Abstract
Aims
Adjuvant radiotherapy is recommended for most patients with early breast cancer (EBC) receiving breast-conserving surgery and those at moderate/high risk of recurrence treated by mastectomy. During the first wave of COVID-19 in England and Wales, there was rapid dissemination of randomised controlled trial-based evidence showing non-inferiority for five-fraction ultra-hypofractionated radiotherapy (HFRT) regimens compared with standard moderate-HFRT, with guidance recommending the use of five-fraction HFRT for eligible patients. We evaluated the uptake of this recommendation in clinical practice as part of the National Audit of Breast Cancer in Older Patients (NABCOP).
Materials and methods
Women aged ≥50 years who underwent surgery for EBC from January 2019 to July 2020 were identified from the Rapid Cancer Registration Dataset for England and from Wales Cancer Network data. Radiotherapy details were from linked national Radiotherapy Datasets. Multivariate mixed-effects logistic regression models were used to assess characteristics influential in the use of ultra-HFRT.
Results
Among 35 561 women having surgery for EBC, 71% received postoperative radiotherapy. Receipt of 26 Gy in five fractions (26Gy5F) increased from <1% in February 2020 to 70% in April 2020. Regional variation in the use of 26Gy5F during April to July 2020 was similar by age, ranging from 49 to 87% among women aged ≥70 years. Use of 26Gy5F was characterised by no known nodal involvement, no comorbidities and initial breast-conserving surgery. Of those patients receiving radiotherapy to the breast/chest wall, 85% had 26Gy5F; 23% had 26Gy5F if radiotherapy included regional nodes. Among 5139 women receiving postoperative radiotherapy from April to July 2020, nodal involvement, overall stage, type of surgery, time from diagnosis to start of radiotherapy were independently associated with fractionation choice.
Conclusions
There was a striking increase in the use of 26Gy5F dose fractionation regimens for EBC, among women aged ≥50 years, within a month of guidance published at the start of the COVID-19 pandemic in England and Wales.
Key words: COVID-19, early breast cancer, hypofractionated radiotherapy, older patient
Introduction
Adjuvant postoperative radiotherapy is a well-established treatment for early breast cancer (EBC). The benefits of its use include reducing the risk of local recurrence. It also significantly decreases death after breast-conserving surgery (BCS) and in patients at intermediate/high risk of recurrence after mastectomy [1,2]. These benefits are balanced against risks of both short- and long-term adverse effects. In the UK, the National Institute for Health and Care Excellence (NICE) recommends that adjuvant treatment decisions should be based on a balance between the risks and benefits of radiotherapy, particularly in patients with comorbidities [3]. Adjuvant radiotherapy is advised for most patients who receive BCS for invasive breast cancer, apart from where the risk of local recurrence is determined to be low (defined as women aged 65 years and older with tumours that are stage 1, node-negative [T1N0], oestrogen receptor [ER]-positive, human epidermal growth factor receptor 2 [HER2]-negative and grade 1 to 2 who are planned to receive 5 years of adjuvant endocrine therapy) [4].
Following evidence generated by the UK Standardisation of Breast Radiotherapy (START) Trial B in 2009, the standard radiotherapy regimen offered to patients in the UK was 40 Gy in 15 fractions (40Gy15F), as it was found to be non-inferior to a previously commonly used regimen of 50 Gy in 25 fractions (50Gy25F) [5,6]. The FAST and FAST-Forward randomised controlled trials both studied the use of five-fraction regimens, with primary analysis being based on non-inferiority to standard 25- and 15-fraction regimens, respectively. Ten-year results from the FAST trial and 5-year results from the FAST-Forward trial for EBC have both shown five fractions to be non-inferior and associated with a low risk of normal tissue effects [7,8].
In March 2020, National Health Service (NHS) organisations were instructed to reduce any unnecessary patient hospital attendance and routine NHS activity in order to support the COVID-19 effort, and (alongside guidance from NICE on the prioritisation of radiotherapy) the Royal College of Radiologists (RCR) published guidance on 24 March 2020 recommending radiotherapy delivery in five fractions over 1 week for all suitable patients [9,10].
Amid uncertainty as to whether the results of the FAST and FAST-Forward trials are applicable to all biological and clinical subsets of patients, and caution from some that this new five-fraction regimen may lack comparable long-term efficacy data, some variation between UK centres in the use of this new regimen would be expected [[11], [12], [13]]. Previous publications have reported on this change to using five-fraction regimens in the UK, but have focused on the national picture, with limited breakdown by subgroups of patient age or geographical region [14,15]. As such, there is limited understanding of the impact of this RCR guidance within the older population of patients with breast cancer. The aim of this study was to investigate temporal and quantitative changes in radiotherapy regimens across England and Wales, using patient-level routine data to examine variation in use by patient/tumour characteristics as well as by geographical region, in women aged 50 years and over diagnosed with EBC. Additionally, we considered factors associated with the receipt of 26 Gy in five fractions (26Gy5F) and include radiotherapy target site.
Materials and Methods
Data
This population-based cohort study was undertaken as part of the National Audit of Breast Cancer in Older Patients (NABCOP). Data for this study are based on patient-level information collected by the NHS, as part of the care and support of patients with cancer. The data for England are collated, maintained and quality assured by the National Cancer Registration and Analysis Service (NCRAS), formerly part of Public Health England and now part of NHS Digital (NHSD). Patient-level data from the Rapid Cancer Registration Dataset (RCRD) were provided, linked to the Hospital Episode Statistics (HES) database, which captures all NHS hospital admissions, for details of surgical procedures; and the national Radiotherapy Dataset (RTDS) for information on radiotherapy. The RCRD represents a relatively new initiative from NCRAS, where contemporaneous data on patients with cancer are provided using a proxy-registration model [16]. Data for Wales are collated, maintained and quality assured by the Wales Cancer Network. Cancer registration, patient-level data were provided, linked to the Patient Episode Database Wales (PEDW), which captures all NHS hospital admissions, for details of surgical procedure, and national radiotherapy data for full information on radiotherapy. The study was exempt from UK National Research Ethics Committee approval as it involved secondary analysis of an existing dataset of pseudonymised data. The NABCOP has approval for processing healthcare information under Section 251 (reference number: 16/CAG/0079) for all NHS patients aged 50 years and over diagnosed with breast cancer in England and Wales.
Study Population
Linked, pseudonymised patient records were provided for all women aged 50 years and over with a diagnosis of breast cancer between 1 January 2019 and 31 July 2020, diagnosed and treated within a NHS trust in England or local health board in Wales. For this analysis, we identified all women receiving surgery for EBC (stage 0–3A International Union Against Cancer [UICC] Tumour Node Metastasis [TNM] staging classification, seventh edition) [17]. Primary surgery was defined as either BCS or mastectomy that occurred within 6 months of the date of diagnosis and was identified from Office of Population Censuses and Surveys procedure codes entered within HES/PEDW patient records (see Supplementary Table S1) [18]. Patients were allocated to the NHS organisation where they were diagnosed. If this information was unavailable, the NHS organisation of surgery was used.
Adjuvant Radiotherapy
The national radiotherapy data provided the date of adjuvant radiotherapy and details of the dose. Treatment with radiotherapy was defined as adjuvant where the first dose was given following surgery (with or without chemotherapy). The radiotherapy target site (region) was provided within pre-defined categories in the English RTDS data (primary only, primary + regional nodes, regional nodes only, metastasis, prophylactic to non-primary site, anatomically non-specific primary); this was not available in the Welsh national radiotherapy data. For patients diagnosed and treated in England, the RTDS includes details on multiple radiotherapy prescriptions and episodes; this level of detail was not available in the radiotherapy data provided for patients diagnosed and treated in Wales. For patients in England, estimated rates of a radiotherapy boost were lower than would be expected in this cohort (<5% compared with around 25% in the FAST-Forward trial cohort) and so this information is not presented.
Sociodemographic and Clinicopathological Variables
The following factors were considered likely to be associated with radiotherapy dosing decisions: age at diagnosis (years), stage (0, 1, 2, 3A), tumour stage (T1, T2, T3), nodal involvement (N0/NX, N+), ER/HER2 status (positive, negative), tumour grade (1, 2, 3, X), type of surgery (BCS, mastectomy among women with N0, small tumours), number of comorbidities (0, 1, 2+), patient fitness (fit, mild-moderate frailty, severe frailty), time from diagnosis to the start of radiotherapy (<3 months, 3 months, 4 months, 5 months, 6 months and over) and whether the patient was diagnosed at an organisation that had recruited patients to the FAST or FAST-Forward trials (no, yes). Additionally, we looked at whether use of 26Gy5F differed by surgical laterality (left, right) based on a previous study published in 2016 that found a difference in adoption of 15–16 fractions according to laterality [19].
Tumour characteristics were not included in the RCRD and therefore were only available for patients in Wales. Consequently, for patients in England, an axillary node dissection within HES or having stage 3A disease (N stage contribution defined as having N1/N2 disease) were used as a proxy for nodal involvement. Unknown nodal involvement was defined as stage 0–2B disease with no recorded axillary node dissection for women in England. For the analysis of post-mastectomy radiotherapy, only women with high-risk breast cancer (defined as N+ or T3N0) were included. The high-risk group for England was defined where women had an axillary node dissection or stage 3A disease recorded (proxies for nodal involvement) or where women had stage 2B disease recorded (where T stage and N stage contributions were T3N0 or N1 disease). Stage was available for both England and Wales. Models considering factors of tumour stage, grade, ER/PR/HER2 status were fitted in the Welsh cohort of patients only.
Social deprivation was measured using the Index of Multiple Deprivation 2019 rank, which was derived from the patient's postcode at diagnosis. The Index of Multiple Deprivation rank was grouped into quintiles from most (group 1) to least (group 5) income deprived. Comorbidity burden was defined using the Royal College of Surgeon's Charlson Comorbidity Index [20]. This counts the presence of specific chronic medical conditions (excluding malignancy), identified using ICD-10 diagnosis codes within patient HES/PEDW records for a period of 2 years prior to diagnosis. Patient fitness was defined using the Secondary Care Administrative Records Frailty index [21]. This describes frailty in relation to 32 different symptoms, signs, diseases and disabilities (referred to as deficits), identified using ICD-10 diagnosis codes within patient HES/PEDW records for a period of 2 years prior to diagnosis.
Statistical Analysis
The percentage of women who received radiotherapy, and of these the percentage who received five-fraction hypofractionated radiotherapy (HFRT), was calculated for the overall cohort and within patient subgroups. These were used to investigate the use of radiotherapy over time and the change to 26Gy5F in 2020. Patients were grouped by week or month based on the date of surgery. The statistical significance of differences between group percentages was assessed using a chi-squared test. Regional variation was examined graphically using the 10 Government Office Regions covering England and Wales.
A multilevel mixed-effects logistic regression model was developed to evaluate the relationship between receipt of five-fraction radiotherapy and associated factors, and was fitted in women who started radiotherapy between April and July 2020. The model included age at diagnosis, patient fitness, number of comorbidities, overall stage, nodal involvement, type of surgery and time since diagnosis. A model fitted only to Welsh patients examined the relationship with the same variables (except overall stage) with the addition of tumour stage, ER/PR/HER2 status and invasive grade. Categories of ‘unknown’ were created where data items had missing, unintelligible or conflicting information.
A multilevel model was used to account for the clustering of patients within geographical regions (Cancer Alliances for England and Wales) [22] due to the relatively low levels of activity at NHS organisations. Each geographical region was fitted as a random intercept, which allowed for the average use of five-fraction radiotherapy to vary between regions.
All data preparation and statistical analyses were conducted using Stata version 17.0.
Results
Of 38 165 women aged 50 years and over diagnosed with EBC in England and Wales and receiving surgery between January 2019 and July 2020, 93% (n = 35 651) had initial surgery within 6 months of diagnosis (of which the majority received surgery within 3 months of diagnosis) and were alive for more than 6 months following initial surgery. Full details of patient selection are shown in Supplementary Figure S1.
Use of Postoperative Radiotherapy in 2020 Compared with 2019
Among 35 651 women receiving surgery for EBC, 71% (n = 25 207) went on to receive radiotherapy. Comparing women receiving surgery in January to July 2020 with those receiving surgery in the same months in 2019, fewer women went on to receive radiotherapy; 68% (n = 7347/10 812) in 2020 compared with 72% (n = 10 333/14 353) in 2019 (P < 0.001). There were also lower rates of radiotherapy in 2020 compared with 2019, which were largely explained by a reduction in the percentage use of radiotherapy among women who had surgery between January and March 2020.
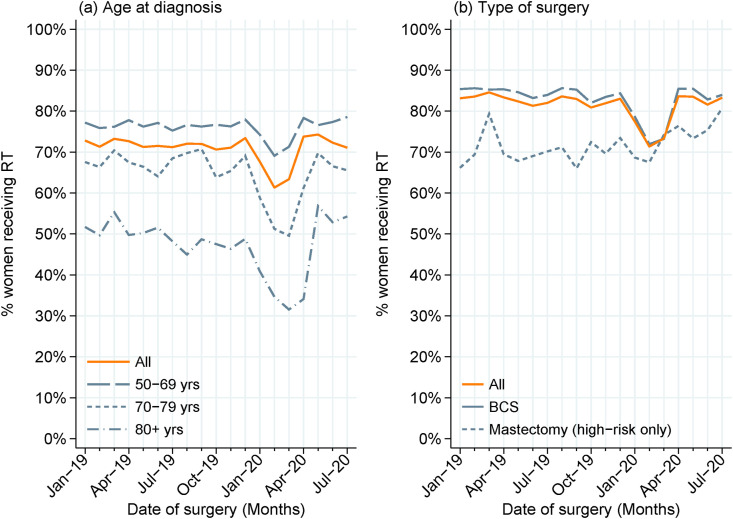
The monthly percentage of women receiving postoperative radiotherapy followed similar patterns across subgroups according to patient fitness (fit versus any frailty). Radiotherapy use in 2020 compared with 2019 was most reduced among older women (Figure 1 a: 70–79 years 59% versus 67%; 80+ years 42% versus 51%) and women having BCS (Figure 1b: 79% versus 85%, P < 0.001). Additionally, radiotherapy use was lower among women with stage 0 EBC (44% in 2020 versus 51% in 2019).
Fig 1.
Use of adjuvant radiotherapy over time, among women receiving surgery for early breast cancer from January 2019 to July 2020, overall and by (a) age at diagnosis and (b) type of surgery. Note: high-risk defined as N+ or T3N0 for women diagnosed in Wales, N+ or stage 2A/3B for women diagnosed in England.
Among women receiving postoperative radiotherapy, the median time between the first surgical resection and the first radiotherapy fraction was slightly shorter among women receiving surgery between April and July 2020, at 10.0 weeks compared with 11.0 weeks among those receiving surgery between April and July 2019 (Supplementary Figure S2a). This pattern was seen among women receiving postoperative radiotherapy in subgroups defined according to patient fitness, age, surgical procedure and stage, as well as among women not having chemotherapy (Supplementary Figure S2c).
Among women who had radiotherapy after having surgery between April and July 2020, the time from initial surgery to starting radiotherapy was typically shorter among those women receiving the 26Gy5F regimen, with the median being 9.1 weeks compared with 16.7 weeks among women starting 40Gy15F (Supplementary Figure S2b). Among women not having chemotherapy, the time from initial surgery to radiotherapy was more comparable, with median times of 8.4 weeks for women receiving 26Gy5F and 9.9 weeks for women receiving 40Gy15F (Supplementary Figure S2d). Of women receiving 26Gy5F, 73% had not had chemotherapy, compared with 39% of women receiving 40Gy15F; this difference was seen regardless of the month of surgery.
Uptake of Ultra-hypofractionated Regimen (26 Gy in Five Fractions over 1 Week) (B Head)
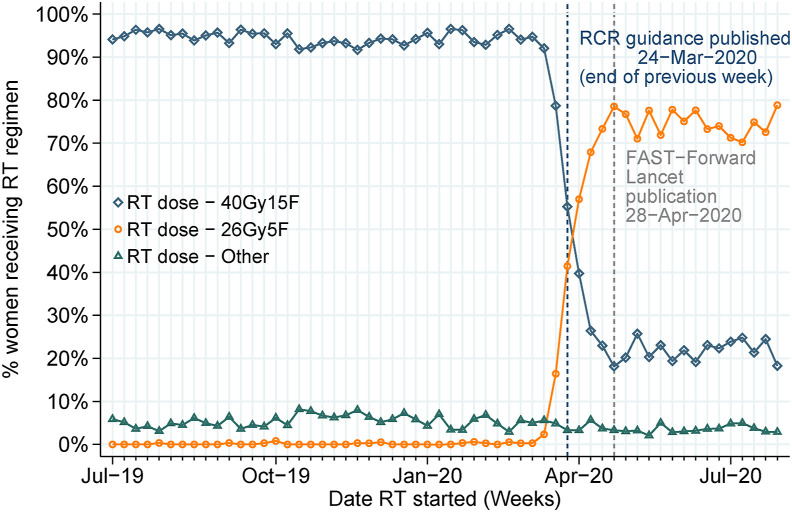
Figure 2 shows the change in reported radiotherapy regimen across July 2019 to July 2020. Where radiotherapy started before February 2020, over 90% was given as 40Gy15F; this dropped to 23% when radiotherapy started between April and July 2020. By contrast, the receipt of 26Gy5F increased from less than 1% of patients starting radiotherapy in February 2020, to 70% in April 2020. Notably, within 3 weeks of the RCR publishing the guidance recommending radiotherapy delivery in five fractions over 1 week for suitable patients (published online 24 March 2020), two-thirds of patients starting radiotherapy received 26Gy5F, increasing to around 80% by the time the FAST-Forward trial results were published online (28 April 2020).
Fig 2.
Reported radiotherapy regimen among women receiving postoperative radiotherapy between July 2019 and July 2020.
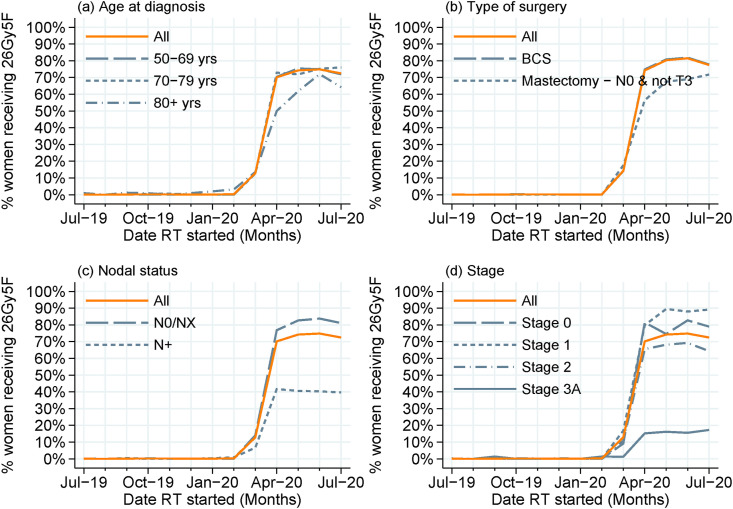
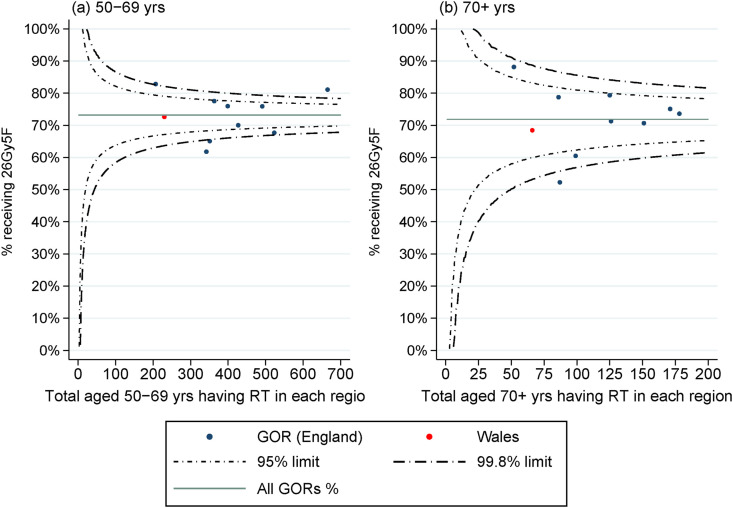
The change of use to 26Gy5F was initially slower within the group of women aged 80 years and over (Figure 3 a) and among those with no known nodal involvement or T3 disease who had a mastectomy (Figure 3b). Use was consistently lower within the group of women who had nodal involvement (Figure 3c), and among those with stage 3A breast cancer, characterised by larger tumours or greater nodal involvement (Figure 3d). The variation across the 10 Government Office Regions in the uptake of 26Gy5F (Figure 4 ) was similar for each age group (noting smaller numbers in the older age group), with percentage use across April to July 2020 ranging from 62% to 83% among women aged 50–69 years and from 49% to 87% among women aged 70 years and over. The lowest rates were seen within one geographical region, where one NHS organisation implemented the breast dose and fractionation change later than other providers, as such use was low in both age groups.
Fig 3.
Reported use of 26 Gy in five fractions (26Gy5F) radiotherapy regimen among women receiving postoperative radiotherapy between July 2019 and July 2020, overall and by (a) age at diagnosis, (b) type of surgery, (c) nodal status and (d) stage. Note: for women diagnosed in England, axillary node dissection recorded within Hospital Episode Statistics or having stage 3A disease (N stage contribution defined as having N1/N2 disease) were used as a proxy for nodal involvement (N+); coded as NX otherwise. ‘Mastectomy – N0 & not T3’ = use following mastectomy for N0 small tumours (<T3) being the cohort for whom 26Gy5F would be the most appropriate.
Fig 4.
Risk-adjusted percentage of women having 26 Gy in five fractions (26Gy5F) radiotherapy regimen among women receiving postoperative radiotherapy between April and July 2020, shown by Government Office Region (GOR), for (a) women aged 50–69 years and (b) women aged 70+ years. Note: percentages risk-adjusted for age at diagnosis, Charlson score, overall stage, nodal involvement, primary surgery and time since diagnosis.
Among 5139 women receiving postoperative radiotherapy between April and July 2020, 73% (n = 3746) received 26Gy5F. Table 1 describes the patient cohort receiving 26Gy5F. The common characteristics were no known nodal involvement, no comorbidities, initial BCS and no chemotherapy. Most women had been diagnosed within the previous 4 months. Additionally, for the Welsh cohort, there were high percentages of use of 26Gy5F among higher invasive grade, tumour stage 1 disease, PR-positive breast cancer and ER-positive HER2-negative breast cancer (Supplementary Table S2). These characteristics were largely consistent regardless of patient age, although there were some minor differences in the use of 26Gy5F among older women in relation to mastectomy, no chemotherapy, use for stage 2 disease and higher levels of comorbidity/frailty. Comparable use of 26Gy5F was observed whether the tumour was on the left (73%) or right (74%). Over two-thirds (71%) of women receiving postoperative radiotherapy between April and July 2020 were diagnosed at NHS organisations that comprised centres participating in the FAST or FAST-Forward trials and the use of 26Gy5F was not associated with trial involvement.
Table 1.
Cohort characteristics among women receiving 26 Gy in five fractions (26Gy5F) between April and July 2020, by country and age at diagnosis
| Wales |
England |
|||||||
|---|---|---|---|---|---|---|---|---|
| All n = 213 | 50–59 years n = 86 | 60–69 years n = 84 | 70+ years n = 43 | All n = 3533 | 50–59 years n = 1441 | 60–69 years n = 1315 | 70+ years n = 777 | |
| Charlson Comorbidity Index | ||||||||
| Reported | 98% | 99% | 98% | 98% | 99% | 99% | 100% | 100% |
| 0 | 93% | 96% | 94% | 86% | 90% | 93% | 90% | 86% |
| 1 | 6% | 2% | 6% | 14% | 8% | 6% | 9% | 10% |
| 2+ | 0% | 1% | 0% | 0% | 2% | 1% | 2% | 4% |
| SCARF Index | ||||||||
| Reported | 98% | 99% | 98% | 98% | 99% | 99% | 100% | 100% |
| Fit | 87% | 92% | 88% | 76% | 83% | 89% | 82% | 75% |
| Mild-moderate | 13% | 8% | 12% | 24% | 15% | 11% | 17% | 22% |
| Severe | 0% | 0% | 0% | 0% | 2% | 1% | 1% | 4% |
| Stage | ||||||||
| Stage 0 | 5% | 5% | 7% | 2% | 10% | 13% | 9% | 8% |
| Stage 1 | 53% | 52% | 56% | 47% | 52% | 54% | 55% | 42% |
| Stage 2 | 40% | 43% | 36% | 44% | 37% | 32% | 35% | 47% |
| Stage 3A | 2% | 0% | 1% | 7% | 2% | 1% | 1% | 3% |
| Nodal involvement∗ | ||||||||
| N0 | 81% | 83% | 82% | 77% | - | - | - | - |
| N+ | 19% | 17% | 18% | 23% | 11% | 10% | 10% | 14% |
| NX | 0% | 0% | 0% | 0% | 89% | 90% | 90% | 86% |
| Surgery type | ||||||||
| Breast-conserving surgery | 92% | 92% | 93% | 88% | 87% | 88% | 90% | 82% |
| Mastectomy | 9% | 8% | 7% | 12% | 8% | 7% | 7% | 14% |
| Missing | 0% | 0% | 0% | 0% | 4% | 5% | 4% | 4% |
| Chemotherapy | ||||||||
| Neoadjuvant chemotherapy | 9% | 8% | 8% | 9% | 3% | 4% | 4% | 2% |
| Adjuvant chemotherapy | 22% | 24% | 20% | 21% | 22% | 24% | 23% | 17% |
| No chemotherapy | 70% | 67% | 71% | 70% | 74% | 72% | 73% | 81% |
| Radiotherapy target site | ||||||||
| Primary | - | - | - | - | 93% | 94% | 94% | 92% |
| Primary + regional nodes | - | - | - | - | 4% | 4% | 4% | 7% |
| Other/unknown | - | - | - | - | 2% | 3% | 2% | 2% |
| Time from diagnosis to radiotherapy | ||||||||
| <3 months | 24% | 24% | 21% | 26% | 24% | 25% | 22% | 24% |
| 3 months | 32% | 30% | 36% | 30% | 30% | 30% | 30% | 29% |
| 4 months | 15% | 15% | 17% | 9% | 15% | 14% | 15% | 18% |
| 5 months | 9% | 6% | 10% | 14% | 8% | 7% | 8% | 11% |
| 6 months and over | 21% | 24% | 17% | 21% | 24% | 25% | 25% | 18% |
SCARF, Secondary Care Administrative Records Frailty.
For women diagnosed in England, axillary node dissection recorded within Hospital Episode Statistics or having stage 3A disease (N stage contribution defined as having N1/N2 disease) were used as a proxy for nodal involvement (N+); coded as NX otherwise.
The use of 26Gy5F in women receiving radiotherapy between April and July 2020 was independently associated with nodal involvement (lower odds for N+), overall stage (increased odds for stage 1), type of surgery (lower odds for mastectomy) and time from diagnosis (months; <3/3/4/5/6+; lower odds as time from diagnosis increased). Adding hormone status, tumour stage and grade to the model that used data from patients in Wales, these tumour characteristics were not found to be associated with the use of 26Gy5F.
Comparing radiotherapy starting in England between April and July 2020 with radiotherapy starting between April and July 2019, there was little difference in radiotherapy target site, with 80% (April to July 2020) of radiotherapy only to the breast or post-mastectomy chest wall and 13% (April to July 2020) additionally including regional nodes. However, for radiotherapy given between April and July 2020 among women having radiotherapy only to the breast or post-mastectomy chest wall, 85% had 26Gy5F and 15% had 40Gy15F. By contrast, where radiotherapy was given to the breast plus regional nodes, 23% had 26Gy5F, whereas 76% had 40Gy15F.
Discussion
This population-based study used routinely collected patient-level data from England and Wales to examine the use of radiotherapy in more than 35 000 women aged 50 years and over receiving surgery for EBC across 2019 and the first 7 months of 2020, together with the initial change in radiotherapy regimens from 40Gy15F to 26Gy5F among women starting radiotherapy between April and July 2020. We found a lower percentage of women with EBC received radiotherapy following surgery between January and July 2020 (68%) compared with January and July 2019 (72%), with a larger difference among older women (aged 70+ years) and those women having initial BCS. The adoption of the 26Gy5F dose fractionation regimen was seen to occur rapidly in response to national guidance related to the COVID-19 pandemic, with two-thirds of those patients having radiotherapy commencing the 26Gy5F regimen within just 3 weeks of published RCR guidance. Although this study looked only at women aged 50 years and over, given the age inclusion for the NABCOP, the characteristics of the patient cohort commencing 26Gy5F were for the most part in line with the characteristics of the patients enrolled in the FAST-Forward trial, which showed non-inferiority of the five-fraction regimen. Specifically, use of 26Gy5F radiotherapy was predominantly in patients with no recorded nodal involvement (81–89% compared with 81% in the trial) and those having BCS (87–92% compared with 94% in the trial). However, 23% of patients who received radiotherapy to the breast and regional nodes were treated with 26Gy5F, despite this regimen primarily being recommended in node-negative patients and nodal radiotherapy not being permitted within the main FAST-Forward study [10]. Negative/unknown nodal status, stage, type of surgery and time from diagnosis were all found to be associated with the use of 26Gy5F among women starting radiotherapy between April and July 2020. Within this cohort of women aged 50 years and over, less than 0.5% were reported to have received 27Gy5F or 28–30Gy5F.
The findings in this study are in agreement with similar research in a UK setting, which has shown rapid adoption in the use of five-fraction radiotherapy regimens. The B-MaP-C study, conducted in 64 UK centres, described changes in breast cancer management during the initial phase of the COVID-19 pandemic, and found that 72% (n = 46/64) were offered five-fraction radiotherapy [14]. A population-based study using RTDS data for patients treated in England examined changes in radiotherapy during the first peak in the COVID-19 pandemic (up to 28 June 2020) and reported a reduction in the number of attendances for breast cancer radiotherapy in 2020, compared with 2019, whilst the use of five-fraction regimens increased to 60.6% in April 2020. They also showed a change in fractionation pattern, with the striking crossover from 15 fractions to five fractions, similar to that demonstrated in this study (Figure 2) [15]. A letter on the impact of COVID-19 on radiotherapy demand in South-East Wales reported that, among patients treated in the first 3 weeks following the first lockdown, 48% received 26Gy5F [23]. Similarly, the Guys Cancer Centre in London reported that among patients having radiotherapy for EBC between 1 March and 7 May 2020, and deemed suitable for ultra-HFRT, 53% (n = 64) received the FAST-Forward regimen [24]. Among studies with a wider country setting, or from single centres outside of the UK, reported use of ultra-HFRT was variable. A survey of 377 breast units from 41 countries (including around 14 from the UK) in April 2020 aiming to ‘provide a real time international snapshot of modifications of breast cancer management during the COVID-19 pandemic’ found that only 28 respondents (7%) said radiotherapy was modified to be given in five fractions. However, no breakdown was given by country [25]. A national survey of radiation oncology departments in Switzerland in April 2020 reported that only one of 22 responding units said they used ultra-HFRT for breast cancer [26]. A survey of 176 radiation oncology department directors in Italy in April 2020 reported that 73.6% of centres promoted the use of HFRT regimens, although this was not specific to breast cancer and it was not clear whether this was moderate or ultra-HFRT. An Italian single-centre study using prospective data collected in May and June 2020 reported that 56.8% of patients with breast cancer received five-fraction radiotherapy regimens [27,28]. A survey with responses from 45 Senology International Society members across 24 countries (0 in the UK) in June and July 2020 reported that 29% of participants reported that radiotherapy protocols were modified to reduce hospital stays (using HFRT), although it was not clear whether this was to moderate or ultra-HFRT [29]. Results from studies in the USA have also reported variable uptake of ultra-HRFT [30,31].
The present study had a number of strengths. It used a large, population-based sample including women diagnosed prior to and through the beginning of the COVID-19 pandemic in NHS organisations across Wales and England. The data also included details on radiotherapy use including attendances, dose and radiotherapy region (the latter being for England only).
However, the study also had some limitations. First, data on tumour characteristics were limited within the RCRD for England, and in particular there was no record of nodal status. The record of axillary nodal dissection or stage 3A disease was used to infer nodal involvement and this probably underestimated the percentage of the English cohort who had node-positive EBC as well as misclassified a small percentage of women as having node-positive EBC. Although these data items were available for Wales, the smaller numbers of patients diagnosed and treated means the results from the analysis of Welsh data is less precise and limits the inferences from the data. Second, the case-ascertainment for the RCRD may be incomplete for some NHS trusts. Although this might produce lower absolute rates of radiotherapy use, there is no a priori reason to believe case-ascertainment would differ by patient or tumour characteristics or by radiotherapy regimen. Another limitation is that details on a radiotherapy boost were limited to England. The use of a boost may have been affected by the COVID-19 pandemic as the RCR guidance published at the start of the pandemic recommended ‘Boost RT should be omitted to reduce fractions and/or complexity in the vast majority of patients unless they are 40 years old and under, or over 40 years with significant risk factors for local relapse’. Information on the risk factors for recurrence were not available in the data used for this analysis and so it was not possible for this study to make any comment on whether the pandemic influenced the use of tumour site boost. Although the study described the use of chemotherapy in the cohort of patients prescribed 26Gy5F, we did not look at the use of other systemic anti-cancer therapies, such as endocrine therapy, largely as these are not well reported within the routine data available. However, use of chemotherapy is probably correlated with the time from diagnosis to radiotherapy, and may explain in part the association between the time from diagnosis and use of 26Gy5F. Finally, the study cohort included women diagnosed with stage 0 breast cancer (or ductal carcinoma in situ), a patient group not included in the FAST-Forward trial. However, at a meeting in October 2020 where the UK breast cancer radiotherapy community voted for five-fraction radiotherapy to be the new national standard for breast radiotherapy, the consensus was that patients with ductal carcinoma in situ could still be offered 26Gy5F for whole-breast radiotherapy [32,33].
Conclusions
The findings of this study show the rapid adoption of the recent randomised controlled trial evidence-based change in traditional adjuvant radiotherapy regimens among women aged 50 years and over diagnosed with EBC across both England and Wales. Seven in 10 women who had radiotherapy in April 2020 received the 26Gy5F ultra-HFRT regimen, just a month after the publication of the RCR guidance recommending the ultra-HFRT regimen. The rapid introduction of the new protocols supported by publication of RCR guidance reflects a striking COVID-19 pandemic-related national health delivery need for alignment of maximum efficiency of radiotherapy resources and minimisation of patient hospital visits coinciding with the publication of trials showing equivalence from less resource demanding treatment schedules. Further work will be needed to establish whether this change is sustained when the pandemic wanes and longer-term data of the efficacy and toxicity of ultra-HFRT dose regimens become available. Outputs from the COVID-19 radiotherapy initiative should provide more comprehensive findings on the full impact of changes in radiotherapy on subsequent patient outcomes [34]. Additionally, it would be valuable to examine the considerable financial impact of a move to five-fraction over a week regimens, as has been noted in several publications [14,35,36].
Data Availability
This work used data that have been provided by patients and collected by the NHS as part of their care and support. The data for England are collated, maintained and quality assured by the National Disease Registration Service (NDRS), which is part of NHS Digital. No additional data are available. Data on English Rapid Cancer Registrations can be accessed via the Office for Data Release. https://www.ndrs.nhs.uk/odr/. The data for Wales are collated, maintained and quality assured by the Wales Cancer Network.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This study was undertaken as part of the work by the National Audit of Breast Cancer in Older Patients (NABCOP). The NABCOP is commissioned by the Healthcare Quality Improvement Partnership (HQIP) as part of the National Clinical Audit and Patient Outcomes Programme and funded by NHS England and the Welsh Government (http://www.hqip.org.uk/national-programmes). Neither the commissioner nor the funders had any involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the article for publication. The authors had full independence from the HQIP. The aim of the NABCOP is to evaluate the care of older women with breast cancer in England and Wales, and support NHS providers to improve the quality of hospital care for these women. More information can be found at http://www.nabcop.org.uk/.
Author Contributions
MRG is the guarantor of integrity of the entire study. MRG, DD, KM, KH, KC, JM, IK and DAC were responsible for study concepts and design. MRG was responsible for literature research and manuscript preparation. MRG, DD, KM, KH, KC, JM, IK and DAC edited the manuscript. All authors were involved in data interpretation, critical appraisal of the draft manuscript and gave final approval on the version to be published.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2022.05.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M., et al. Early Breast Cancer Trialists’ Collaborative Group Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/s0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M., et al. Early Breast Cancer Trialists’ Collaborative Group Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/s0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NICE Early and locally advanced breast cancer: diagnosis and management. 2018. www.nice.org.uk/guidance/ng101 Available at:
- 4.Kunkler I.H., Williams L.J., Jack W.J., Cameron D.A., Dixon J.M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–273. doi: 10.1016/s1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 5.Bentzen S.M., Agrawal R.K., Aird E.G., Barrett J.M., Barrett-Lee P.J., Bentzen S.M., et al. The START Trialists’ Group The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098–1107. doi: 10.1016/s0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarnold J. Changes in radiotherapy fractionation – breast cancer. Br J Radiol. 2019;92(1093) doi: 10.1259/bjr.20170849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunt A.M., Haviland J.S., Sydenham M., Agrawal R.K., Algurafi H., Alhasso A., et al. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38(28):3261–3272. doi: 10.1200/jco.19.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunt A.M., Haviland J.S., Wheatley D.A., Sydenham M.A., Alhasso A., Bloomfield D.J., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395(10237):1613–1626. doi: 10.1016/s0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Royal College of Radiologists Coronavirus (COVID-19): cancer treatment documents. 2020. https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-oncology-resources/coronavirus-covid-19-cancer Available at:
- 10.Coles C.E., Aristei C., Bliss J., Boersma L., Brunt A.M., Chatterjee S., et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol. 2020;32(5):279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krug D., Baumann R., Combs S.E., Duma M.N., Dunst J., Feyer P., et al. Moderate hypofractionation remains the standard of care for whole-breast radiotherapy in breast cancer: considerations regarding FAST and FAST-Forward. Strahlenther Onkol. 2021;197(4):269–280. doi: 10.1007/s00066-020-01744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy A., Rivera S. 1-week hypofractionated adjuvant whole-breast radiotherapy: towards a new standard? Lancet. 2020;395(10237):1588–1589. doi: 10.1016/s0140-6736(20)30978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Untch M., Fasching P.A., Brucker S.Y., Budach W., Denkert C., Haidinger R., et al. Treatment of patients with early breast cancer: evidence, controversies, consensus: German Expert Opinions on the 17th International St. Gallen Consensus Conference. Geburtshilfe Frauenheilkd. 2021;81(6):637–653. doi: 10.1055/a-1483-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dave R.V., Kim B., Courtney A., O'Connell R., Rattay T., Taxiarchi V.P., et al. Breast cancer management pathways during the COVID-19 pandemic: outcomes from the UK 'Alert Level 4' phase of the B-MaP-C study. Br J Cancer. 2021;124(11):1785–1794. doi: 10.1038/s41416-020-01234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer K., Jones C.M., Girdler R., Roe C., Sharpe M., Lawton S., et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22(3):309–320. doi: 10.1016/s1470-2045(20)30743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Registration and Analysis Service (NCRAS) Rapid Cancer Registration Dataset. http://www.ncin.org.uk/collecting_and_using_data/rcrd Available at:
- 17.International Union Against Cancer (UICC) seventh edition. Wiley-Blackwell; Oxford: 2011. TNM classification of malignant tumours. [Google Scholar]
- 18.NHS Digital Classifications browser. https://classbrowser.nhs.uk/#/ Available at:
- 19.Delaney G.P., Gandhidasan S., Walton R., Terlich F., Baker D., Currow D. The pattern of use of hypofractionated radiation therapy for early-stage breast cancer in New South Wales, Australia, 2008 to 2012. Int J Radiat Oncol Biol Phys. 2016;96(2):266–272. doi: 10.1016/j.ijrobp.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Armitage J.N., van der Meulen J.H. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg. 2010;97(5):772–781. doi: 10.1002/bjs.6930. [DOI] [PubMed] [Google Scholar]
- 21.Jauhari Y., Gannon M.R., Dodwell D., Horgan K., Clements K., Medina J., et al. Construction of the secondary care administrative records frailty (SCARF) index and validation on older women with operable invasive breast cancer in England and Wales: a cohort study. BMJ Open. 2020;10(5) doi: 10.1136/bmjopen-2019-035395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Macmillan. Alliances: a crucial first step. 2015. www.macmillan.org.uk/documents/campaigns/canceralliancesreport.pdf Available at:
- 23.Higgins E., Walters S., Powell E., Staffurth J. The impact of the acute phase of COVID-19 on radiotherapy demand in South East Wales. Clin Oncol. 2020;32(10):e217. doi: 10.1016/j.clon.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanumanthappa N., Goldsmith C., Sawyer E., Tutt A., Castell F., Azad G., et al. Adjuvant breast radiotherapy at an academic centre during the COVID-19 pandemic: reassuringly safe. Clin Oncol. 2021;33(4):e221. doi: 10.1016/j.clon.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparri M.L., Gentilini O.D., Lueftner D., Kuehn T., Kaidar-Person O., Poortmans P. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: an international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) Breast. 2020;52:110–115. doi: 10.1016/j.breast.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achard V., Aebersold D.M., Allal A.S., Andratschke N., Baumert B.G., Beer K.T., et al. A national survey on radiation oncology patterns of practice in Switzerland during the COVID-19 pandemic: present changes and future perspectives. Radiother Oncol. 2020;150:1–3. doi: 10.1016/j.radonc.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jereczek-Fossa B.A., Pepa M., Marvaso G., Bruni A., Buglione di Monale E.B.M., Catalano G., et al. COVID-19 outbreak and cancer radiotherapy disruption in Italy: survey endorsed by the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Radiother Oncol. 2020;149:89–93. doi: 10.1016/j.radonc.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galofaro E., Malizia C., Ammendolia I., Galuppi A., Guido A., Ntreta M., et al. COVID-19 pandemic-adapted radiotherapy guidelines: are they really followed? Curr Oncol. 2021;28(5):3323–3330. doi: 10.3390/curroncol28050288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathelin C., Ame S., Anyanwu S., Avisar E., Boubnider W.M., Breitling K., et al. Breast cancer management during the COVID-19 pandemic: the Senologic International Society Survey. Eur J Breast Health. 2021;17(2):188–196. doi: 10.4274/ejbh.galenos.2021.2021-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrigan K.L., Lei X., Ahmad N., Arzu I., Bloom E., Chun S.G., et al. Adoption of ultrahypofractionated radiation therapy in patients with breast cancer. Adv Radiat Oncol. 2022;7(2) doi: 10.1016/j.adro.2021.100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckstein J., Taylor P., Zheng R., Lee L., Chen W., Potters L., et al. Implementation of external beam five-fraction adjuvant breast irradiation in a US center. Cancers. 2022;14(6):1556. doi: 10.3390/cancers14061556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Royal College of Radiologists Postoperative radiotherapy for breast cancer: hypofractionation RCR consensus statements. 2021. https://www.rcr.ac.uk/publication/postoperative-radiotherapy-breast-cancer-hypofractionation-rcr-consensus-statements Available at:
- 33.Brunt A.M., Haviland J.S., Kirby A.M., Somaiah N., Wheatley D.A., Bliss J.M., et al. Five-fraction radiotherapy for breast cancer: FAST-Forward to implementation. Clin Oncol. 2021;33(7):430–439. doi: 10.1016/j.clon.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Lewis P.J., Morris E.J.A., Chan C.S.K., Darley K., Sebag-Montefiore D., Evans M. COVID RT - assessing the impact of COVID-19 on radiotherapy in the UK. A National Cancer Research Institute Clinical and Translational Radiotherapy Research Working Group initiative in partnership with the Royal College of Radiologists, the Society of Radiographers and the Institute of Physics and Engineering in Medicine. Clin Oncol. 2021;33(1):e69–e72. doi: 10.1016/j.clon.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson D.J., Yom S.S., Saeed H., El Naqa I., Ballas L., Bentzen S.M., et al. Radiation fractionation schedules published during the COVID-19 pandemic: a systematic review of the quality of evidence and recommendations for future development. Int J Radiat Oncol Biol Phys. 2020;108(2):379–389. doi: 10.1016/j.ijrobp.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaremko H.L., Locke G.E., Chow R., Lock M., Dinniwell R., Yaremko B.P. Cost minimization analysis of hypofractionated radiotherapy. Curr Oncol. 2021;28(1):716–725. doi: 10.3390/curroncol28010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This work used data that have been provided by patients and collected by the NHS as part of their care and support. The data for England are collated, maintained and quality assured by the National Disease Registration Service (NDRS), which is part of NHS Digital. No additional data are available. Data on English Rapid Cancer Registrations can be accessed via the Office for Data Release. https://www.ndrs.nhs.uk/odr/. The data for Wales are collated, maintained and quality assured by the Wales Cancer Network.