SUMMARY
To examine the prevalence of human pathogens carried by rats in urban areas in Hanoi and Hai Phong, Vietnam, we live-trapped 100 rats in January 2011 and screened them for a panel of bacteria and viruses. Antibodies against Leptospira interrogans (22·0%), Seoul virus (14·0%) and rat hepatitis E virus (23·0%) were detected in rats, but antibodies against Yersinia pestis were not detected. Antibodies against L. interrogans and Seoul virus were found only in adult rats. In contrast, antibodies to rat hepatitis E virus were also found in juvenile and sub-adult rats, indicating that the transmission mode of rat hepatitis E virus is different from that of L. interrogans and Seoul virus. Moreover, phylogenetic analyses of the S and M segments of Seoul viruses found in Rattus norvegicus showed that Seoul viruses from Hai Phong and Hanoi formed different clades. Human exposure to these pathogens has become a significant public health concern.
Key words: Hantavirus, hepatitis E, leptospirosis, surveillance, zoonoses
INTRODUCTION
Rodents play a role as reservoir hosts of causative agents for various bacterial, viral and parasitic zoonoses. Wild rats (Rattus spp.) are a particularly important source of human pathogens because they inhabit areas in the vicinity of human dwellings.
Leptospirosis is caused by spirochaetes belonging to the genus Leptospira. Leptospirosis is an important worldwide zoonosis for which the major reservoir animals are rodents. Although some leptospirosis cases have been diagnosed correctly, leptospirosis is thought to be a major cause of undiagnosed acute febrile illness (AFI) in endemic countries [1]. About 12 outer membrane proteins, including LipL32, OmpL1, Ligb, LenA, LenD and Loa22, have been identified [2]. The major outer membrane lipoprotein, LipL32, is the most abundant protein of the entire cell and is highly conserved in pathogenic Leptospira spp. [3].
Hantavirus infection, haemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) are also known as worldwide rodent-borne viral zoonoses. Hantaviruses are enveloped and negative-sense RNA viruses with a tripartite genome comprising of large (L), medium (M) and small (S) segments [4]. Seoul virus (SEOV) is one of the causative agents of HFRS and is carried by Rattus norvegicus. We previously conducted epidemiological studies on the prevalence of SEOV infection in rodents and AFI patients who were not leptospirosis patients in East Asian countries including Vietnam [5, 6], Indonesia (Ibrahim et al., unpublished data), Thailand [7], and Sri Lanka [8]. The epidemiological results indicate that SEOV infection exists in rodents and non-leptospirosis patients in all of those countries. However, hantavirus antibody-positive rates in non-leptospirosis AFI patients were about 2·3%, which is almost the same as the rate in healthy people in Vietnam [5]. Therefore, although SEOV infection is one of the possible causes of AFI, other causative agents are thought to exist.
Hepatitis E virus (HEV) is a positive-sense, single-stranded RNA virus, and the HEV genome includes two short non-coding regions surrounding three open reading frames (ORF1–ORF3). HEV can generally be divided phylogenetically into four genotypes. A genetically distinct HEV has recently been isolated from rats [9]. Our previous study showed the prevalence of rat HEV antibody in wild rats in Hanoi, Vietnam. Furthermore, the rat HEV genome was seen to be closely associated with rat HEV in Germany [10]. Although a recent study provided evidence of human infection with rat HEV in Germany [11], the relationship between rat HEV and human disease is still unclear.
In this study, we investigated the prevalence of Leptospira interrogans, SEOV and rat HEV in urban rats captured in urban areas in Hai Phong and Hanoi City, which are the second and third most populous cities in Vietnam, respectively. Antibody against Yersinia pestis was also examined as it is an important rodent-borne pathogen.
METHODS
Animals
Two hundred and 220 tomahawk live-traps were set in the evening and checked in the morning after a trapping night in residential districts of urban areas in Hanoi City (+105·84° E, 20·97° N) and inside a warehouse facing the residential district of an urban area in Hai Phong Port (+106·69° E, 20·87° N), in Northern Vietnam in January 2011. A total of 100 small mammals (94 R. norvegicus, 6 R. tanezumi) were captured in Hanoi City and in Hai Phong Port. Weight, sex and species identification were recorded for each animal. Species were identified by external morphology and DNA sequencing of the mitochondrial cytochrome b gene as described previously [5, 12]. Sequence data for cytochrome b obtained in this study were deposited in DDBJ/EMBL/GenBank (accession numbers: AB674753–AB674758, AB746356–AB746367). To investigate the relationship between maturation stage and seroprevalence, R. norvegicus were tentatively categorized by weight as juveniles (<100 g), sub-adults (100–200 g) and adults (>200 g) [13]. A blood sample was collected from each rodent via cardiac puncture under ether anaesthesia. Serum specimens were stored at −80 °C until serological examination. Lung specimens were collected and stored at −80 °C for polymerase chain reaction (PCR) examination of the hantavirus genome.
Antibody detection
Antibody against L. interrogans was detected by an enzyme-linked immunosorbent assay (ELISA) with Escherichia coli-expressed LipL32 of L. interrogans as an antigen according to a previously described method [14]. ELISA was performed essentially by the same procedure as described previously for hantavirus infection [15]. Briefly, wells of a 96-well plate were coated with 1 μg/ml antigen in phosphate-buffered saline (PBS). The plates were then blocked with PBS containing 3% bovine serum albumin (BSA) for 1 h at 37 °C. Rodent sera were diluted 1:200 with ELISA buffer (PBS containing 0·5% BSA and 0·05% Tween-20) and added to the wells. After incubation for 1 h at room temperature, the plates were washed three times with ELISA buffer, and horseradish peroxidase-conjugated goat anti-rat IgG antibody (Zymed Laboratories Inc., USA) was added as the secondary antibody. After incubation for 1 h at room temperature, the plates were washed as described above and colorimetric reaction was developed by the addition of o-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich, USA). Optical density (OD) was measured at 450 nm. A negative antigen that included the Nus-tag protein, made from the pET43b vector, was used as a negative control. The sample OD was calculated by subtracting the average OD for each set of negative antigen duplicates from the average OD for each set of LipL32 duplicates. Serum samples from three wild rats (R. norvegicus), which had been confirmed as antibody-negative to L. interrogans by various diagnoses, were used as negative control sera. The negative control sera were examined in all ELISA experiments, and mean OD value plus three times the standard deviations (s.d.) was used as a cut-off value to distinguish ELISA-positive and ELISA-negative samples. Antibody-positive sera were then subjected to Western blotting (WB) using the same antigen by the procedure described previously [16]. Samples were considered L. interrogans IgG antibody-positive if they were positive by both ELISA and WB.
Antibody to SEOV was detected by IgG ELISA with E. coli-expressed N-terminal 103 amino acids of the nucleocapsid protein as an antigen (HS103) as described previously [7]. Wells of a 96-well plate were coated with 1 μg/ml HS103 in PBS as a capture antigen. ELISA using HS103 antigen was performed using the same procedure as described above. Antibody-positive sera were then subjected to WB using the baculovirus-expressed antigen using the same procedure as described above [17]. Samples were considered SEOV IgG antibody positive if they were positive by both ELISA and WB.
Antibody to rat HEV was detected by ELISA with virus-like particles consisting of baculovirus-expressed rat HEV ORF2 proteins as described previously [10]. Wells of a 96-well plate were coated with 1 μg/ml rat HEV ORF2 proteins in PBS as a capture antigen. ELISA was then performed essentially by the same procedure as described above. However, blocking was performed using 5% skimmed milk dissolved in PBS-T for 1 h at 37 °C.
Antibody to Y. pestis was detected by ELISA with Fraction 1 antigen, which is a capsule-like antigen encoded by the caf1 gene, as described previously [18]. Wells of a 96-well plate were coated with 1 μg/ml Fraction 1 antigen in PBS as a capture antigen. ELISA was then performed using the same procedure as described above.
Molecular characterization of hantaviruses
Total RNA was extracted from lung tissues of all R. norvegicus and R. tanezumi rodents using Isogen (Nippon Gene, Japan) and then reverse-transcribed using a First-Strand cDNA Synthesis kit (GE Healthcare UK Ltd, UK). Real-time PCR followed by PCR for sequencing of the hantavirus genome were performed to amplify the target sequence. Primer and probe sequences for real-time PCR were as follows: Realtime SEOS F (5′-TATGGTTGCCTGGGGAAAG-3′), Realtime SEOS R (5′-GCTCTGGATCCATGTCATCA-3′) and probe no. 86 (5′-GCAGTGGA-3′). Hantavirus sequences were then amplified by PCR using primers for the S and M segments as follows: MurS110F (5′-CAGAAGGTIAIGGATGCAGA-3′), SEOS1589R (5′-ACTTAAGGTGACCTGGCCCT-3′), SEOM1277F (5′-TTTAGAGCAGCTGAGCAGCAGAT-3′) and M12–3161R (5′-AACCACTATGGCCACCTTTC-3′).
PCR products were purified and DNA sequencing was performed as described previously [5]. Phylogenetic relationships among the hantavirus sequences were evaluated using the Neighbour-Joining (NJ) program with the Kimura two-parameter distance in CLUSTALW version 1.83 (European Bioinformatics Institute, UK). The phylogenetic tree was visualized using the NJ plot program. Bootstrap resampling analysis was performed using 1000 replicates.
The viral sequence data obtained in this study were deposited in DDBJ/EMBL/GenBank (accession numbers: AB674759–AB674769).
Statistical analysis
Differences between seroprevalence and body weight were examined for statistical significance by the Mann–Whitney U test. P values <0·05 and <0·01 were considered significant. Differences in seroprevalence, sex and geographical origin were examined for statistical significance by Pearson's χ2 test or Fisher's exact test. To estimate the relationship of co-infection of a human pathogen in the rodents, we obtained an estimate from each study of the odds ratio (OR) with 95% confidence interval (95% CI).
RESULTS
Prevalence of antibodies to rodent-borne pathogens
The trapping rates of rodents in Hanoi City and Hai Phong Port were 32·0% and 15·0%, respectively. Prevalence of antibodies against four rodent-borne pathogens is given in Table 1. Antibodies against L. interogans were detected in 21·7% (13/60) and 26·5% (9/34) of R. norvegicus captured at Hanoi City and Hai Phong Port, respectively (P = 0·60). SEOV antibody-positive R. norvegicus were obtained both in Hanoi City and Hai Phong Port, but the positive rate was higher in Hai Phong (32·4%, 11/34) than in Hanoi (5%, 3/60) (P < 0·01). There were no R. tanezumi seropositive to L. interogans and SEOV. Prevalence of antibodies against rat HEV were 16·7% (10/60) in R. norvegius and 50% (2/4) in R. tanezumi captured in Hanoi and 32·4% (11/34) R. norvegicus captured in Hai Phong. There was no significant difference between rat HEV prevalence rates of R. norvegicus in Hanoi and Hai Phong (P = 0·08). There were no rats seropositive to Y. pestis.
Table 1.
Trapping sites, collected rodent species, and seropositivity for L. interrogans, SEOV, rat HEV and Y. pestis
| Location | Species | No. tested | No. seropositive against (% positive) | |||
|---|---|---|---|---|---|---|
| L. interrogans* | SEOV* | Rat HEV† | Y. pestis† | |||
| Hanoi City | R. norvegicus | 60 | 13 (21·7%) | 3 (5%) | 10 (16·7%) | 0 (0%) |
| R. tanezumi | 4 | 0 (0%) | 0 (0%) | 2 (50%) | 0 (0%) | |
| Hai Phong Port | R. norvegicus | 34 | 9 (26·5%) | 11 (32·4%) | 11 (32·4%) | 0 (0%) |
| R. tanezumi | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Total | 100 | 22 | 14 | 23 | 0 | |
SEOV, Seoul virus; HEV, hepatitis E virus.
Seroprevalence determined by ELISA and Western blotting.
Seroprevalence determined by ELISA.
Body weight, geographical origin, sex and co-infection
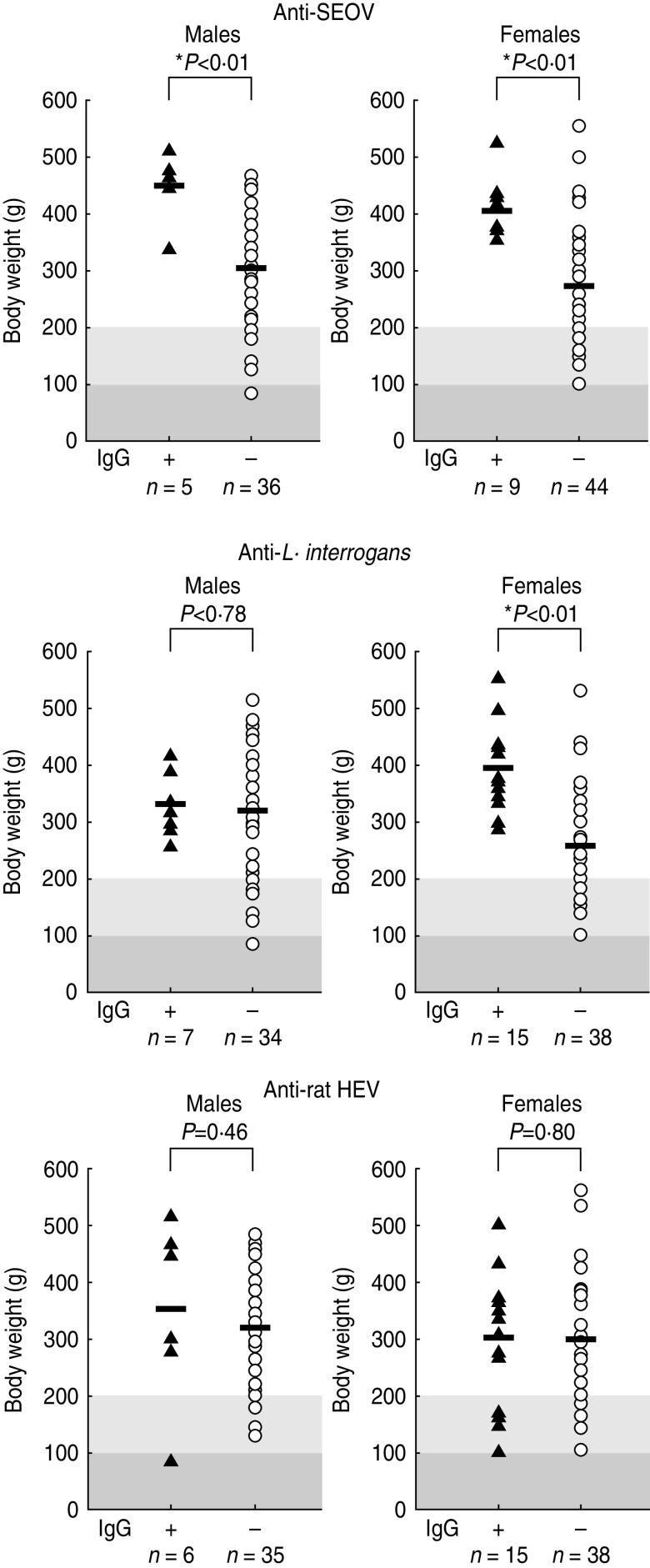
The mean body weight of R. norvegicus in Hai Phong was significantly greater than that of R. norvegicus in Hanoi (336·4 g vs. 289·4 g, P < 0·05). However, no significant difference in sex or rate of maturation stage (juvenile and sub-adults vs. adults) of R. norvegicus was found between rats captured in Hai Phong and rats captured in Hanoi (P = 0·72 and P = 0·78, respectively). The mean body weight of R. norvegicus infected with SEOV was significantly greater in both males and females than in SEOV-negative rats (Fig. 1). L. interrogans antibody-positive females were also significantly heavier than uninfected female rats. However, no significant body-weight difference was found between L. interrogans antibody-positive males and antibody-negative males. No significant body-weight difference was found between HEV-infected rats and uninfected rats.
Fig. 1.
Relationship between seroprevalence and body weight in R. norvegicus. Solid line indicates mean body weight. The grey and light-grey shaded areas indicate juveniles (<100 g) and sub-adults (100–200 g), respectively. An asterisk indicates statistical significance at P < 0·01.
All of the rats with antibodies against L. interrogans and SEOV were adult R. norvegicus with body weights of >260 g and >340 g, respectively. On the other hand, rat HEV antibody-positive R. norvegicus were found in juveniles and sub-adults: one male (84 g) and 4/15 females (100–170 g). There was no infant rat with maternal antibody.
Although no significant difference was found, female R. norvegicus tended to be more frequently infected than males with L. interrogans (28·3% vs. 17·1%, P = 0·20), SEOV (17·0% vs. 12·2%, P = 0·52) and rat HEV (28·3% vs. 14·6%, P = 0·11).
ORs were calculated in seropositive rats to examine the particular combination of co-infection. ORs of co-infection with L. interrogans and SEOV, SEOV and rat HEV, and L. interrogans and rat HEV were 2·0 (95% CI 0·56–6·70, P = 0·20), 1·4 (95% CI 0·35–4·89, P = 0·64), and 1·4 (95% CI 0·43–4·04, P = 0·64), respectively. Thus, no significant ORs were obtained in any combination.
Molecular characterization of SEOV
Lung specimens of all R. norvegicus and R. tanezumi were examined for their virus genome by real-time PCR. All but two of the specimens from seropositive rats were positive by real-time PCR. No real-time PCR-positive specimen was obtained from seronegative rats.
Based on the real-time PCR results, six of the specimens that showed strong positivity were selected and subjected to reverse transcriptase-PCR. Finally, five S-segment sequences and six M-segment sequences were successfully recovered. The phylogenetic trees were drawn using 1378 nt (194–1571) of the S segment and 1101 nt (1966–3066) of the M segment (Fig. 2). All of the sequences were included in the SEOV clade both in the S-segment and M-segment phylogenetic trees. SEOV from Hai Phong (HaiPhong3-11, 17-11, 24-11, 31-11), and SEOV from Southern Vietnam (5CSG and 11CSG), formed one group, which was separated from the other group consisting of SEOV from Hanoi and Singapore.
Fig. 2.
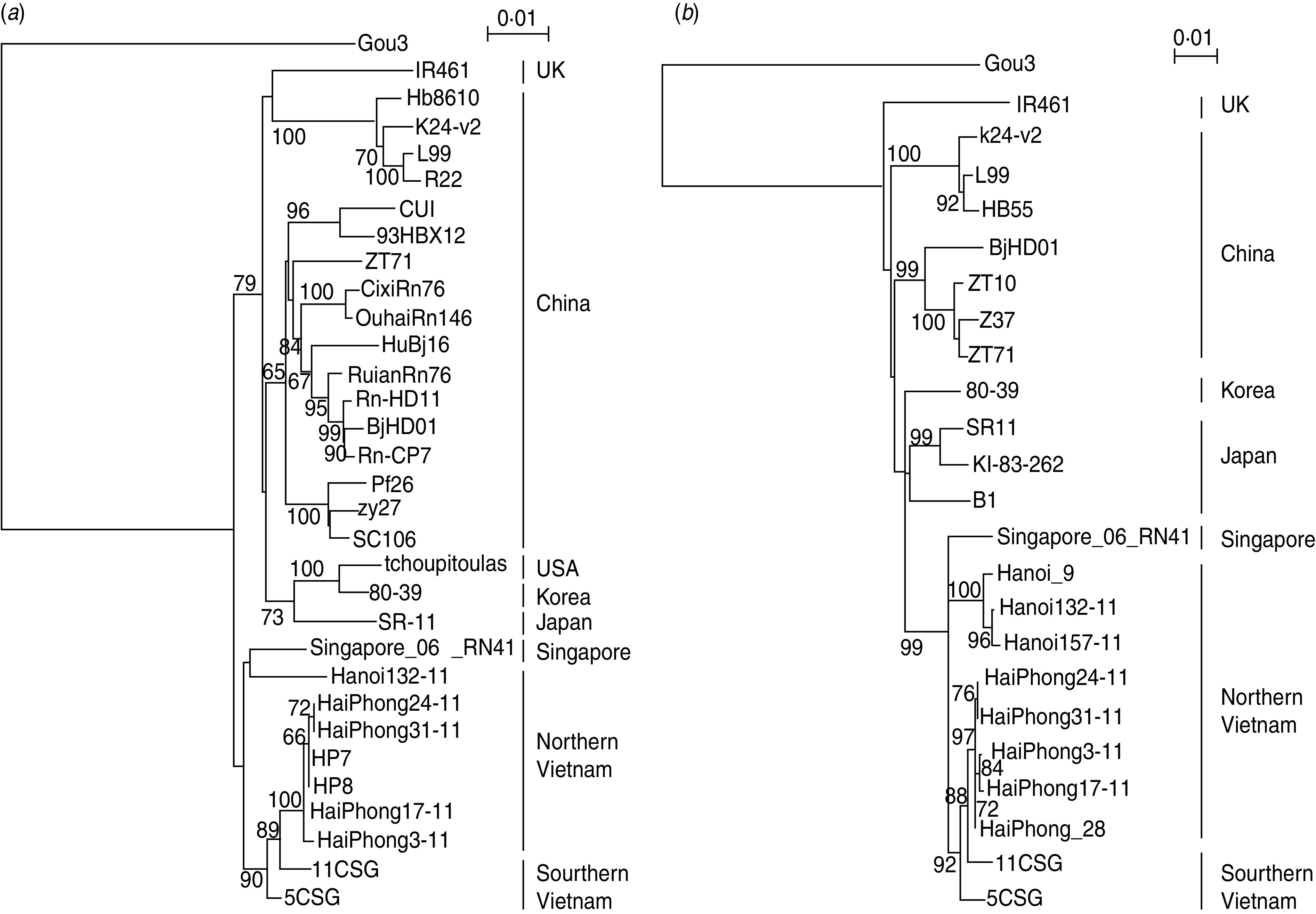
Phylogenetic analysis of SEOV derived from Hanoi City and the Hai Phong Port area. (a) Neighbour Joining (NJ) analysis of hantavirus based on 1378 nt from the S segment. Sequences of SEOV strains Gou3 (AF184988), IR461 (AF329388), Hb8610 (AF288643), K24-v2 (AF288655), L99 (AF288299), R22 (AF288655), CUI (GQ279395), 93HBX12 (EF192308), ZT71 (AY750171), CixiRn76 (FJ803206), OuhaiRn146 (FJ803210), HuBj16 (GQ279380), RuianRn (FJ803216), Rn-HD11 (GQ279392), BjHD01 (AY627049), Rn-CP7 (GQ279383), Pf26 (AY006465), zy27 (AF406965), SC106 (GU361893), tchoupitoulas (AF329389), 80-39 (NC_005236), SR-11 (M34881), Singapore_06_RN41 (GQ274944), 11CSG (AB618113) and 5CSG (AB618112) were used. (b) NJ analysis of hantavirus based on 1103 nt from the M segment. Sequences of SEOV strains Gou3 (AF145977), IR461 (AF458104), k24-v2 (AF288654), L99 (AF288298), HB55 (AF035832), BjHD01 (DQ133505), ZT10 (DQ159911), Z37 (AF187081), ZT71 (EF117248), 80-39 (S47716), SR11 (M34882), KI-83-262 (D17594), B1 (AB457794), Singapore_06_RN41 (GQ274942), Hanoi_9 (AB355732), HaiPhong_28 (AB355731), 11CSG (AB618131) and 5CSG (AB618130) were used. Our sequence data for Vietnamese SEOV derived from R. norvegicus captured in Hanoi City (S segment; Hanoi132-11) (M segment; Hanoi132-11 and Hanoi157-11) and Hai Phong Port (S segment; HP7, HP8, HaiPhong3-11, HaiPhong17-11, HaiPhong24-11 and HaiPhong31-11) (M segment; HaiPhong3-11, HaiPhong17-11, HaiPhong24-11 and HaiPhong31-11) including our previous data were compared with the published sequence.
DISCUSSION
The present study provides information regarding the prevalence of L. interrogans, SEOV and HEV in wild rats in urban areas in Hanoi City and Hai Phong Port in Northern Vietnam. The seroprevalence of L. interrogans and rat HEV in rats was high in both cities. Various prevalence rates of Leptospira and hantavirus infection in wild Rattus spp. have been reported in several countries in Asia: reported prevalence rates of Leptospira were 5–30% [19–25] and those of hantavirus were 5–20% [5, 26–30]. Our results regarding the seroprevalence of L. interrogans and SEOV are consistent with those of previously reported studies. Therefore, our results confirm the potential hazard to humans. A recent study provided evidence for the presence of anti-rat HEV IgG in forestry workers in Germany [11]. However, the relationship between rat HEV and human disease is still unclear. Therefore, further seroepidemiological studies in cryptogenic hepatitis patients should be conducted.
Although the relationship between each seroprevalence and body weight in R. norvegicus was reanalysed with the entry of geographical information to discover the relationship to geographical origin, the mean body weight of male R. norvegicus infected with SEOV, L. interrogans and rat HEV both in Hanoi and Hai Phong was not significantly different from the mean body weight of uninfected rats (P > 0·09) (data not shown). On the other hand, the mean body weight of female R. norvegicus infected with SEOV and L. interrogans both in Hanoi and Hai Phong was significantly greater than the mean body weight of uninfected rats (P < 0·05) (data not shown). Female R. norvegicus infected with rat HEV in Hanoi tended to be heavier than uninfected rats (340·4 g vs. 261·7 g, P = 0·08). Interestingly, rat HEV antibody-positive female R. norvegicus in Hai Phong were lighter than uninfected female rats (261·8 g vs. 363·6 g, P < 0·05). However, the reason for the inverse correlation between body weight of rat HEV antibody-positive female R. norvegicus in Hai Phong and Hanoi is unclear. Further longitudinal studies are needed to clarify the relationships regarding geographical origin, sex and weight factors.
L. interrogans and SEOV were detected only in adult R. norvegicus with body weights of >260 g and >340 g, respectively. Furthermore, antibody-positive rates increased with weight (age), suggesting that L. interrogans and SEOV are maintained in reservoir populations by horizontal transmission [31, 32]. The lower antibody-positive rates in juvenile R. norvegicus might be due to maternal antibodies that prevent vertical transmission [33, 34]. Since the infection rate in sub-adult individuals without maternal antibodies is low, it is speculated that the efficiency of horizontal transmission of the hantavirus is low.
On the other hand, there were juvenile and sub-adult R. norvegicus infected with rat HEV. The manner in which rat HEV is transmitted in rats is still unknown. Our data indicated that rat HEV might have vertical transmission in addition to horizontal transmission in rodents. In human cases, vertical transmission of HEV has been reported [35, 36]. In fact, HEV RNA was detected by PCR in cord or birth blood samples of infants born from acute HEV-infected mothers, indicating that HEV is commonly transmitted from infected mothers to their babies [36, 37]. Further experiments on wild rats or laboratory rats are required to demonstrate vertical transmission of rat HEV in rats.
Nevertheless, the density of R. norvegicus in Hanoi was higher than that in Hai Phong as indicated by the trapping rate, and the seroprevalence of L. interrogans, SEOV and rat HEV in Hanoi was lower than in Hai Phong. It has been reported that the prevalence of hantavirus in Peromyscus maniculatus in North America and that of hantavirus in Myodes glareolus in Europe, in which rodents have a seasonal fluctuation of population density, were higher just after the seasonal high population density [38, 39]. However, seasonal patterns in the prevalence of SEOV and L. interrogans were not observed in R. norvegicus in previous studies [40–42]. Therefore, further longitudinal studies are needed to clarify the relationship between density and R. norvegicus seroprevalence in Vietnam.
Our results show that female R. norvegicus were more frequently infected with SEOV, L. interrogans and rat HEV than males. On the other hand, field studies on SEOV infection in rodents have shown that a higher percentage of infected individuals is commonly observed to be males [43]. Nuttall and Krojgaard et al. found no sexual difference in rates of infection with L. interrogans in rats [44, 45], whereas Easterbrook et al. suggested that female rats are more prone to infection [42]. The reasons for the female-biased SEOV, L. interrogans and rat HEV infections are unknown.
Easterbrook et al. previously reported that there was a correlation between prevalence of L. interrogans infection and HEV infection in R. norvegicus but not between SEOV infection and L. interrogans or HEV infection [42]. In the present study, no significant correlation was found in any of the pathogens tested. The reasons for no correlation in the pathogens are unknown.
All hantavirus genome-positive specimens were also positive by serological assay in this study. This result provides convincing evidence that animals chronically infected with hantavirus have specific antibodies as reported previously [46].
In our previous phylogenetic study, the M segment of SEOV from Hai Phong formed a distinct clade from those of SEOV from Hanoi [5]. Phylogenetic analyses of the S- and M-segment nucleotide sequences indicated that SEOVs from Hai Phong and Hanoi form different clades. Furthermore, the SEOV from Hai Phong was placed more closely to SEOV from Saigon Port in Ho Chi Minh City (11CSG and 5CSG) compared to SEOV from Hanoi. The cytochrome b sequences of R. norvegicus in Saigon and some R. norvegicus in Hai Phong were identical, but there were small differences between cytochrome b sequences of R. norvegicus captured in Saigon and Hanoi and between cytochrome b sequences of R. norvegicus captured in Hanoi and Hai Phong (data not shown). These results indicate that R. norvegicus has recently moved between Saigon and Hai Phong. Together with the phylogenetic tree of SEOV, these results suggest that SEOV in Hai Phong might have been transported from Saigon Port with R. norvegicus. However, since the distance between Hanoi and Hai Phong is only about 90 km, it is also speculated that variable SEOVs were able to be separately maintained.
Taken together, serological evidence of human pathogens, L. interrogans, SEOV and rat HEV, was obtained in Rattus spp. captured in urban areas of Northern Vietnam, Hanoi and Hai Phong. Further differential diagnosis of AFI in humans is needed to determine the number of cases of each infection, and continued rodent surveillance is important to estimate the emergence of rodent-borne diseases.
ACKNOWLEDGEMENTS
Fraction 1 antigen of Y. pestis was kindly supplied by the Centre for Inspection of Imported Foods and Infectious Diseases, Yokohama Quarantine Station.
We thank T. C. Tu and other field staff for supporting the animal sampling in Vietnam. We are also grateful to A. Ohnuma for excellent technical assistance.
This study was supported in part by the Program of Founding Research Centres for Emerging and Reemerging Infectious Diseases and the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID). This work was also supported in part by a grant from the Global COE program (Establishment of International Collaboration Centre for Zoonosis Control) and also supported in part by Grants-in-Aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labour and Welfare including H22-emerging-ippan-006.
We also acknowledge Stewart Chisholm of the Stewart English School (SES) for revising the grammar in the final manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. Journal of Biosciences 2008; 33: 557–569. [DOI] [PubMed] [Google Scholar]
- 2.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nature Reviews Microbiology 2009; 7: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haake DA, Matsunaga J. Leptospira: a spirochaete with a hybrid outer membrane. Molecular Microbiology 2010; 77: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plyusnin A, Vapalahti O, Vaheri A. Hantaviruses: genome structure, expression and evolution. Journal of General Virology 1996; 77: 2677–2687. [DOI] [PubMed] [Google Scholar]
- 5.Truong TT, et al. Molecular epidemiological and serological studies of hantavirus infection in northern Vietnam. Journal of Veterinary Medical Science 2009; 71: 1357–1363. [DOI] [PubMed] [Google Scholar]
- 6.Huong VT, et al. Hemorrhagic fever with renal syndrome, Vietnam. Emerging Infectious Diseases 2010; 16: 363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pattamadilok S, et al. Geographical distribution of hantaviruses in Thailand and potential human health significance of Thailand virus. American Journal of Tropical Medicine and Hygiene 2006; 75: 994–1002. [PubMed] [Google Scholar]
- 8.Gamage CD, et al. Serological evidence of Thailand virus-related hantavirus infection among suspected leptospirosis patients in Kandy, Sri Lanka. Japanese Journal of Infectious Diseases 2011; 64: 72–75. [PubMed] [Google Scholar]
- 9.Johne R, et al. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. Journal of General Virology 2010; 91: 750–758. [DOI] [PubMed] [Google Scholar]
- 10.Li T, et al. Characterization of self-assembled virus-like particles of rat hepatitis E virus generated by recombinant baculoviruses. Journal of General Virology 2011; 92: 2830–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dremsek P, et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Medical Microbiology and Immunology 2012; 201: 189–200. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda SP, et al. Phylogeographic patterning of mtDNA in the widely distributed harvest mouse (Micromys minutus) suggests dramatic cycles of range contraction and expansion during the mid- to late Pleistocene. Canadian Journal of Zoology 2005; 83: 1411–1420. [Google Scholar]
- 13.Webster JP, Macdonald DW. Parasites of wild brown rats (Rattus norvegicus) on UK farms. Parasitology 1995; 111: 247–255. [DOI] [PubMed] [Google Scholar]
- 14.Flannery B, et al. Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. Journal of Clinical Microbiology 2001; 39: 3303–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koma T, et al. Truncated hantavirus nucleocapsid proteins for serotyping Sin Nombre, Andes, and Laguna Negra hantavirus infections in humans and rodents. Journal of Clinical Microbiology 2010; 48: 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimatsu K, et al. Production of recombinant hantavirus nucleocapsid protein expressed in silkworm larvae and its use as a diagnostic antigen in detecting antibodies in serum from infected rats. Laboratory Animal Science 1995; 45: 641–646. [PubMed] [Google Scholar]
- 17.Araki K, et al. Truncated hantavirus nucleocapsid proteins for serotyping Hantaan, Seoul, and Dobrava hantavirus infections. Journal of Clinical Microbiology 2001; 39: 2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker EE, et al. Studies on immunization against plague. I. The isolation and characterization of the soluble antigen of Pasteurella pestis. Journal of Immunology 1952; 68: 131–145. [PubMed] [Google Scholar]
- 19.Kollars TM Jr., et al. Antibodies to leptospirosis in rodents from Thailand using a modified human diagnostic assay. Journal of the Medical Association of Thailand 2002; 85: 67–70. [PubMed] [Google Scholar]
- 20.Wangroongsarb P, et al. Survey of leptospirosis among rodents in epidemic areas of Thailand. Journal of Tropical Medicine and Parasitology 2002; 25: 55–58. [Google Scholar]
- 21.Gamage CD, et al. Prevalence and carrier status of leptospirosis in smallholder dairy cattle and peridomestic rodents in Kandy, Sri Lanka. Vector-Borne and Zoonotic Diseases 2011; 11: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 22.Doungchawee G, et al. Survey of leptospirosis of small mammals in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 2005; 36: 1516–1522. [PubMed] [Google Scholar]
- 23.Yalin W, et al. High prevalence of pathogenic Leptospira in wild and domesticated animals in an endemic area of China. Asian Pacific Journal of Tropical Medicine 2011; 4: 841–845. [DOI] [PubMed] [Google Scholar]
- 24.Kim HC, et al. Seroepidemiological survey of rodents collected at a U.S. military installation, Yongsan Garrison, Seoul, Republic of Korea. Military Medicine 2007; 172: 759–764. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, et al. Leptospiral carrier state and seroprevalence among animal population – a cross-sectional sample survey in Andaman and Nicobar Islands. Epidemiology and Infection 2003; 131: 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang JF, et al. Study on the association between hantavirus infection and Rattus norvegicus. Zhonghua Liu Xing Bing Xue Za Zhi 2006; 27: 196–199. [PubMed] [Google Scholar]
- 27.Kosasih H, et al. Evidence of human hantavirus infection and zoonotic investigation of hantavirus prevalence in rodents in western Java, Indonesia. Vector-Borne and Zoonotic Diseases 2011; 11: 709–713. [DOI] [PubMed] [Google Scholar]
- 28.Jiang JF, et al. Prevalence and genetic diversities of hantaviruses in rodents in Beijing, China. American Journal of Tropical Medicine and Hygiene 2008; 78: 98–105. [PubMed] [Google Scholar]
- 29.Huong V, et al. Hantavirus infection in human and rodents in central highlands and southern Vietnam during 2006–2009. BMC Proceedings 2011; 5. [Google Scholar]
- 30.Lin XD, et al. Migration of norway rats resulted in the worldwide distribution of seoul hantavirus today. Journal of Virology 2011; 86: 972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills JN, et al. A longitudinal study of hantavirus infection in three sympatric reservoir species in agroecosystems on the Argentine Pampa. Vector-Borne and Zoonotic Diseases 2007; 7: 229–240. [DOI] [PubMed] [Google Scholar]
- 32.Mohan RA. Preventive measures for leptospirosis: rodent control. Indian Journal of Medical Microbiology 2006; 24: 325–328. [DOI] [PubMed] [Google Scholar]
- 33.Morita C, et al. Inability of a strain of Seoul virus to transmit itself vertically in rats. Japanese Journal of Medical Science and Biology 1993; 46: 215–219. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XK, Takashima I, Hashimoto N. Role of maternal antibody in protection from hemorrhagic fever with renal syndrome virus infection in rats. Archives of Virology 1988; 103: 253–265. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, et al. Mother-to-child transmission of hepatitis E virus infection. Indian Journal of Pediatrics 2003; 70: 37–39. [DOI] [PubMed] [Google Scholar]
- 36.Sookoian S. Liver disease during pregnancy: acute viral hepatitis. Annals of hepatology 2006; 5: 231–236. [PubMed] [Google Scholar]
- 37.Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet 1995; 345: 1025–1026. [DOI] [PubMed] [Google Scholar]
- 38.Madhav NK, et al. Delayed density-dependent prevalence of Sin Nombre virus antibody in Montana deer mice (Peromyscus maniculatus) and implications for human disease risk. Vector-Borne and Zoonotic Diseases 2007; 7: 353–364. [DOI] [PubMed] [Google Scholar]
- 39.Escutenaire S, et al. Spatial and temporal dynamics of Puumala hantavirus infection in red bank vole (Clethrionomys glareolus) populations in Belgium. Virus Research 2000; 67: 91–107. [DOI] [PubMed] [Google Scholar]
- 40.Li HY, Davis DE. The prevalence of carriers of Leptospira and Salmonella in Norway rats of Baltimore. American Journal of Hygiene 1952; 56: 90–91. [DOI] [PubMed] [Google Scholar]
- 41.Klein SL, et al. Environmental and physiological factors associated with seoul virus infection among urban populations of Norway rats. Journal of Mammalogy 2002; 83: 478–488. [Google Scholar]
- 42.Easterbrook JD, et al. A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiology and Infection 2007; 135: 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein SL, Bird BH, Glass GE. Sex differences in Seoul virus infection are not related to adult sex steroid concentrations in Norway rats. Journal of Virology 2000; 74: 8213–8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuttall GHF. Leptospira icterohaemorrhagiae in Oxford rats. Journal of Hygiene 1929; XXIX: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krojgaard LH, et al. High prevalence of Leptospira spp. in sewer rats (Rattus norvegicus). Epidemiology and Infection 2009; 137: 1586–1592. [DOI] [PubMed] [Google Scholar]
- 46.Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clinical Microbiology Reviews 2010; 23: 412–441. [DOI] [PMC free article] [PubMed] [Google Scholar]