of L. paracasei subsp. paracasei on three gels, with cluster analysis being performed as described above.
RESULTS
Compositional profiles of cheeses.
The 17 cheeses analyzed were between 9 and 27 months old. The averages ± standard deviations of the four compositional parameters of pH, percent salt in moisture, percent moisture in nonfat substance, and percent fat in dry matter were 5.10 ± 0.15, 5.27% ± 0.75%, 55.46% ± 2.40%, and 52.82% ± 1.64%, respectively (Table 3). NSLAB counts averaged log 7.42 ± 0.43 CFU · g−1. No correlation was found between commercial grading score and compositional parameters, since the latter could not separate premium-quality cheeses (A to R) from downgraded cheeses (1 to 3).
TABLE 3.
Compositional analysis of mature Cheddar cheeses from six factories
| Factory | Cheese | Cheese age (yr) | NSLAB (log CFU · g−1) | pHa | % S/Mb | % MNFSc | % FDMd |
|---|---|---|---|---|---|---|---|
| I | A | 0.92 | 7.95 | 5.04 | 5.12 | 54.32 | 52.44 |
| I | B | 1.83 | 7.65 | 5.22 | 6.95 | 53.93 | 50.53 |
| I | K | 2.25 | 7.60 | 5.34 | 6.23 | 53.54 | 50.89 |
| I | 1 | 1.25 | 7.60 | 5.29 | 5.30 | 51.40 | 51.01 |
| I | 2 | 1.25 | 7.60 | 5.13 | 4.83 | 53.70 | 53.66 |
| I | 3 | 1.25 | 7.60 | 5.14 | 4.29 | 55.14 | 53.45 |
| II | N | 0.83 | 6.38 | 5.00 | 4.54 | 54.35 | 50.18 |
| II | O | 1.67 | 7.43 | 5.03 | 4.70 | 55.90 | 52.76 |
| III | H | 0.83 | 7.59 | 5.16 | 5.26 | 54.58 | 53.70 |
| IV | C | 0.75 | 7.04 | 4.91 | 5.31 | 56.55 | 51.42 |
| IV | D | 0.92 | 7.43 | 4.93 | 5.03 | 59.60 | 54.94 |
| IV | E | 1.92 | 7.18 | 4.97 | 6.30 | 55.11 | 53.43 |
| V | F | 1.67 | 7.70 | 5.10 | 4.67 | 61.69 | 56.25 |
| V | G | 1.67 | 7.64 | 5.04 | 5.44 | 53.89 | 53.32 |
| VI | P | 2.0 | 7.11 | 5.08 | 4.56 | 56.17 | 54.33 |
| VI | Q | 2.08 | 6.63 | 5.38 | 6.26 | 56.09 | 52.30 |
| VI | R | 1.0 | 7.99 | 4.86 | 4.88 | 56.94 | 53.35 |
The suggested range of pHs for first-grade cheeses was 5.1 to 5.3; that for second-grade cheeses was 5.0 to 5.4 (27).
S/M (percent salt in moisture) = 100 × (x g of salt/100 g of cheese/(x g of moisture/100 g of cheese). The suggested range of percentages for first-grade cheeses was 4.7 to 5.7%; that for second-grade cheeses was 4.0 to 6.0% (27).
MNFS (percent moisture in nonfat substance) = 100 × (percent moisture)/(100 − percent fat). The suggested range of percentages for first-grade cheeses was 52 to 54%; that for second-grade cheeses was 50 to 56% (27).
FDM (percent fat in dry matter) = 100 × (percent fat)/(percent dry matter), where percent dry matter = 100 − percent moisture. The suggested range of percentages for first-grade cheeses was 52 to 56%; that for second-grade cheeses was 50 to 57% (27).
Phenotypic characterization. (i) Type and collection strains.
Biochemical characterization, based on sugar fermentation patterns and end products of glucose metabolism, classified the collection strains as expected. However, comparison of phenotypic characteristics with those reported in the literature revealed some anomalies: LMG 6904T (ATCC 393) did not ferment ribose (12), LMG 12586 (ATCC 334) fermented raffinose (9), and LMG 6907T (ATCC 14917) fermented rhamnose (7). Two different colony morphologies (a and b) were observed for the following LMG isolates: 6400T, 14769, 9191T, and 13724.
(ii) Cheese isolates.
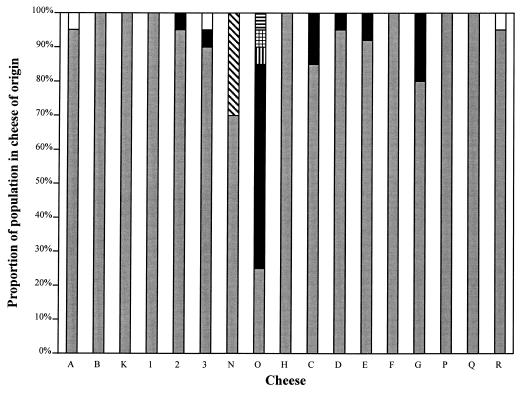
All 331 NSLAB were found to be catalase-negative, nonmotile rods, indicating that they were members of the genus Lactobacillus. Phenotypic characterization of this population revealed a predominance (96.4%) of the species L. paracasei (Fig. 1). The other species identified were L. plantarum (2.1%), L. curvatus (0.3%), and L. brevis (0.3%). The NSLAB population of 7 of the 17 cheeses analyzed was comprised solely of L. paracasei strains. An additional five cheeses also contained ribose-negative variants of L. paracasei, such that 70% of the sampled cheeses had an NSLAB flora wholly comprised of strains from the L. paracasei complex. L. curvatus was found only in cheese O, which also contained L. brevis and a raffinose-negative variant of L. plantarum (tentatively identified as Lactobacillus paraplantarum). The six L. plantarum strains were all isolated from cheese N, which was obtained from the same factory as cheese O. The fermentation profiles of three strains, which were mutually dissimilar, were such that these isolates could not be phenotypically identified.
FIG. 1.
Survey of NSLAB populations in mature Irish Cheddar cheeses. Species designations are based on phenotypic characteristics. ░⃞, L. paracasei; ■, ribose-negative L. paracasei; ▧, L. plantarum; ▤, L. paraplantarum; , L. curvatus; ▥, L. brevis; □, unidentified.
DNA amplification with random primers.
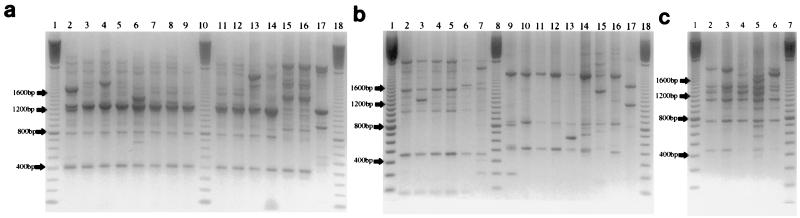
Dilution of DNA from 100 to 10−2 resulted in identical RAPD patterns for four strains (data not shown); thus, the undiluted template was subsequently used in all PCR preparations. Primers P3 to P6 did not give PCR products, despite extended annealing times at low temperatures, probably due to their low melting temperatures. The remaining primers, P1, P2, P7, and P8, all gave acceptable PCR products; P1 gave the largest number of bands, while P2 gave easily identifiable species-specific profiles (Fig. 2). Optimal primer concentrations for P1 and P2 were 1 and 0.5 μM, respectively, and the optimal annealing temperature was 44°C, based on the intensities of low-molecular-weight fragments.
FIG. 2.
(a) RAPD profiles of type and collection strains of L. paracasei subsp. paracasei (lanes 2 to 9, LMG 14766, LMG 11473, LMG 13087T, LMG 9193, LMG 11965, LMG 13722, LMG 13717, and LMG 13724a, respectively, and lanes 11 to 14, LMG 13724b, LMG 13732, LMG 12586, and LMG 9192T, respectively), L. paracasei subsp. tolerans (lanes 15 and 16, LMG 9191aT and LMG 9191bT, respectively), and L. casei (lane 17, LMG 6904T). Lanes 1, 10, and 18 contain 100-bp molecular size markers. (b) RAPD profiles of type and collection strains of L. rhamnosus (lanes 2 to 7, LMG 10769, LMG 10775, LMG 6400Ta, LMG 6400Tb, LMG 8153, and LMG 12166, respectively), L. plantarum (lanes 9 to 16, LMG 11475, LMG 14769a, LMG 14769b, LMG 6907T, LMG 1284, LMG 14760, LMG 9205, and LMG 11460, respectively), and L. pentosus (lane 17, LMG 10755T). Lanes 1, 8, and 18 contain 100-bp molecular size markers. (c) RAPD profiles of type and collection strains of L. curvatus (lanes 2 to 6, LMG 12009, LMG 12007, LMG 9198T, LMG 12008, and LMG 11479, respectively). Lanes 1 and 7 contain 100-bp molecular size markers.
Reproducibility of RAPD.
Triplicate loadings of triplicate PCR products from three individual strains of L. paracasei subsp. paracasei were separable, following cluster analysis, at the 80% similarity level (data not shown); i.e., separation above this level of similarity was insignificant.
Resolving power of RAPD.
RAPD profiles exhibiting species-specific bands were generated with the P2 primer for L. paracasei (Fig. 2a); Lactobacillus rhamnosus, L. plantarum, and Lactobacillus pentosus (Fig. 2b); and L. curvatus (Fig. 2c). Differentiation of isolates to the subspecies and strain levels was possible by RAPD analysis.
Genotypic characterization of cheese and collection strains.
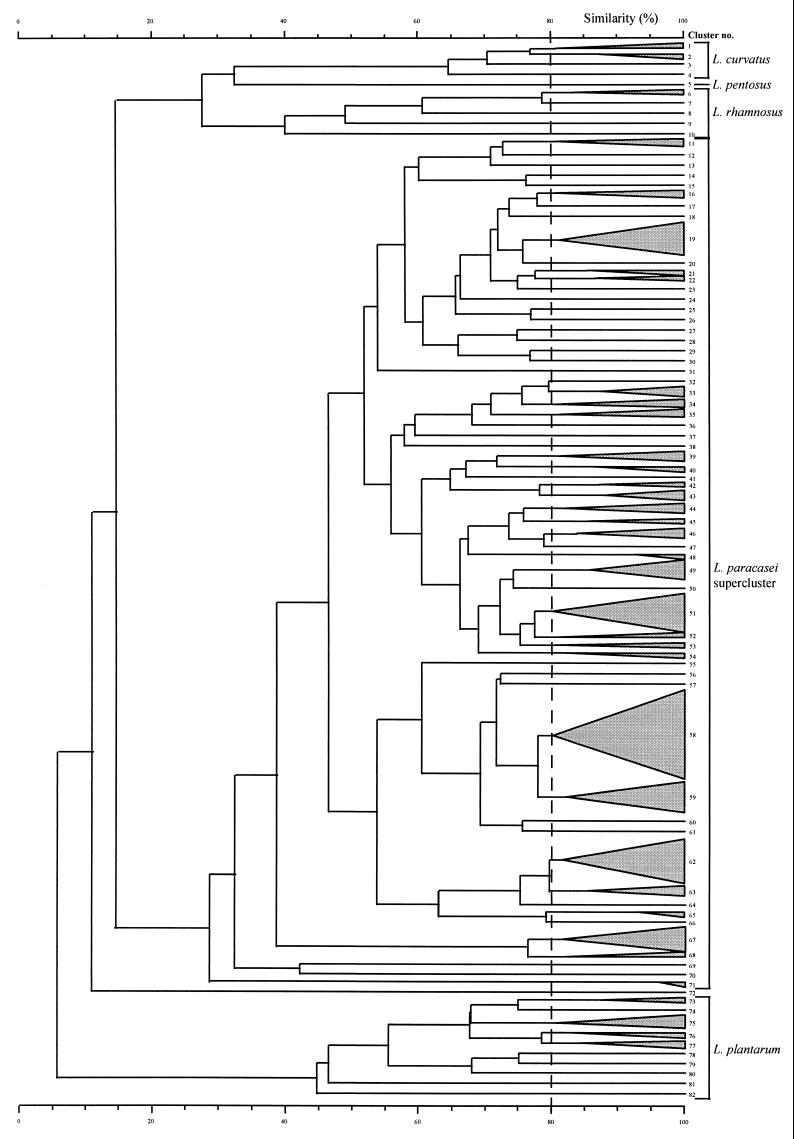
The dendrogram in Fig. 3 is based on the combined RAPD profiles generated by P1 and P2 for nine cheeses from three factories (including three poor-quality cheeses from factory I) and was abridged at the 80% similarity level to indicate individually separated strains only. Details of each cluster are given in Table 1. To test the discriminating power of RAPD analysis at the species or subspecies and strain levels, 31 collection isolates of mesophilic lactobacilli, previously typed as 25 individual strains by protein profiling, were analyzed (6). Typed strains of each species clustered together. Cluster analysis of RAPD profiles for collection strains resulted in distinct species clusters (Fig. 3). Fingerprints, generated with the P2 primer for multiple strains of the same species demonstrated the presence of a number of species-specific bands. There were three common bands for all L. paracasei strains (Fig. 2a, lanes 2 to 9 and 11 to 16) at 0.4, at 0.9, and at 1.2 and 1.3 kb (the last being a doublet). Fingerprint bands for L. rhamnosus strains (Fig. 2b, lanes 2 to 7) occurred at 0.47, 1.05, and 1.55 kb, while those for L. plantarum strains (lanes 9 to 16) were at 0.52 (faint band for LMG 9205 [lane 15]), 0.85, and 2 kb. All five L. curvatus strains had a faint band at 0.44 kb, with more intense bands appearing at 0.77, 1.1, and 1.4 kb (Fig. 2c, lanes 2 to 6). Since only one strain of L. pentosus (Fig. 2b, lane 17) was subjected to RAPD analysis, identification of species-specific bands was not possible. However, there were two intense bands at 1.23 and 1.7 kb. This result implies that RAPD analysis has potential application in the rapid classification of mesophilic lactobacilli to the species level. A band at 0.8 kb, present in all L. paracasei subsp. paracasei profiles (Fig. 2a, lanes 2 to 9 and 11 to 13), was not found for the L. paracasei subsp. tolerans isolate (lanes 15 and 16; two different colony morphologies), which displayed a faint doublet at 1.2 and 1.3 kb, relative to that of the L. paracasei subsp. paracasei strains. These data suggest that RAPD analysis has the capacity to distinguish between subspecies of lactobacilli. Although the type strain of L. casei, LMG 6904T (Fig. 2a, lane 17), reclassified as Lactobacillus zeae (12), displayed the intense doublet at 1.2 and 1.3 kb common to L. paracasei strains, a distinctly different fingerprint was obtained with bands at 0.49, 0.56, 0.98 kb.
FIG. 3.
Abridged dendrogram, obtained from combined RAPD patterns with two primers, of Lactobacillus strains found in mature Irish Cheddar cheese, with cluster analysis having been performed with the Pearson product moment correlation coefficient (r) and by the unweighted pair group algorithm method with arithmetic averages.
RAPD analysis had the capacity to distinguish between strains of the same species, as demonstrated by the four different banding patterns obtained for the 12 strains within the L. paracasei subsp. paracasei group (Fig. 2a, lanes 2, 3, 6, and 13), the four banding patterns for five L. rhamnosus strains (Fig. 2b, lanes 2, 3, 6, and 7), the five banding patterns for seven L. plantarum isolates (Fig. 2b, lanes 9, 10, 13, 15, and 16), and the four banding patterns for five L. curvatus strains (Fig. 2c, lanes 2 and 4 to 6). Characteristic banding patterns were also obtained when these strains were analyzed with RAPD and the P1 primer, although the banding profiles were more complex, which enabled the differentiation of more strains within a species than was possible with the P2 primer. The resolving power of RAPD analysis, however, was not as great as protein profiling, as the latter resolved a greater number of the reference isolates to the strain level.
The heterogeneity of the L. paracasei strains is demonstrated by the separation of strains at the 29% similarity level for the supercluster formed (clusters 11 to 71, inclusive). The type strain of L. casei, LMG 6904T (ATCC 393), in cluster 69 was only 32% similar to L. paracasei subsp. paracasei strains, while the type strain of L. paracasei subsp. tolerans, LMG 9191T (cluster 71; two different colony morphologies), had the lowest homology (29%) within the supercluster. Differentiation of the L. paracasei subsp. paracasei strains occurred at 32%.
Genotypic characterization of the isolates correlated well with their biochemical classification obtained. Cluster analysis demonstrated an average of seven strains per cheese (based on the number of individually separated strains at the 80% similarity level for combined gels of P1 and P2 profiles). Cheese N had the greatest strain diversity, with 13 strains being identified, while both cheeses H and K contained only 4 strains. For the most part, isolates from the same factory grouped together. Analysis of cheese isolates from factories I to III indicated that four strains (clusters 51, 53, 58, and 59) occurred in different cheeses made in factory I at different times (Table 3); two of these strains were found in both premium-quality and sensorially defective cheese.
Most cheese isolates (∼79%) produced l-lactate from glucose. However, no correlation could be found between the isomer of lactate produced and RAPD profiles, as demonstrated both by the clustering of the dl-lactate-producing type strain of the former L. casei subsp. pseudoplantarum with l-lactate producers in clusters 55 to 66, inclusive, and by the grouping of dl and l producers in clusters 11, 19, 22, 43, 46, and 49. Most strains of the ribose-negative phenotype grouped together (clusters 17 to 19 and 25 to 26). The raffinose-negative variant of L. plantarum in cluster 74 had the biochemical profile of L. paraplantarum 61D, as reported by Curk et al. (7), and grouped with those collection and cheese isolates biochemically classified as strains of L. plantarum. Of the three isolates unidentified by phenotypic characterization, the two on which cluster analysis was performed clustered apart from each other (clusters 31 and 43), in agreement with the unique fermentation profile obtained for each. However, both isolates clustered within the L. paracasei complex.
DISCUSSION
NSLAB were isolated from 14 mature, premium-quality and from three defective-quality Cheddar cheeses, obtained from six different commercial manufacturers. Each cheese was graded by the in-house sensory analyst of the factory of origin. Comparison of compositional data (Table 3) with recommended ranges for percent salt in moisture, percent moisture in nofat substance, percent fat in dry matter, and pH for first- and second-grade Cheddar cheese, as devised by Lawrence and Gilles (27), revealed that no cheese, including one awarded a prestigious prize, fulfilled all four premium-grade criteria; only cheeses G and H satisfied three of these criteria. Five cheeses, namely, A, G, H, N, and O, complied with second-grade parameters. However, compositional analysis of cheeses was carried out at least one year postmanufacture, while suggested ranges for compositional parameters are based on analyzes at 14 days postmanufacture. The composition ranges across cheeses A to R were quite wide: the range for moisture was 35.3 to 41.3%, that for salt was 1.69 to 2.55%, and that for pH was 4.86 to 5.38. Similar results were reported by Fox (14) in a study of 27 mature Cheddar samples.
The mean NSLAB level in the cheeses, which were all at least 9 months old, was log 7.42 ± 0.43 CFU · g−1. All isolates were found to be Lactobacillus species. This was in agreement with the results of a previous study of commercial Cheddar of Irish origin (23), indicating that the absence of other genera of NSLAB may be a feature of Irish Cheddar cheese. Pediococci, which can survive for 12 months in cheese (24), were found to be the dominant non-starter flora in a series of English Cheddar cheeses (16) but constituted far smaller proportions of New Zealand (8) and American (31) Cheddar cheeses. Feagan and Dawson (13) noted that micrococci, which are not lactic acid bacteria and thus not normally considered part of the NSLAB flora of Cheddar cheese, comprised from 16 to 68% of the non-starter flora of 6-month-old Australian Cheddar.
Phenotypic characterization to the species level of the 331 isolates from 17 cheeses indicated that 319 (96.4%) were L. paracasei (including 23 ribose-negative variants), 7 (2.1%) were L. plantarum, including a raffinose-negative variant which is possibly L. paraplantarum (7), 1 (0.3%) was L. curvatus, 1 (0.3%) was L. brevis, and 3 could not be identified, either from Bergey’s manual (24) or from information provided with API 50CH kits for identification of lactobacilli (bioMérieux s.a., Marcy-l’Etoile, France). Cheese O from factory II displayed the greatest species diversity and contained five different species. It is interesting that the population of 8-week-old Cheddar cheeses studied by Jordan and Cogan (23), comprising 160 isolates, had a smaller proportion of L. paracasei (55%) and larger proportions of L. plantarum and L. curvatus (28 and 14%, respectively) strains than the cheeses investigated in this study. These data imply that the composition of the NSLAB population of Cheddar changes with age, as was reported from an investigation by Naylor and Sharpe (35), who found differences in the distributions of serological types of lactobacilli, both within a block and within a pair of duplicate cheeses, during ripening.
A study of the changes in the microbial ecosystem during the ripening of raw milk Swiss-type minicheeses (11) revealed that the species identified evolved from a great diversity at the start of ripening to two dominating subgroups of L. paracasei at 24 weeks postmanufacture. A comparison of the relative occurrence of L. plantarum with that of L. casei during the ripening of Cheddar (13) indicated that similar proportions of L. plantarum and L. casei were found in both good- and defective-quality cheeses and that variations in the population of lactobacilli in cheese of comparable ages could not be correlated with particular flavor changes.
In an effort to validate the biochemical classifications to species level, a selection of 31 collection isolates of mesophilic lactobacilli were subjected to the tests outlined in Materials and Methods and Table 2. All were designated to the correct species on the basis of these biochemical tests. Fermentation patterns for the majority of these were as reported in the literature. However, a few anomalies were noted, highlighting the main disadvantage of phenotyping: the designation of isolates to one species or another on the basis of a selection of phenotypic markers, which may not be stably expressed under certain environmental or culture conditions. The difficulty associated with using changes in pH as indicators of growth in the presence of different sugars is determining the cutoff point for a positive or negative reaction. For example, LMG 10755T (ATCC 8041), reported as fermenting rhamnose, gave a weakly positive reaction for this carbon source that was indicated by a change in pH of 0.69 after 7 days of incubation at 30°C, compared to mean changes in pH of 1.43 and 0.27 for positive and negative reactions, respectively, for collection strains.
Although phenotypic tests provide evidence of the metabolic capabilities of strains, they have problems such as nonreproducibility and lack of discriminatory power. The advantages of genotyping include the stability of genomic DNA, its composition being independent of cultural conditions or preparation methods, and amenability to automation and statistical data analysis. The designation of certain neotype strains on the basis of phenotypic characteristics alone has resulted in confusion in the nomenclature of strains of L. casei, which is being resolved with genotypic data (12). The most accurate results are obtained when both phenotypic and genotypic attributes are used to make the correct appellation.
RAPD analysis has previously been reported for rapid typing of L. plantarum strains (21), but this procedure has not been used to study lactobacilli in cheese. To complete the present study, it was necessary to determine if this technique could be extended to other species of mesophilic lactobacilli. Distinct species clusters were obtained following cluster analysis of RAPD patterns for 31 collection isolates (Fig. 3). The P2 primer generated species-specific bands for multiple strains of the same species (Fig. 2).
Cluster analysis of RAPD fingerprints of cheese isolates demonstrated that there was an average of seven strains per cheese (based on the number of individually separated strains at the 80% similarity level for combined gels of P1 and P2 profiles). The dendrogram in Fig. 3 illustrates the relationships between collection strains and isolates from cheeses, obtained from three different commercial producers. The majority of strains isolated from cheeses made in the same factory clustered together: isolates from factory I cheeses fell in clusters 32 to 54 and 58 to 61, isolates from factory II cheeses fell in clusters 16 to 30 and 73 to 80, and isolates from factory III cheeses fell in clusters 62 to 64 (Fig. 3 and Table 1). For the most part, isolates from premium-quality cheeses clustered together (87 of 103, in clusters 11 to 54), as did isolates from defective-quality cheeses (clusters 58, 59, and 67). Clustering of biochemically characterized isolates from cheeses of adequate and defective quality within the L. paracasei complex was reported by Lindberg et al. (30) in a study of mature Norwegian and Swedish semihard cheese.
The most striking feature of the cluster structure in Fig. 3 is the heterogeneity of the L. paracasei supercluster (clusters 11 to 71) (Table 1). The type strains of L. paracasei subsp. paracasei (LMG 13087T, cluster 30) and L. casei subsp. casei (LMG 6904T [ATCC 393, reclassified as L. zeae], cluster 69) and the proposed neotype strain of L. casei subsp. casei ATCC 334 (LMG 12586, cluster 57), in place of ATCC 393 (12), were all well separated (Fig. 3). Six collection strains of L. paracasei subsp. paracasei (cluster 58) had a very high homology with poor cheese isolates from factory I (clusters 58 and 59). The type strain (LMG 13087T) was most similar to isolates from factory II (clusters 16 to 28), while the type strain of L. casei subsp. casei (LMG 6904T) was only 32% similar to the majority of isolates in the L. paracasei supercluster, a fact which supports its revised taxonomic status (12).
Cluster analysis of RAPD profiles of cheese isolates from three factories provided evidence for the recurrence of strains in cheeses K and 1 to 3, manufactured 1 year apart at factory I (clusters 51, 53, 58, and 59). Visual observation of fingerprints for the remaining isolates revealed that similar banding profiles were found for isolates from cheeses Q and R, produced by factory VI 1 year apart; however, no common strain was isolated from cheeses P and Q, manufactured 1 month apart at factory VI (data not shown). Although L. plantarum strains were found only in cheeses N and O, produced at factory II in consecutive years, the latter exhibited greater species diversity. This reappearance of certain strains of NSLAB in cheese over time has previously been reported (35), prior to the advent of RAPD analysis.
The majority (∼79%) of cheese isolates were l-lactate producers. No correlation could be found between the RAPD profile and isomer of lactate produced; the dl-producing type strain of the former subspecies L. casei subsp. pseudoplantarum LMG 9192T (DSM 20008T, cluster 66) grouped together with isolates in clusters 55 to 66, all of which were l-lactate producers. Furthermore, strains of the ribose-negative phenotype divided into two distinct groups (clusters 17 to 19 and 25 to 26). These findings are in contrast to those of Lindberg et al. (30), who reported that strains of the same phenotype clustered together on the basis of biochemical parameters.
In conclusion, RAPD analysis proved to be a very rapid, effective, and reproducible method of differentiating the NSLAB floras of mature Irish Cheddar cheeses to the strain level. Application of this technique to isolates from mature Cheddar demonstrated that the NSLAB flora was dominated by L. paracasei and that major strain heterogeneity existed among isolates.
ACKNOWLEDGMENTS
This work was supported by European Regional Development Fund, Measure 3(ii), of the Food Sub-Programme. N. Fitzsimons was in receipt of a Walsh Fellowship.
The assistance of the staff of the Department of Food Technology, Chemical Centre, University of Lund, Lund, Sweden, is gratefully acknowledged.
REFERENCES
- 1.Bhowmilk T, Marth E H. Role of Micrococcus and Pediococcusspecies in cheese ripening: a review. J Dairy Sci. 1990;73:859–866. [Google Scholar]
- 2.British Standard. Chemical analysis of cheese. Part 5. Determination of pH value. British Standard 770. Milton Keyes, United Kingdom: British Standards Institution; 1976. [Google Scholar]
- 3.Broome M C, Krause D A, Hickey M W. The isolation and characterization of lactobacilli from Cheddar cheese. Aust J Dairy Technol. 1990;45:60–66. [Google Scholar]
- 4.Coakley M, Ross R P. Application of the polymerase chain reaction to the rapid analysis of brewery yeast strains. J Inst Brew. 1996;102:349–354. [Google Scholar]
- 5.Collins M D, Phillips B A, Zanoni P. Deoxyribonucleic acid homology studies of Lactobacillus casei, Lactobacillus paracasei sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosussp. nov., comb. nov. Int J Syst Bacteriol. 1989;39:105–108. [Google Scholar]
- 6.Costas M, Pot B, Vandamme P, Kersters K, Owen R J, Hill L R. Interlaboratory comparative study of the numerical analysis of one-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoretic protein patterns of Campylobacterstrains. Electrophoresis. 1990;11:467–474. doi: 10.1002/elps.1150110606. [DOI] [PubMed] [Google Scholar]
- 7.Curk M-C, Hubert J-C, Bringel F. Lactobacillus paraplantarum sp. nov., a new species related to Lactobacillus plantarum. Int J Syst Bacteriol. 1996;46:595–598. doi: 10.1099/00207713-46-2-595. [DOI] [PubMed] [Google Scholar]
- 8.Dacre J C. A note on pediococci in New Zealand Cheddar cheese. J Dairy Res. 1958;25:414–417. [Google Scholar]
- 9.Dellaglio F, Dicks L M T, du Toit M, Torriani S. Designation of ATCC 334 in place of ATCC 393 (NCDO 161) as the neotype strain of Lactobacillus casei subsp. casei and rejection of the name Lactobacillus paracasei(Collins et al., 1989). Request for an opinion. Int J Syst Bacteriol. 1991;41:340–342. [Google Scholar]
- 10.deMan J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 11.Demarigny Y, Beuvier E, Dasen A, Duboz G. Influence of raw milk microflora on the characteristics of Swiss-type cheese. I. Evolution of microflora during ripening and characterisation of facultatively heterofermentative lactobacilli. Lait. 1996;76:371–387. [Google Scholar]
- 12.Dicks L M T, Du Plessis E M, Dellaglio F, Lauer E. Reclassification of Lactobacillus casei subsp. casei ATCC 393 and Lactobacillus rhamnosus ATCC 15820 as Lactobacillus zeae nom. rev., designation of ATCC 334 as the neotype of L. casei subsp. casei, and rejection of the name Lactobacillus paracasei. Int J Syst Bacteriol. 1996;46:337–340. doi: 10.1099/00207713-46-1-337. [DOI] [PubMed] [Google Scholar]
- 13.Feagan J T, Dawson D J. Bacteriology of Cheddar cheese. Some observations on the microflora during maturing. Aust J Dairy Technol. 1959;14:59–66. [Google Scholar]
- 14.Fox P F. Influence of cheese composition on quality. Ir J Agric Res. 1975;14:33–42. [Google Scholar]
- 15.Franklin J G, Sharpe M E. The incidence of bacteria in cheesemilk and Cheddar cheese and their association with flavour. J Dairy Res. 1963;30:87–99. [Google Scholar]
- 16.Fryer T F, Sharpe M E. Pediococci in Cheddar cheese. J Dairy Res. 1966;33:325–331. [Google Scholar]
- 17.Irish Standard. Determination of the percentage fat in cheese. Irish Standard 69. Dublin, Ireland: Institute for Industrial Research and Standards; 1955. [Google Scholar]
- 18.International Dairy Federation. Cheese and processed cheese. Determination of chloride content: potentiometric titration method. Standard 88. Brussels, Belgium: International Dairy Federation; 1979. [Google Scholar]
- 19.International Dairy Federation. Determination of the total solids content (cheese and processed cheese). Standard 4A. Brussels, Belgium: International Dairy Federation; 1982. [Google Scholar]
- 20.Jayarao B M, Oliver S P. Polymerase chain reaction-based DNA fingerprinting for identification of Streptococcus and Enterococcusspecies isolated from bovine milk. J Food Prot. 1994;57:240–245. doi: 10.4315/0362-028X-57.3.240. [DOI] [PubMed] [Google Scholar]
- 21.Johansson M-L, Quednau M, Molin G, Ahrné S. Randomly amplified polymorphic DNA (RAPD) for rapid typing of Lactobacillus plantarumstrains. Lett Appl Microbiol. 1995;21:155–159. doi: 10.1111/j.1472-765x.1995.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 22.Johns C K, Cole S E. Lactobacilli in Cheddar cheese. J Dairy Res. 1959;26:157–161. [Google Scholar]
- 23.Jordan K N, Cogan T M. Identification and growth of non-starter lactic acid bacteria in Irish Cheddar cheese. Ir J Agric Food Res. 1993;32:47–55. [Google Scholar]
- 24.Kandler O, Weiss N. Regular, non-sporing Gram-positive rods. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of determinative bacteriology. 8th ed. Vol. 2. Baltimore, Md: Williams and Wilkins Co.; 1986. pp. 1208–1234. [Google Scholar]
- 25.Laleye L C, Simard R E, Lee B H, Holley R A. Quality attributes of Cheddar cheese containing added lactobacilli. J Food Sci. 1990;55:114–118. [Google Scholar]
- 26.Law B A, Castanon M, Sharpe M E. The effect of non-starter bacteria on the chemical composition and the flavour of Cheddar cheese. J Dairy Res. 1976;43:117–125. [Google Scholar]
- 27.Lawrence R C, Gilles J. Cheddar cheese and related dry-salted varieties. In: Fox P F, editor. Cheese: chemistry, physics and microbiology. Vol. 2. London, United Kingdom: Elsevier Applied Science; 1987. pp. 1–44. [Google Scholar]
- 28.Lee B H, Laleye L C, Simard R E, Holley R A, Emmons D B, Giroux R N. Influence of homofermentative lactobacilli on physicochemical and sensory properties of Cheddar cheese. J Food Sci. 1990;55:386–397. [Google Scholar]
- 29.Lemieux L, Puchades R, Simard R E. Size-exclusion HPLC separation of bitter and astringent fractions from Cheddar cheese made with added Lactobacillusstrains to accelerate ripening. J Food Sci. 1989;54:1234–1237. [Google Scholar]
- 30.Lindberg A-M, Christiansson A, Rukke E-O, Eklund T, Molin G. Bacterial flora of Norwegian and Swedish semi-hard cheese after ripening, with special reference to Lactobacillus. Neth Milk Dairy J. 1996;50:563–572. [Google Scholar]
- 31.Litopoulou-Tzanetaki E, Graham D C, Beyatli Y. Detection of pediococci and other non-starter organisms in American Cheddar cheese. J Dairy Sci. 1989;72:854–858. [Google Scholar]
- 32.Lynch C M, McSweeney P L H, Fox P F, Cogan T M, Drinan F D. Manufacture of Cheddar cheese with and without adjunct lactobacilli under controlled microbiological conditions. Int Dairy J. 1996;6:851–867. [Google Scholar]
- 33.Martley F G, Crow V L. Interactions between non-starter microorganisms during cheese manufacture and ripening. Int Dairy J. 1993;3:461–483. [Google Scholar]
- 34.Naylor J, Sharpe M E. Lactobacilli in Cheddar cheese. I. The use of selective media for isolation and of serological typing for identification. J Dairy Res. 1958;25:92–103. [Google Scholar]
- 35.Naylor J, Sharpe M E. Lactobacilli in Cheddar cheese. II. Duplicate cheeses. J Dairy Res. 1958;25:421–430. [Google Scholar]
- 36.Naylor J, Sharpe M E. Lactobacilli in Cheddar cheese. III. The source of lactobacilli in cheese. J Dairy Res. 1958;25:431–438. [Google Scholar]
- 37.Peterson S D, Marshall R T. Non-starter lactobacilli in Cheddar cheese: a review. J Dairy Sci. 1990;73:1395–1410. [Google Scholar]
- 38.Pot B, Vandamme P, Kersters K. Analysis of electrophoretic whole-organism protein fingerprints. In: Goodfellow M, O’Donnell A G, editors. Chemical methods in prokaryotic systematics. J. Chichester, United Kingdom: Wiley & Sons; 1994. pp. 493–521. [Google Scholar]
- 39.Puchades R, Lemieux L, Simard R E. Evolution of free amino acids during the ripening of Cheddar cheese containing added lactobacilli strains. J Food Sci. 1989;54:885–888. [Google Scholar]
- 40.Reiter B, Sharpe M E. Relationship of the microflora to the flavour of Cheddar cheese. J Appl Bacteriol. 1971;34:63–80. doi: 10.1111/j.1365-2672.1971.tb02269.x. [DOI] [PubMed] [Google Scholar]
- 41.Rogosa M, Mitchell J A, Wiseman R F. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951;62:132–133. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan M P, Rea M C, Hill C, Ross R P. An application in Cheddar cheese manufacture for a strain of Lactococcus lactisproducing a novel broad-spectrum bacteriocin, lacticin 3147. Appl Environ Microbiol. 1996;62:612–619. doi: 10.1128/aem.62.2.612-619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tailliez P, Quénée P, Chopin A. Estimation de la diversité parmi les souches de la collection CNRZ: application de la RAPD àun groupe de lactobacilles. Lait. 1996;76:147–158. [Google Scholar]
- 44.Trépanier G, El Abboudi M, Lee B H, Simard R E. Accelerated maturation of Cheddar cheese: influence of added lactobacilli and commercial protease on composition and texture. J Food Sci. 1992;57:898–902. [Google Scholar]
- 45.Trépanier G, El Abboudi M, Lee B H, Simard R E. Accelerated maturation of Cheddar cheese: microbiology of cheeses supplemented with Lactobacillus casei subsp. caseiL2A. J Food Sci. 1992;57:345–349. [Google Scholar]
- 46.Trépanier G, Simard R E, Lee B H. Effect of added lactobacilli on composition and texture of Cheddar cheese during accelerated maturation. J Food Sci. 1991;56:696–700. [Google Scholar]
- 47.Walsh E M, McSweeney P L H, Fox P F. Use of antibiotics to inhibit non-starter lactic acid bacteria in Cheddar cheese. Int Dairy J. 1996;6:425–431. [Google Scholar]