SUMMARY
In this study within-herd prevalence of Salmonella Dublin was investigated in three age groups (calves, young stock, adult cows) during five herd visits at 3-month intervals of 14 endemically infected dairy herds. A total of 10162 paired faecal cultures and antibody measurements were used to calculate the age and temporal dynamics of seroprevalence and prevalence of positive faecal cultures. Faecal culture-positive prevalence was generally low. It was highest (5·4%) in calves during December to February. Seroprevalence varied from 0% to 70% between herds, but was generally more stable in young stock and adult cows than in calves. Hierarchical mixed-model results showed that seroprevalence was associated with the bacteriological status in calves and cows, but not in young stock. These results can be used to develop and validate theoretical infection dynamics models and to design effective control programmes for Salmonella Dublin in dairy herds.
Key words: Antibody, cattle, faecal culture, prevalence, Salmonella Dublin
INTRODUCTION
Salmonella enterica subsp. enterica serovar Dublin (S. Dublin) is a gastrointestinal bacterial infection of concern in intensive cattle rearing farms because it leads to increased morbidity and mortality as well as production losses [1–3]. In order to design effective control programmes, good estimates of within-herd prevalence of infection are required [4, 5]. Furthermore, within-herd prevalence estimates are needed for development and validation of theoretical models of S. Dublin infection dynamics [6–8]. There are, however, very few published studies available that provide good insight into within-herd prevalence and dynamics of S. Dublin, in particular for persistently infected cattle herds.
Veling et al. [9] investigated seroprevalence in 79 dairy herds 24 months after confirmed outbreaks of S. Dublin. The seroprevalence varied between 10% to almost 60%; however, averaged seroprevalence estimates >30% across all herds were only found in calves aged between 3 and 7 months. In adult cattle seroprevalence was on average 12%. The authors of that study also reported that the seroprevalence in young stock did not differ between infected herds with or without clinical signs, indicating that serology of young stock is a good indicator of subclinical S. Dublin infection in the herd. However, a subsequent study in some of the same herds showed large variations in prevalence between herds ranging from 0% to 70% in both young stock and adults [5].
In another study conducted in California, of a large persistently infected dairy herd with clinical problems associated with S. Dublin, the prevalence of seropositive adult cows was 3·5%, whereas in calves it was 52%. In that study, 11% of the calves were found to be faecal shedders of S. Dublin bacteria [10]. However, neither herd size nor management reported in that study were representative of Danish dairy herds, therefore a field study was performed to gain more knowledge about the occurrence of S. Dublin within endemically infected dairy herds in Denmark. The objective of this study was therefore to investigate age- and time-related dynamics of within-herd seroprevalence and faecal excretion in endemically infected dairy herds.
MATERIALS AND METHODS
Selection of herds and sampling
In 2000, a total of 14 dairy herds were selected to participate in a field study on the basis of them having high bulk-tank milk enzyme-linked immunosorbent assay (ELISA) results, i.e. above 50 background-corrected optical density values (ODC%) [11, 12]. Herd size varied between 38 and 154 lactating cows. S. Dublin was isolated from faecal samples of all of these herds at least once during the study period from the beginning of 2000 to the beginning of 2002 [13], with indications of the herd being endemically infected throughout the project period (i.e. continued serological responses in all age groups of cattle and bulk-tank milk throughout the study period). Each of the 14 herds was visited five times – except one that was visited four times – within a time-frame of ∼3-month intervals. At each visit, blood samples were collected from all accessible calves, young stock and dry cows; and milk samples were collected from all lactating cows at the morning milking for serological analysis. Faecal samples were collected rectally from all accessible animals and placed into marked faecal transport containers with a snap cap (549263 NUNC A/S, Denmark), with the aim of obtaining at least 50 g from each animal.
All samples were transported directly to the Danish Cattle Health Laboratory (DCHL) in Ladelund, and stored at <5 °C until analysis could be performed within a few days of the samples' arrival. At the laboratory faecal samples were pooled five at a time using 5 g per sample. This was then mixed in a 25 g pool before analysis. The blood samples were spun to extract the serum fraction for analysis.
Bacteriological culture method
Pooled faecal samples were examined at DCHL for the presence of Salmonella bacteria by mixing the 25 g faecal material in a 225 ml peptone buffer which was then left for pre-enrichment at 37 °C for 18–24 h. A volume of 0·1 ml of the test material was added to modified semi-solid Rappaport–Vassiliadis medium base (MSRV agar) plates and 1 ml of the test material was placed into 9 ml of selenite cystine broth and incubated for 18–24 h at 41·5 °C. Material from the selenite cystine tubes was inoculated on modified Brilliant-green Phenol-red lactose sucrose agar (BPLS agar) plates and incubated at 37 °C for 18–24 h. Positive test results from MSRV were inoculated onto BPLS agar plates and confirmed using triple sugar iron agar tests and lysine iron agar tests. Serotyping and confirmation of positive isolates were conducted at the Danish Veterinary Institute (now the National Food Institute at the Danish Technical University in Copenhagen).
If the pool was found to be positive for Salmonella, then the individual samples would be cultured using 25 g faecal material to try to identify those animals that were positive in the pool. The diagnostic sensitivity of this faecal culture procedure has been evaluated to be about 6–14% in subclinically infected cattle [14].
Antibody measurements by ELISA
The serum S. Dublin ELISA that was used in this study, was performed at DCHL as slightly modified from a previously described ELISA method [15] and described in detail in Nielsen & Ersbøll [16]. Briefly, an O-antigen based S. Dublin lipopolysaccharide (LPS) preparation produced at the Danish Veterinary Institute in Copenhagen was used to coat microtitration plates. Sera were diluted 1:200 and added to microtitration plate wells in duplicate. Known positive and negative reference sera were added in quadruplicate. The plates were incubated overnight at 4 °C, and washed three times. For detection of immunoglobulins, affinity purified horseradish peroxidase-labelled goat anti-bovine IgG (H + L) conjugate was added. Following incubation for 1 h at 37 °C the plates were washed three times. Substrate and indicator solution was added to the wells and incubated in the dark at room temperature for 10–20 min. The reaction was then stopped when the optical density of the positive reference wells was visually evaluated to be about 2·000 OD values. The OD was read at 492 nm and 620 nm as reference using an ELISA plate reader. Plates were considered valid if the four negative reference wells had an average OD of <0·300, and the four positive reference wells had an average OD of 1·200–2·500. An ODC% value, which is a background-corrected proportion of the test sample OD to a positive reference sample, was calculated as follows:
where 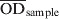 is the mean value of two test wells,
is the mean value of two test wells, 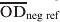 and
and 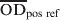 are the mean of ELISA plate readings of four test negative and test positive reference wells, respectively.
are the mean of ELISA plate readings of four test negative and test positive reference wells, respectively.
Serum and milk samples with ODC% >50 were considered seropositive in the consecutive calculations for within-herd and within-age group prevalence estimations. At this cut-off, the sensitivity of the ELISA has previously been estimated to be 0·16–0·26 for calves aged <100 days, 0·66–0·88 in calves and young stock aged 100–300 days, and 0·50–0·68 in cattle aged >300 days old. The specificity was estimated to be 0·93–0·98, 0·93–0·98, and 0·88–0·91 for the same age groups, respectively [14].
Statistical analyses
SAS version 9.2 (SAS Institute Inc., USA) was used for the data management as well as the descriptive and statistical analyses. Within-herd prevalence of seropositive and bacteriologically positive animals was calculated for rolling intervals of age across all herds. Intervals of serological prevalence contained 200 observations. Due to the low number of faecal culture-positive animals, the intervals used for descriptive statistics of the bacteriological results contained 500 observations in each interval. For each interval, 95% confidence limits were calculated.
For further analysis, the dataset was split into three age groups based on typical management and housing structures in Danish dairy herds: calves aged 0–180 days (usually housed in calf barns with single housing followed by small groups of calves), young stock aged 181 days to 2 years (growing and breeding heifers often kept in larger groups) and adult cattle aged >2 years (adult heifers close to calving or cows). The correlation between seroprevalence and faecal culture-positive prevalence within each age group and across all age groups was investigated using Spearman's correlation. Furthermore, the correlation between the faecal culture-positive prevalences at one visit and the seroprevalences at the next visit for each age group and across all age groups were investigated using Spearman's correlation, because it could be expected that the increase in seroprevalence would be delayed by at least 2–4 weeks compared to the point in time of the shedding of the bacteria [17–19].
Factors affecting seroprevalence at each herd visit were investigated for each of the age groups using three hierarchical mixed models with seroprevalence as the outcome and season and bacteriological status of the age group on the given visit date as potential risk factors in the model. Repeated sampling at herd level was taken into account in the analysis, and herd was included as a fixed effect in order to determine the actual predicted seroprevalence for each herd and level of significant predictors. Two-way interactions between predictors were tested in the models. Predictors and interactions were considered significantly associated with the outcome if P < 0·05.
RESULTS
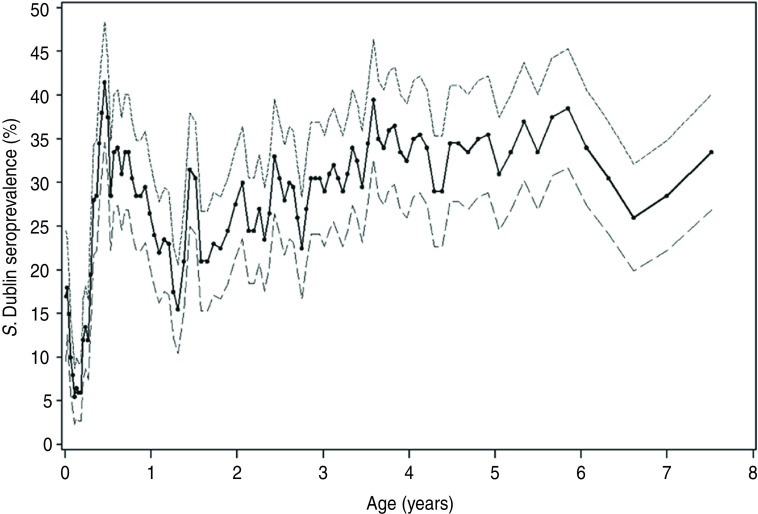
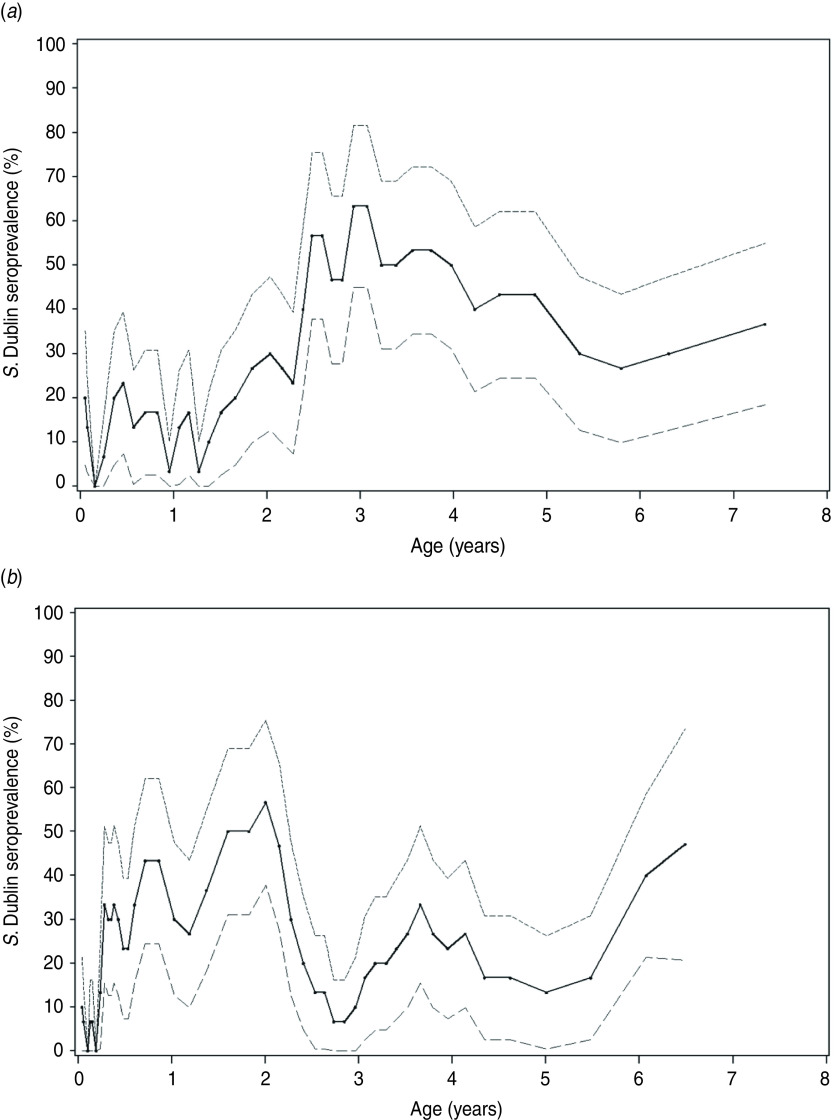
There were a total of 10162 observations with paired faecal cultures and ELISA results from all ages of cattle in the 14 study herds. Table 1 shows the distribution of seropositive and faecal culture-positive cattle in each season stratified by age groups across the 14 dairy herds. In the descriptive statistics and univariable analysis, seroprevalence was lower (below 25%) in summer and autumn than in spring and winter (above 30%) for calves; whereas for young stock the seroprevalence remained around 25–30% for all seasons. For cows, seroprevalence varied significantly with season. The seroprevalence was lower in autumn than in spring, summer and winter. The dynamic changes of seroprevalence with age across all study herds are illustrated in more detail in Figure 1, and examples of how different these patterns were for each herd are illustrated for two herds in Figure 2.
Table 1.
Descriptive statistics of Salmonella Dublin seroprevalence and faecal culture-positive prevalence by season, in three age groups of 14 endemically infected dairy herds
| Factors | Distribution of animals and P values from univariable analysis of the effect of season within each age group | |||
|---|---|---|---|---|
| Age group, season and age distribution within each group (mean; 5th and 95th percentiles) | No. of observations | % seropositive (95% CI) | % faecal culture positive (95% CI) | P values serology/bacteriology |
| Calves | 0·001/0·02 | |||
| Spring (135; 95–177 days) | 279 | 33 (27–39) | 1·1 (0–2·3) | |
| Summer (132; 94–176 days) | 132 | 19 (12–26) | 2·3 (0–4·8) | |
| Autumn (135; 93–177 days) | 180 | 24 (18–31) | 1·1 (0–2·7) | |
| Winter (131; 93–176 days) | 185 | 37 (30–44) | 5·4 (2·1–8·7) | |
| Young stock | 0·25/0·08 | |||
| Spring (435; 197–687 days) | 1118 | 25 (22–28) | 0·9 (0·3–1·5) | |
| Summer (386; 187–684 days) | 244 | 26 (20–31) | 2·1 (0·3–3·8) | |
| Autumn (351; 197–625 days) | 463 | 30 (26–34) | 1·5 (0·4–2·6) | |
| Winter (439; 212–696 days) | 792 | 27 (24–30) | 2·3 (1·2–3·3) | |
| Cows | <0·001/0·07 | |||
| Spring (4·0; 2·2–7·4 years) | 2027 | 33 (31–35) | 0·4 (0·2–0·7) | |
| Summer (4·1; 2·3–7·3 years) | 1071 | 32 (29–35) | 0 | |
| Autumn (4·1; 2·3–7·5 years) | 1264 | 26 (24–29) | 0·2 (0–0·5) | |
| Winter (4·0; 2·2–7·3 years) | 1376 | 34 (32–37) | 0·5 (0·1–0·9) | |
CI, Confidence interval.
P values provided are from χ2 tests of the effects of season on prevalence in univariable analyses for each age group.
Fig. 1.
Salmonella Dublin seroprevalence in 14 endemically infected dairy herds tested repeatedly during 2000–2002. The solid line shows the mean seroprevalence and the dashed lines the 95% confidence intervals. The various points represent average age in rolling intervals, each containing 200 observations.
Fig. 2.
Salmonella Dublin seroprevalence in two endemically infected dairy herds tested repeatedly during 2000–2002. The seroprevalence dynamics in a herd that was (a) bacteriologically positive only among cows; (b) bacteriologically positive only among calves and young stock. The solid line shows the mean seroprevalence and the dashed lines the 95% confidence intervals. The points represent average age in rolling intervals, each containing 200 observations.
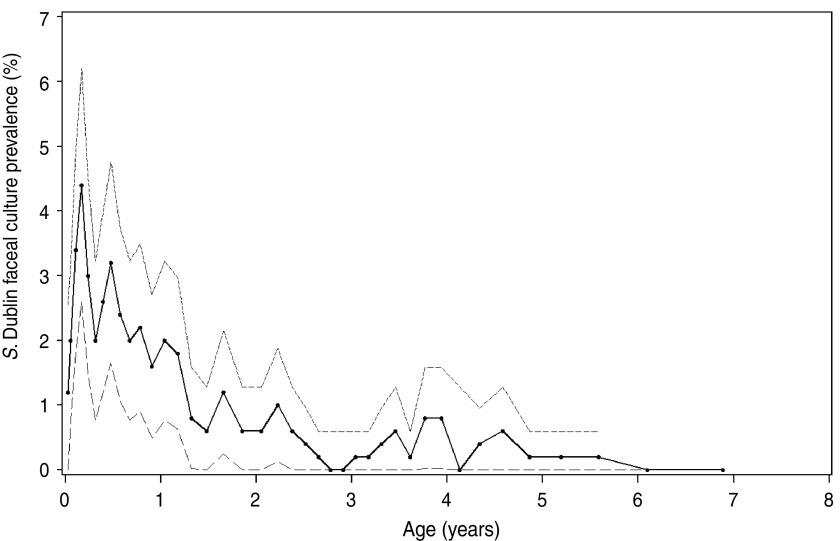
The prevalence of positive faecal cultures differed between seasons in calves (varied from 1·1% in spring and autumn to 5·4% in winter, P = 0·02). In young stock (0·9% in spring to 2·3% in winter, P = 0·08) and cows (0% in summer to 0·5% in winter, P = 0·07) a similar tendency was observed in the univariable analyses. The dynamics of faecal culture-positive prevalence with age are illustrated in more detail in Figure 3. Hence, faecal shedding prevalence was up to twice as high in calves as in young stock, and up to 10 times higher in calves than in cows. In contrast, seroprevalence was generally at comparable levels in all age groups.
Fig. 3.
Prevalence of Salmonella Dublin faecal-culture positive cattle 14 endemically infected dairy herds tested repeatedly during 2000–2002. The solid lines show the mean prevalence in rolling intervals and the dashed lines the 95% confidence intervals. Points show average age of the rolling intervals, each containing 500 observations.
Spearman's correlations between seroprevalence and faecal culture-positive prevalence were generally low: at herd-visit level (n = 69 herd visits) the correlation coefficient was ρ = 0·232 (P = 0·05). At age group-visit level it was ρ = 0·349 (P = 0·003) in calves, ρ = 0·285 (P = 0·02) in young stock and ρ = 0·372 (P = 0·002) in cows. Spearman's correlations between faecal culture-positive prevalence and seroprevalence at the following herd visit (n = 55) were similar to those found when comparing seroprevalence and faecal culture-positive prevalence at the same visit, and graphic displays of seroprevalence vs. faecal culture-positive prevalence at the same visit and at offset herd visits did not suggest specific patterns that would be of interest for further analysis (data not shown), it was therefore decided not to explore these patterns any further.
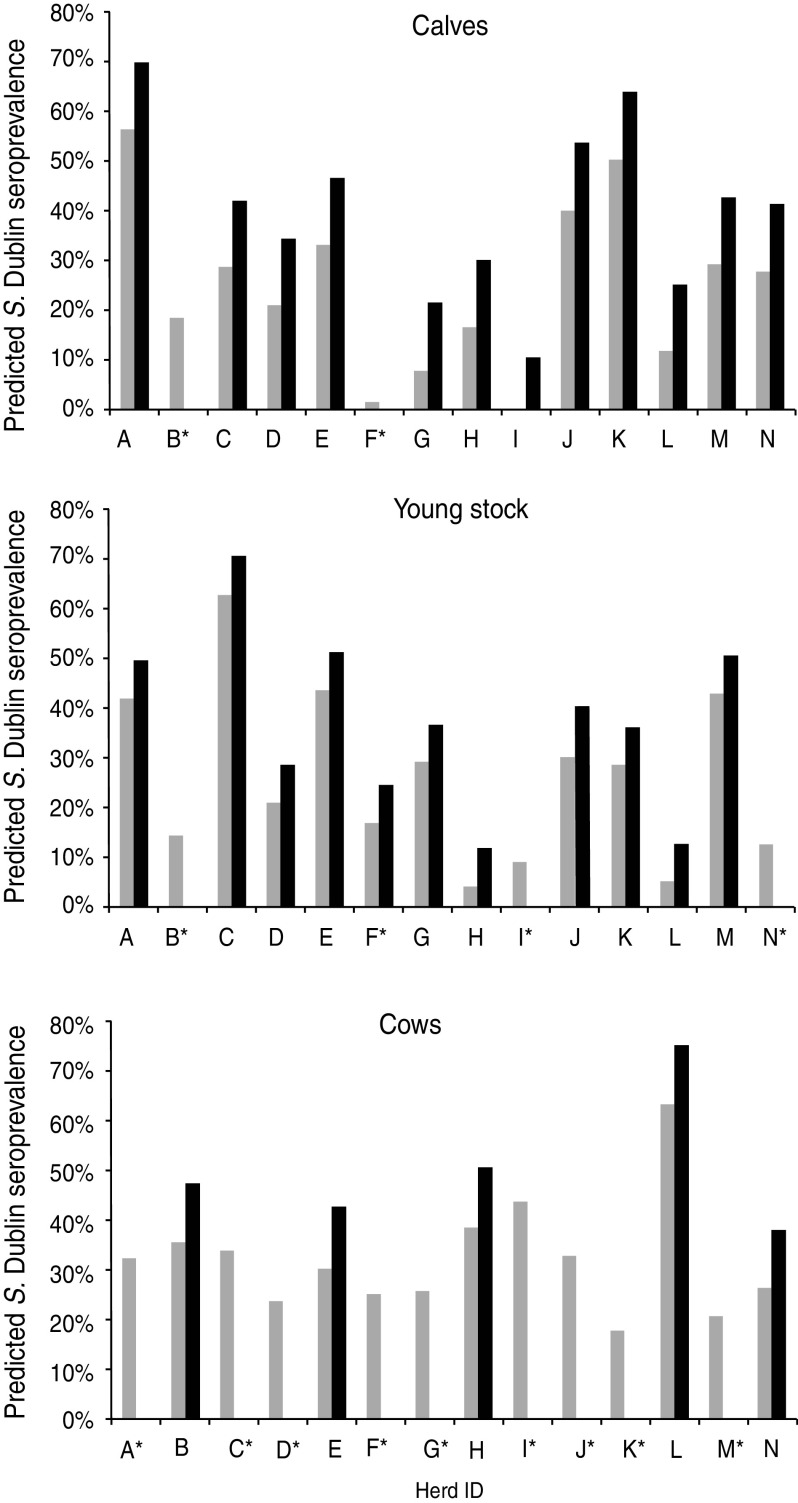
The model-predicted seroprevalence varied significantly between herds (i.e. up to 70% difference between the lowest to the highest predicted seroprevalence in calves) (model results not shown). On average seroprevalence was 13·4% higher in calves, 7·4% higher in young stock and 11·3% higher in cows if S. Dublin was isolated from at least one faecal sample in the same age group at the same herd visit. Model-predicted seroprevalence for each herd and underlying bacteriological status is illustrated in Figure 4 for the three age groups. According to these models, seroprevalence was not affected by season when taking into account the underlying bacteriological status of the age group.
Fig. 4.
Model-predicted seroprevalences in three age groups in 14 Salmonella Dublin-infected dairy herds. The black bars (▪) indicate seroprevalence where there was at least one positive faecal culture sample; the grey bars ( ) indicate no positive faecal culture samples in the age group. * Indicates no positive faecal cultures in that herd and age group.
) indicate no positive faecal culture samples in the age group. * Indicates no positive faecal cultures in that herd and age group.
DISCUSSION
Using a large field data collection from 14 endemically infected dairy herds, this study has provided new detailed information on the level and dynamics of faecal culture-positive prevalence and seroprevalence for S. Dublin. The main finding was that the seroprevalence of S. Dublin varied tremendously between herds. Generally it was higher in herds with positive faecal cultures at the same herd visit. Seroprevalence was found to be significantly associated, but not highly correlated with the faecal culture-positive prevalence in calves and cows. In young stock the correlation appeared to be significant by Spearman's correlation analysis. However, in the mixed-model analysis, which took into account herd variation and repeated sampling of animals, there appeared to be no association between seroprevalence and faecal excretion of bacteria. The correlation between faecal shedding prevalence and seroprevalence at the following visit could have been higher than the correlation between the serology and the current bacteriology results for each visit due to the delay in serological responses upon infection. However, neither the descriptive nor the statistical analyses of these correlations showed any notable difference in the correlations for any of the age groups or across the herds when taking into account the time delay between faecal shedding and serology (data not shown). This may be because these herds were endemically infected leading to continuous low-dose exposure of the animals and to little faecal shedding and fluctuating serology in all age groups. This is also reflected in the fact that the seroprevalence generally became more stable with increasing age as illustrated in Figure 1, hovering around 25–35% in adult cows. Surprisingly, this was markedly higher than the average seroprevalence of 3·5% reported by House et al. [10] from a study of a large persistently infected dairy herd with clinical problems associated with S. Dublin, and the seroprevalence of 12% reported by Veling et al. [9] for adult cows in recent outbreak herds. However, a subsequent study with some of the same herds showed large variation between herds with seroprevalences ranging from 0% to 70% in both young stock and adults [5]. These results have more similarities to the variations found in the present study (Figs 2, 4). Together these studies indicate that the seroprevalence may be higher in adult cattle in persistently infected herds without clinical signs than in herds with outbreaks and clinical problems. Even though antibodies are not necessarily protective in individual animals [20], but rather an indication of previous exposure which may have evoked cell-mediated immunity [21, 22], a high seroprevalence may be an indication of a high level of herd immunity, which would explain the lack of clinical signs in age groups with high seroprevalence [7, 23].
Overall, faecal culture-positive prevalence was low, but generally higher the younger the age. This corresponds well with previous studies on clinical expression of S. Dublin in cattle herds, where calves were more frequently and more severely affected by the disease than older cattle [3, 24]. One of the reasons for the varying prevalence observed in field studies is that S. Dublin is a very dynamic infection within cattle herds. Moreover, in some barn sections the cattle populations are also very dynamic with a continuous or fluctuating number of new animals with varying susceptibility to the infection being introduced to the age groups over time [7, 25].
Furthermore, a seasonal trend was seen, in that faecal culture-positive prevalence was generally highest in the winter season (i.e. December to February). This differs from the pattern observed for outbreaks of S. Dublin in cattle herds, which tend to have the highest incidence from August to November [26, 27]. Again this may be explained by differences between outbreak and endemic situations which may be related to variations in infectious doses and immunity levels in different age groups, management, hygiene, herd size and other diseases in the herd [7, 22, 28].
This is the first study providing this level of longitudinal and extensive data collection for investigation of within-herd S. Dublin epidemiology. All cattle present in the barns were sampled at each visit (i.e. animals on pastures were excluded from the sampling rounds) leading to a total of 10162 paired samples for analysis of antibodies and bacteriology. Samples consisted of rectally collected faecal material making it possible to follow the excretion patterns of each individual animal over time. However, the low diagnostic sensitivity of the faecal culture method is generally problematical in studies of S. Dublin infections [14]. This may have affected the associations and correlations found between faecal culture-positive prevalence and seroprevalence. It is likely that the associations and correlations would have been stronger if there had been more positive faecal culture samples. However, the seasonal and age difference would most likely have been the same, as it is unlikely that biased sensitivities and specificities of the laboratory tests were present in this study. A cut-off of 50 ODC% was used to differentiate between serologically negative and positive samples in the subsequent descriptive and statistical analyses. Sensitivity and specificity of the laboratory tests used depend on age as described in previous studies [14, 16]. The sensitivity was very low for young calves. Therefore, the observations from calves aged <90 days were omitted for the statistical analyses of correlations and associations. In Denmark, it is recommended not to use serology for calves aged <3 months in order to avoid sensitivity issues with the test.
In conclusion, this study provided detailed estimates and illustration of dynamics and factors affecting seroprevalence and faecal culture-positive prevalence of S. Dublin in all age groups of endemically infected Danish dairy herds. These results can be used in modelling of infection dynamics and control scenarios, as well as planning of test strategies to support surveillance and control programmes at herd and national levels.
ACKNOWLEDGEMENTS
The author thanks the farmers for participating in the Kongeå-project. Thanks are also due to the consultants from the Danish Dairy Board, who collected all of the samples and the technicians from the Danish Cattle Health Laboratory for handling and analysing the samples. The work was funded by the Danish Dairy Board and was performed as part of the Kongeå-project.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Nielsen TD, et al. Association between bulk-tank milk Salmonella antibody level and high calf mortality in Danish dairy herds. Journal of Dairy Science 2010; 93: 304–310. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen TD, et al. Evaluation of milk yield losses associated with Salmonella antibodies in bulk-tank milk in bovine dairy herds. Journal of Dairy Science 2012; 95: 4873–4885. [DOI] [PubMed] [Google Scholar]
- 3.Richardson A, Watson WA. A contribution to the epidemiology of Salmonella Dublin infection in cattle. British Veterinary Journal 1971; 127: 173–182. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen LR, Nielsen SS. A structured approach to control of Salmonella Dublin in 10 Danish dairy herds based on risk scoring and test-and-manage procedures. Food Research International 2011; 45: 1158–1165. [Google Scholar]
- 5.Veling J. Diagnosis and control of Salmonella Dublin infections on Dutch dairy farms (PhD thesis). Animal Health Service, Deventer, The Netherlands, 2004, pp. 1–173. [Google Scholar]
- 6.Lanzas C, et al. The effect of heterogeneous infectious period and contagiousness on the dynamics of Salmonella transmission in dairy cattle. Epidemiology and Infection 2008; 136: 1496–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen LR, Kudahl AB, Østergaard S. Age-structured dynamic, stochastic and mechanistic simulation model of Salmonella Dublin infection within dairy herds. Preventive Veterinary Medicine 2012; 105: 59–74. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Y, et al. Understanding the dynamics of Salmonella infections in dairy herds: a modelling approach. Journal of Theoretical Biology 2005; 233: 159–175. [DOI] [PubMed] [Google Scholar]
- 9.Veling J, et al. Herd-level diagnosis for Salmonella enterica subsp. enterica serovar Dublin infection in bovine dairy herds. Preventive Veterinary Medicine 2002; 53: 31–42. [DOI] [PubMed] [Google Scholar]
- 10.House JK, et al. Enzyme-linked immunosorbent assay for serologic detection of Salmonella dublin carriers on a large dairy. American Journal of Veterinary Research 1993; 54: 1391–1399. [PubMed] [Google Scholar]
- 11.Andersen HJ, et al. Integration of research, development, health promotion, and milk quality assurance in the Danish dairy industry. In: Salman MD, Morley PS, Ruch-Galie R, eds. Proceedings of the 9th Symposium of ISVEE. Breckenridge, Colorado 2000, pp. 258–260. [Google Scholar]
- 12.Nielsen LR. Salmonella Dublin in dairy cattle: use of diagnostic tests for investigation of risk factors and infection dynamics (PhD thesis). The Royal Veterinary and Agricultural University, 2003, pp. 1–219. [Google Scholar]
- 13.Nielsen LR, Ersbøll AK. Factors associated with variation in bulk-tank-milk Salmonella Dublin ELISA ODC% in dairy herds. Preventive Veterinary Medicine 2005; 68: 165–179. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen LR, Toft N, Ersbøll AK. Evaluation of an indirect serum ELISA and a bacteriological faecal culture test for diagnosis of Salmonella serotype Dublin in cattle using latent class models. Journal of Applied Microbiology 2004; 96: 311–319. [DOI] [PubMed] [Google Scholar]
- 15.Hoorfar J, et al. Serodiagnosis of Salmonella dublin infection in Danish dairy herds using O-antigen based enzyme-linked immunosorbent assay (published Erratum appears in Can. J. Vet. Res. 1995, 59, 25). Canadian Journal of Veterinary Research 1994; 58: 268–274. [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen LR, Ersbøll AK. Age stratified validation of an indirect Salmonella Dublin serum ELISA for individual diagnosis in cattle. Journal of Veterinary Diagnostic Investigation 2004; 16: 205–211. [DOI] [PubMed] [Google Scholar]
- 17.Da Roden L, et al. Effect of calf age and Salmonella bacterin type on ability to produce immunoglobulins directed against Salmonella whole cells or lipopolysaccharide. American Journal of Veterinary Research 1992; 53: 1895–1899. [PubMed] [Google Scholar]
- 18.Jordan D, Nielsen LR, Warnick LD. Modelling a national programme for the control of foodborne pathogens in livestock: the case of Salmonella Dublin in the Danish cattle industry. Epidemiology and Infection 2008; 136: 1521–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertsson JA. Humoral antibody responses to experimental and spontaneous Salmonella infections in cattle measured by ELISA. Zentralblatt fur Veterinarmedizin, B 1984; 31: 367–380. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi GC, Sharma VK. Cell-mediated immunoprotection in calves immunized with rough Salmonella dublin. British Veterinary Journal 1981; 137: 421–430. [PubMed] [Google Scholar]
- 21.Steinbach G, et al. Course of infection and humoral immune reaction in calves infected orally with different salmonella serovars. Journal of Veterinary Medicine Series B 1993; 40: 515–521. [DOI] [PubMed] [Google Scholar]
- 22.Steinbach G, et al. Influence of prior infection on the dynamics of bacterial counts in calves experimentally infected with Salmonella dublin. Veterinary Microbiology 1996; 48: 199–206. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen LR. Review of pathogenesis and diagnostic methods of immediate relevance for epidemiology and control of Salmonella Dublin in cattle. Veterinary Microbiology 2012; Published online: 9 August 2012. doi: 10.1016/j.vetmic.2012.08.003). [DOI] [PubMed] [Google Scholar]
- 24.McDonough PL, et al. Salmonella enterica serotype Dublin infection: an emerging infectious disease for the northeastern United States. Journal of Clinical Microbiology 1999; 37: 2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen LR, van den Borne B, van Schaik G. Salmonella Dublin infection in young dairy calves: Transmission parameters estimated from field data and an SIR model. Preventive Veterinary Medicine 2007; 79: 46–58. [DOI] [PubMed] [Google Scholar]
- 26.Steffensen M, Blom JY. Incidence of Salmonella infections in Danish cattle herds 1992–1998 [in Danish]. Dansk Veterinærtidsskrift 1999; 82: 966–970. [Google Scholar]
- 27.Carrique-Mas JJ, et al. Salmonella infection in cattle in Great Britain, 2003 to 2008. Veterinary Record 2010; 167: 560–565. [DOI] [PubMed] [Google Scholar]
- 28.Wray C, Sojka WJ. Salmonella Dublin infection of calves: use of small doses to simulate natural infection on the farm. Journal of Hygiene 1981; 87: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]