SUMMARY
A panel of 223 faecal samples was analysed to determine the clinical utility of the Seeplex® Diarrhea ACE Detection multiplex PCR system (Seeplex system; Seegene, Korea), a qualitative multiplexing PCR technology that enables simultaneous multi-pathogen detection of four viruses and/or ten bacteria associated with acute gastroenteritis. Conventional diagnostic methods and a norovirus-specific multiplex real-time RT–PCR detected 98 pathogens in 96 samples. The Seeplex system detected 81 pathogens in 75 samples. All samples positive for adenovirus, norovirus, Campylobacter spp., Escherichia coli O157, Shigella spp. or Vibrio spp. were detected by the Seeplex system. Rotavirus, Clostridium difficile toxin B, and Salmonella spp. were not detected in 12·5%, 50% and 15·8% of samples, respectively. Additional multiple infections were detected in 19 samples by the Seeplex system. The Seeplex system provides significant additional diagnostic capability for the syndromic diagnosis of acute gastroenteritis with increased sensitivity for the majority of pathogens.
Key words: Clinical microbiology, gastroenteritis, molecular biology
INTRODUCTION
Acute gastroenteritis is associated with bacterial, viral, and parasitic infections. It is estimated that there are up to 17 million sporadic, community cases of infectious intestinal disease (IID) and 1 million General Practitioner (GP) consultations annually in the UK [1]. Campylobacter spp., predominantly Campylobacter jejuni, is the most frequently detected bacterial species among enteric pathogens with 500 000 cases and 80 000 GP consultations annually [1]. Most infections are sporadic and may be linked to the ingestion of contaminated poultry, milk, or water. The genus Salmonella comprises two species, S. enterica and S. bongori, which have more than 2500 serotypes or serovars. S. enterica with its six subspecies is of most clinical relevance for humans. Most Salmonella infections are confined to the gastrointestinal tract. Enterotoxigenic Escherichia coli, such as E. coli O157, are associated with bloody diarrhoea and haemolytic uraemic syndrome and are transmitted via contaminated food and water or the ingestion of undercooked meat contaminated by animal faeces. Clostridium (Cl.) difficile is the most common cause of antibiotic-associated colitis and is typically hospital acquired. The bacteria secrete both a cytotoxin and an enterotoxin.
Rotavirus and norovirus are the most common viral enteric pathogens. Rotaviruses are a major cause of acute gastroenteritis in children aged <5 years worldwide [2], while noroviruses are the leading cause of epidemics of gastroenteritis and an important cause of sporadic gastroenteritis in both children and adults [3], accounting for 3 million cases and 130 000 GP consultations annually [1]. Noroviruses, which are spread directly from person to person or via contaminated food, water and environmental surfaces, are associated with outbreaks in semi-closed communities such as hospitals, nursing homes, cruise ships, and hotels [4–8].
Giardia intestinalis, a flagellate protozoan, and Cryptosporidium parvum, a coccidian protozoan, are associated with gastroenteritis. Indirect person-to-person or zoonotic transmission may occur through the ingestion of contaminated food, drinking or recreational water [9–13].
Conventional diagnostic methods for routine detection of enteric pathogens within the clinical microbiology setting rely on microscopy, culture, and enzyme immunoassays. Protozoa are detected by direct microscopy and antigen detection, whereas bacteria are detected through culture, with or without enrichment, and pathogen-specific toxin detection and viruses by viral antigen and genome detection. Bacterial culture may take 48–96 h and commensal flora may overgrow a pathogen while biochemical tests may be unreliable as not all strains within a species may share the same biochemical characteristics and cross-reactivity may be present when serological techniques are used. Microscopy and antigen detection for parasites and viruses may lack sensitivity, particularly in samples taken several days after the onset of the illness.
Molecular methodologies based on polymerase chain reaction (PCR) and reverse-transcription (RT)–PCR provide powerful tools [14], which have markedly improved the detection of enteric pathogens [15]. These methods are rapid, have increased or equivalent sensitivity compared to culture, antigen detection or direct microscopy [14, 16–21], are less labour intensive, and do not require viable organisms, but are generally limited to the detection of a specific viral, bacterial or parasitic pathogen [17, 19, 21–27]. However, new molecular technologies are emerging for use within the routine setting, which enable the simultaneous detection and identification of multiple groups of enteric pathogens [28, 29], which in combination with automated systems available for nucleic acid extraction, PCR reagent preparation, and assay set up allow high sample throughput. In addition, PCR products are available for molecular characterization allowing speciation, genogrouping or genotyping.
This retrospective cross-sectional study compared routine diagnostic methods including culture, and antigen, toxin and genome detection to the Seeplex® Diarrhea ACE Detection multiplex PCR system (hereafter referred to as the Seeplex system) (Seegene, Korea), a new multiplexing PCR technology that enables simultaneous multi-pathogen detection of four viruses, nine bacteria and a bacterial toxin using three multiplex assays, the Seeplex Diarrhea-V, -B1 and -B2 assays.
MATERIALS AND METHODS
Clinical samples and routine diagnostic microbiology
Identification of enteric pathogens was performed at the Microbiology Department, Norfolk and Norwich University Hospital using a combination of culture, antigen detection, toxin detection and genome detection (Table 1). An initial 10% suspension of all semi-formed or liquid faeces was made in 0·1% peptone water and used to inoculate standard media and enrichment broths in accordance with UK Standards for Microbiology Investigations B30 [30], for Campylobacter spp., Salmonella spp., Shigella spp. and verocytotoxin-producing E. coli (VTEC) (Table 1). In addition, faecal specimens were screened for individual target bacteria or parasites as indicated by clinical details (Table 1) and in accordance with UK Standards for Microbiology Investigations B30 and B31, respectively [30, 31].
Table 1.
Target organisms: primary diagnostic methods
| Methods | Reference | |
|---|---|---|
| Bacteria | ||
| Salmonella spp.* | Direct plating – xylose lysine deoxycholate (XLD) agar. Enrichment culture – mannitol selenite broth and subculture onto XLD agar | National standard operating procedure B30 |
| Shigella spp.* | Direct plating – XLD agar | National standard operating procedure B30 |
| Campylobacter spp.* | Direct plating – modified cefeoperazone, charcoal deoxycholate (CCD) agar | National standard operating procedure B30 |
| Escherichia coli O157* | Direct plating on cefixime tellurite sorbitol MacConkey (CT-SMAC) agar. Enrichment in modified tryptone soya broth with novobiocin | National standard operating procedure B30 |
| Clostridium difficile | C.DIFF CHEK-60 and TOX A/B QUIK CHEK (TechLab, USA) | National standard operating procedure B10 |
| Yersinia enterocolitica* | Direct plating – cefsulodin irgasin novobiocin (CIN) selective agar | National standard operating procedure B30 |
| Vibrio spp.* | Direct plating – thiosulphate citrate bile salt sucrose (TCBS) agar and alkaline peptone water (APW) enrichment | National standard operating procedure B30 |
| Aeromonas spp. | Direct plating – Aeromonas selective agar | National standard operating procedure B30 |
| Clostridium perfringens* | Direct plating – neomycin fastidious anaerobe agar | National standard operating procedure B30 |
| Protozoa | ||
| Cryptosporidium spp. | Stain using auramine-phenol and examined using an incident-light fluorescent microscope for characteristic oocysts | National standard operating procedure B31 |
| Ova, cysts and parasites* | Direct and concentration microscopy | National standard operating procedure B31 |
| Viruses | ||
| Adenovirus | ProSpecT Adenovirus EZ Microplate assay (Oxoid Ltd, UK) | |
| Rotavirus | ProSpecT Adenovirus EZ Microplate assay (Oxoid Ltd, UK) | |
| Norovirus | Primary test: internally controlled, one-step, real-time RT–PCR assay for norovirus detection and genogrouping | Rolfe et al. [33] |
| Confirmatory test: RIDA®GENE Norovirus V real-time RT–PCR (R-Biopharm Rhône Ltd, UK) | ||
All positive isolates were sent to the relevant reference laboratory and confirmed.
Notes: (1) All patients screened for Salmonella spp., Shigella spp., Campylobacter spp. and Escherichia coli O157, except convalescent cases of previous isolates, and contacts of positive cases. (2) If clinically indicated, patients who fulfilled any of the following criteria were screened for Clostridium difficile: antibiotic-associated diarrhoea, diarrhoea in hospitalized patients aged ⩾2 years; pseudomembranous colitis; and diarrhoea in patients aged >65 years, children with Hirschsprung's Disease. (3) If clinically indicated, patients who fulfilled any of the following criteria were screened for Yersinia enterocolitica: acute diarrhoea, mesenteric lymphadenitis, terminal ileitis, pseudo-appendicitis, septicaemia, metastatic infections, immunological sequelae (e.g. reactive arthritis). (4) If the clinical information indicated a history of foreign travel then specimens were tested for Vibrio spp. (5) If the clinical information indicated a history of contact with untreated water then specimens were tested for Aeromonas spp. (6) Investigation of suspected food poisoning caused by Clostridium perfringens was performed if clinical information was relevant, after consultation with medical microbiologist. (7) If clinically indicated, patients who fulfilled any of the following criteria were screened for Cryptosporidium: symptomatic patients from the community, emergency hospital admissions; paediatric wards; immunocompromised patients. (8) If clinically indicated, patients who fulfilled any of the following criteria were screened for ova, cysts and parasites: liquid stools from the community, emergency hospital admissions; paediatric wards; a history of foreign travel; persistent diarrhoea; eosinophilia; malabsorption or AIDS. (9) Specimens from children aged ⩽6 years with a history of diarrhoea and vomiting were examined for rotavirus and adenovirus serotypes 40, 41. (10) Norovirus was only investigated in association with outbreaks in the community and healthcare setting.
Faecal specimens from patients suspected of having Cl. difficile disease were screened in accordance with UK Standards for Microbiology Investigations B10 [32] using two commercial enzyme immunoassays. The C.DIFF CHEK-60® test (TechLab, USA) was used as a screening test to detect Cl. difficile antigen, glutamate dehydrogenase while the TOX A/B QUIK CHEK® test (TechLab) was used to detect Cl. difficile toxins A and B in specimens that were positive by the C.DIFF CHEK-60 test.
Specimens from children aged ⩽6 years with a history of diarrhoea and/or vomiting were examined for rotavirus and adenovirus serotypes 40, 41 using the ProSpecT® Adenovirus EZ Microplate assay and ProSpecT® Rotavirus EZ Microplate assay (Oxoid Ltd, UK), respectively while faecal specimens from patients with suspected norovirus were screened using an internally controlled, one-step real-time RT–PCR assay developed by Rolfe et al. [33]. Confirmation of the presence of norovirus in faecal samples, which were positive in the primary diagnostic test was performed using RIDA®GENE Norovirus V real-time RT–PCR (R-Biopharm Rhône Ltd, UK).
All isolates of the major enteric pathogens were submitted to the HPA Centre for Infections for confirmation and strain characterization.
Nucleic acid extraction
Total nucleic acid was extracted from 200 μl of a 10% faecal suspension prepared in normal saline and eluted in a final volume of 60 μl elution buffer using the QIAsymphony® Virus/Bacteria Mini kit (Qiagen, UK) on the QIAsymphony® SP instrument (Qiagen), according to the manufacturer's instructions.
Reverse transcription
All reverse transcription reactions were performed using the TaqMan® RevertAid™ First Strand cDNA Synthesis kit (Fermentas GmbH, Germany). The reverse transcription reaction mix was prepared on ice by combining 8 μl total RNA, 1 μl of 0·2 μg/μl random hexamers and 3 μl nuclease-free water in a reaction tube. The reaction mix was incubated at 80 °C for 3 min before the tubes were placed on ice for 2 min. Next, 4 μl of 5× reaction buffer, 2 μl of 10 nm dNTP mix, 1 μl of 20 U/μl RiboLock RNase inhibitor, and 1 μl of 200 U/μl RevertAid M-MuLV reverse transcriptase were added. The reaction tubes were loaded into the chamber of a GeneAmp 2400 Thermal Cycler (PerkinElmer, UK) programmed with the following cycling parameters: 37 °C for 90 min, 94 °C for 2 min and 4 °C for 2 min. After thermocycling, the cDNA was stored at 2 − 8 °C overnight or at −20 °C for prolonged storage.
Amplification
A working reaction mix was prepared by combining 4 μl of 5× primer mixture, 3 μl 8-MOP solution, and 10 μl of 2× multiplex master mix. Next, 17 μl of the working reaction mix were dispensed into 0·2 μl tubes before 3 μl DNA/cDNA were added. For the negative control 3 μl Diarrhea ACE negative control was added and for the positive control 3 μl Diarrhea ACE positive control was added. Amplification was performed using a Rotor-Gene 6000 real-time PCR system (Corbett Research Ltd, UK) programmed with the following cycling parameters: 94 °C for 15 min followed by 40 cycles at 94 °C for 0·5 min, 60 °C for 1·5 min, 72 °C for 1·5 min with a final cycle at 72 °C for 10 min. After thermocycling, the PCR product was stored at 2 − 8 °C overnight or at −20 °C for prolonged storage.
Seeplex system assays
All samples were retested using three multiplex assays, the Seeplex Diarrhea-V, -B1 and -B2 assays, according to the manufacturer's instructions. The Seeplex Diarrhea-V assay permitted the simultaneous amplification of target DNA/cDNA of astrovirus (with a PCR product of 650 bp), enteric adenovirus (411 bp), group A rotavirus (541 bp), genogroup I and genogroup II noroviruses (304 bp and 214 bp, respectively). The Seeplex Diarrhea-B1 assay permitted the simultaneous amplification of target DNA of Salmonella spp. (395 bp) (S. enterica, S. bongori), Shigella (Sh.) spp. (330 bp) (Sh. flexneri, Sh. boydii, Sh. Sonnei, Sh. dysenteriae), Vibrio spp. (651 bp) (V. cholerae, V. parahaemolyticus, V. vulnificus), Cl. difficile toxin B, (476 bp) Campylobacter spp. (227 bp) (C. jejuni and C. coli). The Seeplex Diarrhea-B2 assay permitted the simultaneous amplification of target DNA of Cl. perfringens (700 bp), Yersinia enterocolitica (580 bp), Aeromonas spp. (217 bp) (A. salmonicida, A. sobria, A. bivalvium, A. hydrophila), E. coli O157 (476 bp), E. coli H7 (370 bp), and VTEC (291 bp). The simultaneous amplification of both the 476 bp and 370 bp amplicons by Seeplex Diarrhea-B2 assay confirmed E. coli O157:H7 detection. An internal control (1000 bp) was included in each multiplex assay.
Analysis of PCR products
PCR products were separated by capillary electrophoresis using the MCE-202 MultiNA Microchip Electrophoresis System (Shimadzu Corporation, Japan). The MCE-202 MultiNA was used to generate a size calibration curve by ladder analysis using the DNA-1000 Reagent kit, which was in turn used to conduct high accuracy DNA size analysis for 100–1000 bp fragments. The size calibration curve was interpreted using Seegene Viewer software, according to the manufacturer's instructions (Fig. 1).
Fig. 1.
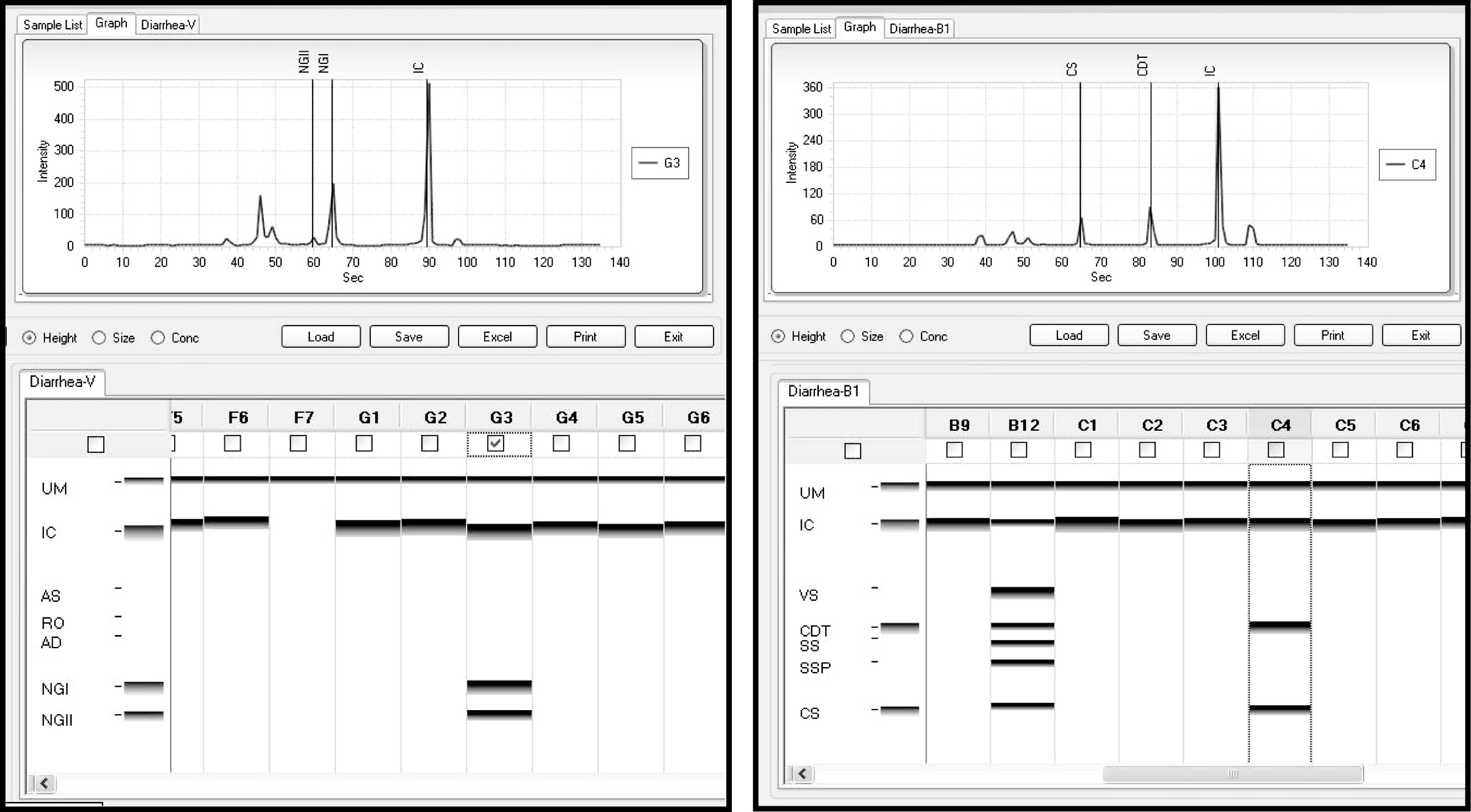
Computer generated graphical representation of the results of capillary electrophoresis. The Seeplex Diarrhea-V ACE detection multiplex assay shows sample G3 containing a mixture of genogroup I and genogroup II norovirus amplicons. Sample F7 shows inhibition of the PCR reaction as the internal control (IC) had failed (PCR inhibitors were removed through re-extraction). The Seeplex Diarrhea-B1 ACE detection multiplex assay shows the positive control (B12) containing amplified DNA from Campylobacter spp. (CS), Shigella spp. (SSP), Salmonella spp. (SS), Clostridium difficile toxin B (CDT), Vibrio spp. and the IC. Results of sample C4 revealed a mixture containing amplicons for Campylobacter spp. and C. difficile toxin B.
RESULTS
Conventional viral, bacterial, and protozoan diagnostic tests
For conventional viral, bacterial and protozoan detection different laboratory testing profiles were used (Table 1) and 98 enteric pathogens were detected in 96/223 (43·0%) samples (Table 2). Multiple enteric pathogens were detected in two faecal samples.
Table 2.
Performance of the Seeplex® Diarrhea ACE Detection multiplex PCR system (Seeplex system) compared to the routine diagnostic testing algorithm for detection of enteric pathogens
| Pathogen | No. specimens tested | No. specimens with results by | |
|---|---|---|---|
| Routine diagnostic testing algorithm | Seeplex system | ||
| Salmonella spp. | 201 | 19 | 16 |
| Shigella spp. | 201 | 4 | 7 |
| Campylobacter spp. | 201 | 16 | 18 |
| Escherichia coli O157 | 201 | 2 | 2 |
| Clostridium difficile | 63 | 10 | 5 |
| Vibrio spp. | 5 | 1 | 1 |
| Cryptosporidium spp. | 161 | 5 | n.a. |
| Giardia lamblia | 71 | 10 | n.a. |
| Adenovirus | 64 | 11 | 13 |
| Rotavirus | 64 | 8 | 7 |
| Norovirus | 14 | 12 | 12 |
| Total | 98 | 81 | |
Crytosporidium spp. and Giardia lamblia are not available (n.a.) in the Seeplex system.
The Seeplex system
The Seeplex system detected 81 enteric pathogens in 75/223 (33·6%) faecal samples, which were tested by the routine diagnostic testing algorithm (Table 2). All samples positive in routine diagnostic tests for adenovirus, VTEC, norovirus, Shigella spp. Campylobacter spp. or Vibrio spp. were positive using Seeplex assays. The Seeplex system detected Shigella spp., Campylobacter spp. and adenovirus in an additional six faecal samples, which were previously negative by the routine diagnostic testing algorithm (Table 2). The Seeplex Diarrhea-V assay failed to detect rotavirus in one out of eight positive samples, which was positive by the ProSpecT Rotavirus EZ Microplate assay. The Seeplex Diarrhea-B1 assay failed to detect Cl. difficile toxin B in 5/10 samples, which were positive by the C.DIFF CHEK-60 and TOX A/B QUIK CHEK immunoassays, and Salmonella spp. in 3/19 positive samples (Table 2). The diagnostic sensitivity and specificity of the Seeplex system relative to the routine diagnostic testing algorithm for the diagnosis of IID caused by enteric pathogens common to both diagnostic tests is shown in Table 3. The sensitivity of the Seeplex system was 100% for all pathogens common to both diagnostic tests with the exception of Salmonella spp., Cl. difficile and rotavirus. This probably reflects the enhanced sensitivity of the Seeplex system in comparison to conventional diagnostic methods for routine detection of enteric pathogens, which rely on microscopy, culture, and enzyme immunoassays. Alternative methods to confirm discordant results were not available with the exception of norovirus. An additional 53/223 (23·8%) enteric pathogens were identified in faecal samples for which testing was not performed as part of the routine diagnostic testing algorithm or not available (Table 4). Interestingly, the detection of Cl. perfringens in about 10% of faecal samples tested, none of which met the criteria for testing in the primary diagnostic algorithm, possibly reflects the lack of relevant clinical and epidemiological information that often accompanies clinical samples.
Table 3.
The diagnostic sensitivity and specificity of the Seeplex® Diarrhea ACE Detection multiplex PCR system (Seeplex system) relative to the routine diagnostic testing algorithm for diagnosis of IID. The Seeplex system was compared to the routine diagnostic testing algorithm as the gold standard for enteric pathogens common to both diagnostic tests
| Pathogen | No. specimens tested | Concordant | Discordant | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|---|
| +/+ | −/− | +/− | −/+ | ||||
| Salmonella spp. | 201 | 16 | 182 | 0 | 3 | 84·21 | 100·00 |
| Shigella spp. | 201 | 4 | 194 | 3 | 0 | 100·00 | 98·48 |
| Campylobacter spp. | 201 | 16 | 184 | 2 | 0 | 100·00 | 98·92 |
| Escherichia coli O157 | 201 | 2 | 199 | 0 | 0 | 100·00 | 100·00 |
| Clostridium difficile | 63 | 5 | 53 | 0 | 5 | 50·00 | 100·00 |
| Vibrio spp. | 5 | 1 | 4 | 0 | 0 | 100·00 | 100·00 |
| Adenovirus | 64 | 11 | 51 | 2 | 0 | 100·00 | 96·23 |
| Rotavirus | 64 | 7 | 56 | 0 | 1 | 87·50 | 100·00 |
| Norovirus | 14 | 12 | 2 | 0 | 0 | 100·00 | 100·00 |
+/ + , Positive by the Seeplex system and routine diagnostic testing algorithm.
−/−, Negative by the Seeplex system and routine diagnostic testing algorithm.
+/−, Positive by the Seeplex system and negative by the routine diagnostic testing algorithm.
−/ + , Negative by the Seeplex system and positive by the routine diagnostic testing algorithm.
Table 4.
Enteric pathogens were identified in faecal samples by the Seeplex® Diarrhoea ACE Detection multiplex PCR system (Seeplex system) for which testing was not performed as part of the routine diagnostic testing algorithm or not available
| Pathogen | No. specimens tested by the Seeplex system only | No. pathogens detected by the Seeplex system |
|---|---|---|
| Salmonella spp. | 22 | 0 |
| Shigella spp. | 22 | 0 |
| Campylobacter spp. | 22 | 1 |
| Escherichia coli O157 | 22 | 0 |
| Clostridium difficile | 160 | 3 |
| Yersinia enterocolitica | 223 | 2 |
| Vibrio spp. | 218 | 0 |
| Aeromonas spp. | 223 | 6 |
| Clostridium perfringens | 223 | 24 |
| Adenovirus | 145 | 1 |
| Astrovirus* | 223 | 2 |
| Rotavirus | 145 | 0 |
| Norovirus | 209 | 14 |
| Total | 53 |
Test not available within Microbiology Department, Norfolk and Norwich University Hospital.
The Seeplex system identified multiple enteric pathogens in 20/223 (9·0%) faecal samples, which were previously tested by the routine diagnostic testing algorithm (Table 5). These included a co-infection with Shigella spp., and Campylobacter spp. that was detected by the routine diagnostic testing algorithm. A second co-infection with Campylobacter spp. and Cryptosporidium spp. was not detected as no option is available within the current Seeplex system for detection of human diarrhoeal parasites although the Seeplex system detected Shigella spp., Cl. perfringens, Aeromonas spp. in addition to Campylobacter spp. in this sample. Campylobacter spp., Cl. perfringens, and norovirus were the most common enteric pathogens detected in mixed infections by the Seeplex system. An additional 4/223 (1·8%) samples were positive for multiple enteric pathogens by the Seeplex system, for which testing was not performed as part of the routine diagnostic testing algorithm or not available. These included a dual infection with norovirus GI and norovirus GII, two dual infections with norovirus GII and Cl. difficile toxin B and a dual infection with norovirus GII and Aeromonas spp.
Table 5.
Multiple enteric pathogens detected in faecal samples by the Seeplex® Diarrhea ACE Detection multiplex PCR system that were previously screened using the routine diagnostic testing algorithm
| Pathogen | No. multiple infections |
|---|---|
| Salmonella spp. and Shigella spp. | 1 |
| Salmonella spp. and Clostridium (Cl.) perfringens | 2 |
| Salmonella spp., Campylobacter spp. and norovirus GII | 1 |
| Shigella spp. and Cl. perfringens | 1 |
| Shigella spp. and Campylobacter spp. | 1 |
| Shigella spp., Campylobacter spp., Cl. perfringens and Aeromonas spp. | 1 |
| Campylobacter spp. and Cl. perfringens | 3 |
| Verocytotoxin Escherichia coli and Aeromonas spp. | 1 |
| Cl. difficile and Cl. perfringens | 1 |
| Cl. difficile and Campylobacter spp. | 1 |
| Vibrio spp. and norovirus GII | 1 |
| Adenovirus and Cl. perfringens | 1 |
| Adenovirus and Yersinia enterocolitica | 1 |
| Adenovirus and norovirus | 1 |
| Norovirus GII and Aeromonas spp. | 1 |
| Norovirus GII and Cl. perfringens | 2 |
| Total | 20 |
DISCUSSION
The plethora of methods for the detection of enteric pathogens has led to the development of testing profiles or algorithms, which attempt to select the most appropriate laboratory methods to use based on clinical symptoms, age of the patient, previous antibiotic use, travel history, and epidemiology such as seasonality. Pathogens can be associated with unusual clinical presentations, may infect out of season, or clinical information could omit recent travel history, contact with infected individuals or previously prescribed antibiotics. For these reasons, the aetiological agents or mixed infections associated with many cases of acute diarrhoea remain unidentified.
Multiplex PCR has provided a rapid, sensitive, specific, and cost-effective alternative to traditional methods and mono-specific PCR since it permits the simultaneous detection and identification of multiple infectious agents in a single reaction [34]. Furthermore, automated systems for nucleic acid extraction, reagent preparation, assay set up, and the analysis of PCR products are ideally suited to syndromic-based laboratory diagnosis.
When corrected for organisms not available in the Seeplex system (Cryptosporidium spp., Giardia intestinalis), 83 enteric pathogens were detected by the routine diagnostic testing algorithm in 82/223 (36·8%) faecal samples collected from patients with acute gastroenteritis whereas the Seeplex system retrospectively detected 81 enteric pathogens in 33·6% faecal samples included in the routine diagnostic testing algorithm. Furthermore, the implementation of a generic approach for the detection of enteric pathogens through utilization of the Seeplex system offered improved detection of enteric pathogens as an additional 53 (23·8%) enteric pathogens were detected in samples for which testing was not performed as part of the routine diagnostic testing algorithm or not available. These findings reiterate that the application of molecular methodologies to the diagnosis of IID is likely to reduce the diagnostic gap [14] and provide further insight into the relative frequency of different enteric pathogens in cases of gastroenteritis for which testing was not performed as part of the routine diagnostic testing algorithm or not available.
The sensitivity and specificity of the Seeplex system was comparable to the routine diagnostic testing algorithm for the majority of enteric pathogens. The Seeplex system detected Shigella spp., Campylobacter spp. and adenovirus in an additional six faecal samples but failed to detect rotavirus, Salmonella spp. and Cl. difficile toxin B, in one, three, and five samples, respectively, which may be due to sample quality, although this is unlikely as the inhibition control designed to identify inhibition in the PCR reaction was detected in all samples. Rotavirus was detected by enzyme immunoassay. Non-specific reactivity has been reported in immunoassays used to detect rotavirus antigens [35, 36]. Higgins et al. [37] reported the diagnostic sensitivity and specificity for rotavirus after discordant analysis was 100% when evaluating the Seeplex Diarrhea-V assay. Similarly, glutamate dehydrogenase and toxins A and B of Cl. difficile were detected routinely using two enzyme immunoassays. The first, C.DIFF CHEK-60 used mouse monoclonal antibodies specific for the glutamate dehydrogenase produced by both toxigenic and non-toxigenic strains of Cl. difficile as a screening test. The status of samples that were positive by the C.DIFF CHEK-60 test was confirmed using the TOX A/B QUIK CHEK test, which uses mouse monoclonal antibody specific for toxin A coupled to horseradish peroxidise and goat monoclonal antibody specific for toxin B coupled to horseradish peroxidise. The specificities of the C.DIFF CHEK-60 and the TOX A/B QUIK CHEK were 91·2% (95% CI 88·5–93·4) and 99·7% (95% CI 98·8–99·9), respectively. It is a limitation of the Seeplex system that detection of Cl. difficile toxin A is not included in the Seeplex Diarrhea-B1 assay, which may provide a plausible explanation for failure to detect Cl. difficile in all faecal samples that were positive by the two C. difficile-specific enzyme immunoassays in routine use. However, the inability of the Seeplex system to detect the gene encoding C. difficile toxin B in 50% of samples and Salmonella spp. in 16% of samples may be related to the choice of gene/genome region or characteristics of the primer and reaction conditions. Interestingly, Salmonella culture methods, through the inclusion of selection and enrichment, may be more sensitive than the Seeplex Diarrhea-B1 assay. Nevertheless, failure to detect three Salmonella strains with the Seeplex Diarrhea-B1 assay was not dependent on Salmonella group as two of seven group B strains and one of seven group D strains were undetected.
The ability of newer multiplexed methodologies to confirm the presence of multiple infectious agents in samples considered to contain a single pathogen or no pathogen is of public health importance. The Seeplex system identified multiple enteric pathogens in more than 5% of faecal samples, which were tested previously by the routine diagnostic testing algorithm. The frequency of mixed infection within the current study is higher than previous studies [38, 39], which may reflect the inclusion of enteric pathogens that were excluded from these studies such as Aeromonas spp. but more likely reflects the selection of faecal samples that were positive by the routine diagnostic testing algorithm. The identification of multiple enteric pathogens may reflect common routes of infection [14, 39]. For example, the identification of norovirus, Salmonella spp. and Campylobacter spp. by the Seeplex system may indicate ingestion of food contaminated with faeces or raw sewage. Similarly, a multiple infection with Cryptosporidium spp., Campylobacter spp., Shigella spp., Cl. perfringens and Aeromonas spp. may suggest contaminated water as the source, whereas, a dual infection with norovirus and toxin B-positive Cl. difficile may indicate hospital-acquired infections.
There are several disadvantages to the Seeplex system. First, no option is available within the current system for the detection of human diarrheal parasites and although the Seeplex system incorporates quality controls, the internal control is only available for inclusion in each PCR master mix, which does not allow validation of the nucleic acid extraction or reverse transcription processes. Moreover, reverse transcription was performed as a separate step, which in turn increased the duration of the assay. Nevertheless, the Seeplex system streamlined IID diagnosis through the simultaneous amplification and detection of multiple enteric pathogens compared to the routine diagnostic testing algorithm and offered the ability to detect multiple infections more frequently. Turnaround time (TAT) was calculated based on 96 samples/run. The average TAT to process 96 samples using the Seeplex system incorporating 10 bacterial targets, four viral targets and internal controls was 9–10 h or 0·6 h per target in a run of 96 samples compared to 24–48 h for culture depending on the bacterial target; 2 h for C.DIFF CHEK-60 and TOX A/B QUIK CHEK immunoassays and Rotavirus/Adenovirus EZ Microplate assay, and 24 h for direct and concentration microscopy.
Although a formal cost-benefit analysis is not available for this study, it is clear that the syndromic molecular diagnostic approach offered by the Seeplex system provides benefits in terms of pathogen coverage, a common sample preparation technique with reduced labour and the associated costs of performing the detection of bacteria, viruses and parasites in separate laboratories.
There are several limitations to this study notwithstanding its retrospective nature and the unavailability of alternative methods to confirm discordant results excluding norovirus. These include the absence of studies to investigate the limit of detection as well as inter- and intra-assay variation of the Seeplex system. However, the reproducibility of the Seeplex system was determined by calculation of the coefficient of variation from the means and standard deviations of replicates of each positive control panel (10 replicates of the Diarrhea-V positive control and 12 replicates of Diarrhea-B1 and-B2 positive controls) included in the Diarrhea-V, -B1 and -B2 assays. The coefficient of variation for the Diarrhea-V, -B1, and -B2 assays ranged from 2·1% (norovirus GII) to 5·1% (rotavirus), 1·2% (Vibrio spp.) to 4·7% (Shigella spp.), and 0·6% (Y. enterocolitica) to 5·4% (Aeromonas spp.), respectively.
In conclusion, the Seeplex system provides significant additional diagnostic capability for the syndromic diagnosis of acute gastroenteritis with increased sensitivity for the majority of pathogens, which may improve the diagnosis of nosocomial transmissions, and improve clinical management by earlier intervention after diagnosis.
ACKNOWLEDGEMENTS
The authors thank Mast Group Ltd, Merseyside, UK for the provision of diagnostic kits and equipment and colleagues at the Microbiology Department, Norfolk and Norwich University Hospital for technical assistance and support.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Tam CC, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012; 61: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desselberger U, et al. Rotaviruses and rotavirus vaccines. British Medical Bulletin 2009; 90: 37–51. [DOI] [PubMed] [Google Scholar]
- 3.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. New England Journal of Medicine 2009; 361: 1776–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godoy P, et al. High incidence of outbreaks of norovirus GGII.4 in hospitals and nursing homes in Catalonia. Journal of Hospital Infection 2009; 72: 275– 277. [DOI] [PubMed] [Google Scholar]
- 5.Guzman-Herrador B, et al. Outbreak of norovirus infection in a hotel in Oslo, Norway, January 2011. Eurosurveillance 2011; 16: pii = 19 928. [PubMed] [Google Scholar]
- 6.Kanerva M, et al. Prolonged norovirus outbreak in a Finnish tertiary care hospital caused by GII.4-2006b subvariants. Journal of Hospital Infection 2009; 71: 206–213. [DOI] [PubMed] [Google Scholar]
- 7.Medici MC, et al. An outbreak of norovirus infection in an Italian residential-care facility for the elderly. Clinical Microbiology and Infection 2009; 15: 97–100. [DOI] [PubMed] [Google Scholar]
- 8.Vivancos R, et al. Norovirus outbreak in a cruise ship sailing around the British Isles: investigation and multi-agency management of an international outbreak. Journal of Infection 2010; 60: 478–485. [DOI] [PubMed] [Google Scholar]
- 9.Coetzee N, et al. A swimming pool-associated outbreak of cryptosporidiosis in Staffordshire, England, October to December 2007. Eurosurveillance 2008; 13: 19028. [PubMed] [Google Scholar]
- 10.Hoek MR, et al. Outbreak of Cryptosporidium parvum among children after a school excursion to an adventure farm, south west England. Journal of Water and Health 2008; 6: 333–338. [DOI] [PubMed] [Google Scholar]
- 11.Persson K, Svenungsson B, de Jong B. An outbreak of cryptosporidiosis at a day-care centre in Sweden. Eurosurveillance 2007; 12: E070823.3. [DOI] [PubMed] [Google Scholar]
- 12.Smith S, et al. Value of syndromic surveillance in monitoring a focal waterborne outbreak due to an unusual Cryptosporidium genotype in Northamptonshire, United Kingdom, June–July 2008. Eurosurveillance 2010; 15: 19643. [DOI] [PubMed] [Google Scholar]
- 13.Yoder JS, et al. Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events – United States, 2005–2006. Morbidity and Mortality Weekly Report. Surveillance summaries 2008; 57: 1–29. [PubMed] [Google Scholar]
- 14.Amar CF, et al. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996). European Journal of Clinical Microbiology and Infectious Diseases 2007; 26: 311–323. [DOI] [PubMed] [Google Scholar]
- 15.de Boer RF, et al. Improved detection of five major gastrointestinal pathogens by use of a molecular screening approach. Journal of Clinical Microbiology 2010; 48: 4140–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amar CF, et al. Blinded application of microscopy, bacteriological culture, immunoassays and PCR to detect gastrointestinal pathogens from faecal samples of patients with community-acquired diarrhoea. European Journal of Clinical Microbiology and Infectious Diseases 2004; 23: 529–534. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham SA, et al. Three-hour molecular detection of Campylobacter, Salmonella, Yersinia, and Shigella species in feces with accuracy as high as that of culture. Journal of Clinical Microbiology 2010; 48: 2929–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni SP, et al. Detection of campylobacter species: a comparison of culture and polymerase chain reaction based methods. Journal of Clinical Pathology 2002; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Leary J, Corcoran D, Lucey B. Comparison of the EntericBio multiplex PCR system with routine culture for detection of bacterial enteric pathogens. Journal of Clinical Microbiology 2009; 47: 3449–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ten Hove R, et al. Detection of diarrhoea-causing protozoa in general practice patients in The Netherlands by multiplex real-time PCR. Clinical Microbiology and Infection 2007; 13: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 21.Wiemer D, et al. Real-time multiplex PCR for simultaneous detection of Campylobacter jejuni, Salmonella, Shigella and Yersinia species in fecal samples. International Journal of Medical Microbiology 2011; 301: 577–584. [DOI] [PubMed] [Google Scholar]
- 22.Kittigul L, et al. Molecular characterization of rotaviruses, noroviruses, sapovirus, and adenoviruses in patients with acute gastroenteritis in Thailand. Journal of Medical Virology 2009; 81: 345–353. [DOI] [PubMed] [Google Scholar]
- 23.Li W, et al. A novel multiplex PCR coupled with Luminex assay for the simultaneous detection of Cryptosporidium spp., Cryptosporidium parvum and Giardia duodenalis; Veterinary Parasitology 2010; 173: 11–18. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, et al. Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. Journal of Clinical Virology 2011; 50: 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang XL, Preiksaitis JK, Lee B. Multiplex real time RT-PCR for the detection and quantitation of norovirus genogroups I and II in patients with acute gastroenteritis. Journal of Clinical Virology 2005; 33:168–171. [DOI] [PubMed] [Google Scholar]
- 26.Stark D, et al. Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. Journal of Clinical Microbiology 2011; 49: 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svraka S, et al. Novel approach for detection of enteric viruses to enable syndrome surveillance of acute viral gastroenteritis. Journal of Clinical Microbiology 2009; 47: 1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughn M, et al. Evaluation of the FilmArray® Gastrointestinal Pathogen Detection System for Infectious Diarrhea. Poster presented at the 112th General Meeting of the American Society for Microbiology, San Francisco, 16–19 June 2012 (http://www.biofiredx.com/pdfs/Posters/2012/Rapid%20Multi-target%20Detection%20of%20Gastrointestinal%20Pathogens%20in%20Patients%20Presenting%20with%20Diarrhea.pdf). Accessed 15 August 2012.
- 29.Wessels E, et al. Prospective application of the Luminex xTAG-GPP multiplex PCR in diagnosing infectious gastro-enteritis. Poster presented at the 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Milan, 7–10 May 2011 (http://www.luminexcorp.com/prod/groups/public/documents/lmnxcorp/gpp-poster-claas.pdf). Accessed 15 August 2012.
- 30.Health Protection Agency. Investigation of faecal specimens for bacterial pathogens. UK Standards for Microbiology Investigations. B30, Issue 7.1, 2012. (http://ww.hpa.org.uk/SMI/pdf).
- 31.Health Protection Agency. Investigation of specimens other than blood for parasites. UK Standards for Microbiology Investigations. B31, Issue 3.1, 2012. (http://www.hpa.org.uk/SMI/pdf).
- 32.Health Protection Agency. Processing of faeces for Clostridium difficile. UK Standards for Microbiology Investigations. B10, Issue 1.4, 2012. (http://www.hpa.org.uk/SMI/pdf).
- 33.Rolfe KJ, et al. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. Journal of Clinical Virology 2007; 39: 318–321. [DOI] [PubMed] [Google Scholar]
- 34.Templeton KE, et al. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. Journal of Clinical Microbiology 2004; 42: 1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bendall RP, Gray JJ. Haemorrhagic colitis and haemolytic-uraemic syndrome: false-positive reaction with a rotavirus latex agglutination test. Journal of Clinical Pathology 1991; 44: 609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prey MU, et al. Evaluation of three commercially available rotavirus detection methods for neonatal specimens. American Journal of Clinical Pathology 1988; 89: 675–678. [DOI] [PubMed] [Google Scholar]
- 37.Higgins RR, et al. Evaluation and verification of the Seeplex Diarrhea-V ACE assay for simultaneous detection of adenovirus, rotavirus, and norovirus genogroups I and II in clinical stool specimens. Journal of Clinical Microbiology 2011; 49: 3154–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olesen B, et al. Etiology of diarrhea in young children in Denmark: a case-control study. Journal of Clinical Microbiology 2005; 43: 3636–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam CC, et al. Changes in causes of acute gastroenteritis in the United Kingdom over 15 years: microbiologic findings from 2 prospective, population-based studies of infectious intestinal disease. Clinical Infectious Diseases 2012; 54: 1275–1286. [DOI] [PubMed] [Google Scholar]


