Abstract
Differences in expression of the Escherichia coli stress protein HtpG were found following exposure of exponentially growing cells to heat or chemical shock when cells were grown under different environmental conditions. With an htpG::lacZ reporter system, htpG expression increased in cells grown in a complex medium (Luria-Bertani [LB] broth) following a temperature shock at 45°C. In contrast, no HtpG overexpression was detected in cells grown in a glucose minimal medium, despite a decrease in the growth rate. Similarly, in pyruvate-grown cells there was no heat shock induction of HtpG expression, eliminating the possibility that repression of HtpG in glucose-grown E. coli was due to catabolite repression. When 5 mM phenol was used as a chemical stress agent for cells growing in LB broth, expression of HtpG increased. However, when LB-grown cells were subjected to stress with 10 mM phenol and when both 5 and 10 mM phenol were added to glucose-grown cultures, repression of htpG expression was observed. 2-Chlorophenol stress resulted in overexpression of HtpG when cells were grown in complex medium but repression of HtpG synthesis when cells were grown in glucose. No induction of htpG expression was seen with 2,4-dichlorophenol in cells grown with either complex medium or glucose. The results suggest that, when a large pool of amino acids and proteins is available, as in complex medium, a much stronger stress response is observed. In contrast, when cells are grown in a simple glucose mineral medium, htpG expression either is unaffected or is even repressed by imposition of a stress condition. The results demonstrate the importance of considering differences in growth environment in order to better understand the nature of the response to an imposed stress condition.
Since the original description of the heat shock response (44), a wide range of adverse environmental conditions have been found to induce expression of stress proteins. The function of these induced proteins is to protect the cell against the harmful effects of altered environmental conditions. Many of the induced proteins facilitate the adaptation of metabolism to growth under the altered conditions or enable the cell to adapt in order to enhance survival mechanisms (28, 52). The most intensively investigated stress condition is that of heat shock, and among the bacteria, the best-characterized response is that for Escherichia coli (30). Among the E. coli heat shock proteins (HSPs) are some which function as molecular chaperones or have functions associated with DNA replication, cell division, and maintenance of active protein conformation (7, 21). Other stress conditions have also been shown to result in induction of specific groups of proteins. Such stimulons include those induced due to nutrient starvation (13, 27, 33, 34), nutrient exhaustion (12, 31, 35, 39, 52), heavy metal stress (5, 10, 38, 47), and phage shock (50, 51), as well as those induced following exposure to a range of organic solvents (5, 29, 32, 47). Many of these stimulons contain proteins which overlap especially with proteins belonging to the heat shock stimulon. The fact that specific patterns of proteins are expressed for a particular stress has led to the development of the use of stress proteins to monitor environmental samples for the presence of particular pollutants (4, 16, 47, 49).
In E. coli, regulation of the HSPs involves an alternative sigma factor, ς32, which when bound to the DNA polymerase holoenzyme recognizes the promoter sequence of the HSPs. Under conditions where accumulation of misfolded or dissociated proteins occurs in the cytoplasm, the amount, stability, and activity of ς32 increase (6, 15, 42, 54). In addition, another sigma factor, ςE, which recognizes the E. coli ς32 gene and several other heat shock promoters, is generated in response to signals of stress, such as unfolded proteins, in the periplasm (36, 40).
One of the originally described E. coli HSPs is the protein HtpG (C62.5). This protein comprises a large fraction (0.36%) of all proteins in E. coli growing at 37°C (20). HtpG possesses a noticeable sequence homology among prokaryotes and eukaryotes (2), with 41% identity in amino acids between the E. coli HtpG and its analog in Drosophila melanogaster. htpG deletion mutants in E. coli grow normally at 37°C but require the protein for growth above 46°C (3, 43). The protein is a dimeric phosphoprotein which has been shown to act as a suppressor of the secY24 mutation (45) and to suppress growth retardation of an ftsH mutant (41). In E. coli, in addition to heat, htpG expression has been shown to be induced by treatment with a variety of chemicals including ethanol, cadmium chloride, and 2,4-dinitrophenol (11, 46). In yeasts and humans, the analog of HtpG is the Hsp90 protein. This is also a highly abundant molecular chaperone which serves to protect proteins from denaturation on temperature upshifts. The protein is thermally stable up to 50°C but is very sensitive to the levels of divalent cations (22).
Since a stress or a shock involves a change from one environmental condition to another, the nature of the original condition plays a major role in defining the response which is required. Nevertheless, most of the work that has been carried out on stress has focused more on the nature of the stress than on the environment within which the stress is applied. With HtpG as an HSP marker, it has already been shown elsewhere that, in continuous culture, the growth environment has a strong influence on the nature and extent of stress gene expression (19). The work presented here extends this approach and examines how the nature of the growth environment affects the stress response to both a temperature-induced stress and a chemically induced stress. The results show that when a large pool of amino acids and proteins is available, as in a complex medium, a much stronger stress response is observed. In contrast, when cells are growing in a simple glucose mineral medium htpG expression either is unaffected by or is even repressed by imposing a stress condition.
MATERIALS AND METHODS
Bacterial strains.
E. coli JB23 (MC1655 F− lacZ::Tn10 zba315::kan ΔhtpG1::lacZ) and JB22 (MC1655 F− lacZ::Tn10 zba315::kan) were kindly provided by E. A. Craig. JB23 contains a chromosomal substitution deletion mutation where the coding sequence of the htpG gene has been replaced by the coding sequence of the lacZ gene in a lacZ mutant, resulting in an in-frame fusion between the codons for amino acid 15 of HtpG and amino acid 8 of β-galactosidase. JB22 is identical, except that the htpG gene is intact. A detailed description of the strains and their construction is given by Bardwell and Craig (3). Control experiments comparing growth of E. coli JB22 and that of E. coli JB23 showed that there was no difference in the growth rates of the two strains, indicating that the htpG deletion had no effects on normal growth (data not shown). Similarly, it has been shown previously that growth of the mutant strain was indistinguishable from that of the wild type at temperatures below 46°C (43).
Growth conditions.
Bacteria were grown in batch culture in a defined mineral salts medium (8) modified by substituting 29.3 mg of Na2-EDTA/liter for citrate. The medium was supplemented with 0.2% of the respective carbon source (glucose, glycerol, and pyruvate). For experiments carried out with complex medium, the Luria-Bertani (LB) medium containing (per liter) 3 g of K2HPO4, 1 g of KH2PO4, 10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl was used. For routine growth, kanamycin (100 mg/liter) was added to the culture. Stress experiments were carried out in the absence of kanamycin. Growth was monitored by measuring the absorption of samples at 546 nm in a Uvikon spectrophotometer (Kontron, Zurich, Switzerland).
Stress experiments.
Parallel cultures of E. coli JB23 were inoculated in Erlenmeyer flasks from cultures transferred twice in either modified Evans medium or LB medium. Cultures were grown at 37°C until an optical density at 546 nm (OD546) of 0.4 was attained. For heat shock experiments, whole flasks were transferred to a second water bath operating at 45°C and either maintained at this temperature for the duration of the experiment or transferred back to 37°C after 15 min. For chemical stress experiments, appropriate quantities of phenol or 2-chlorophenol were added directly to the cultures once an OD546 of 0.4 had been attained. 2,4-Dichlorophenol was added together with ethanol as the solvent. Samples of the culture were withdrawn periodically for determination of β-galactosidase and were immediately frozen in liquid nitrogen and stored at −18°C.
β-Galactosidase assay.
Samples were thawed on ice at 4°C. Five milliliters of the sample was washed twice by centrifugation for 6 min at 36,000 × g in a buffer containing 0.02 M Na2HPO4 at pH 7. Three milliliters of the washed-cell suspension was then transferred to a precooled glass tube, and the cells were disrupted by sonification (Branson sonifier 450; Skan) with a duty cycle of 40% and an output control of 2. The container holding the cells was maintained in ice during the sonification period (three times, 2 min each) to ensure that the sample remained cool. The samples were maintained on ice prior to subsequent analysis. β-Galactosidase was assayed according to the method described by Miller (37), modified so that the rate of increase in the absorbance was measured at 420 nm with a Uvikon 860 UV-visible light (UV/VIS) spectrophotometer (Kontron). Relative specific activities are expressed in arbitrary units and defined as enzyme activity per unit of OD546, normalized to the specific activity measured in the control unstressed cultures. Duplicate measurements within an experiment gave less than 10% variation.
Data analysis.
Shown in the figures are data from single representative experiments. All experiments were repeated several times to ensure reproducibility of the results. Statistical analysis was performed with the independent t test to determine the significance of differences between conditions tested, significance being ascribed at P > 0.1.
RESULTS
Heat shock in rich medium.
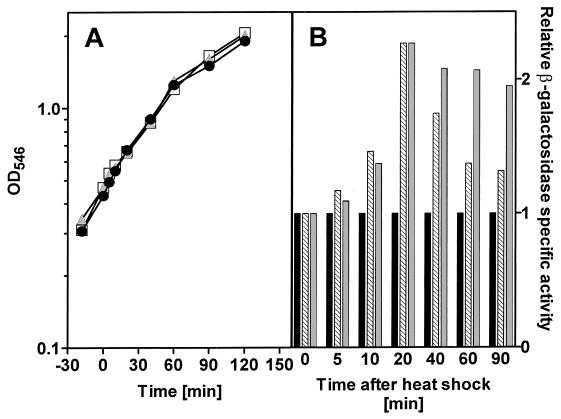
The macroscopic effects of growth perturbations are most easily seen as changes in growth rate. Representative batch growth curves from experiments where E. coli JB23 was subjected to either a prolonged or a temporary heat shock are shown in Fig. 1A. In these experiments, E. coli JB23 was grown in a rich nutrient medium (LB broth). Analysis of the growth rates following the stress (Table 1) shows that the prolonged shock had a more substantial effect on the growth rate than did the transient heat shock. Batch growth became limited, presumably by oxygen, in the Erlenmeyer shake flasks used during the late exponential phase as indicated by the gradual decline in the growth rate following the extended period of logarithmic growth. Figure 1B shows a good example of the classical heat shock response exemplified here by the expression of the heat shock gene htpG measured as β-galactosidase expression from an htpG promoter. A transient heat shock resulted in a transient expression of β-galactosidase. This peaked 20 min after the temperature upshift at a level that was 2.27 times that of the control and then decreased. Similarly, the level of β-galactosidase measured in the culture that was maintained at 45°C peaked after 20 min, after which it remained at an elevated level equivalent to 1.95 times that of the untreated control after 90 min.
FIG. 1.
(A) Growth of E. coli JB23 in complex medium exposed to a temperature shock. Growth curves are shown for three cultures. At time zero, one culture ( ) was transferred to 45°C and then returned to 37°C after 15 min; a second culture (□) was transferred to 45°C for the remainder of the experiment, while the third culture (●) was maintained at 37°C. (B) Expression of the htpG gene was determined as specific β-galactosidase activity of the htpG-lacZ fusion protein. ■, control; ▧, transient heat shock; , long-term heat shock.
TABLE 1.
Relative growth ratesa of E. coli JB23 following physical and chemical stress
| Stress | Condition(s) | Relative rate in growth
medium:
|
|||
|---|---|---|---|---|---|
| LB | Glucose | Pyruvate | Glycerol | ||
| Heat shock | 37 → 45→ 37°C | 0.93 | 0.77 | 0.89 | NDb |
| 37 → 45°C | 0.85 | 0.50 | 0.62 | ND | |
| Chemical stress: | |||||
| Phenol | 5 mM | 0.81 | 0.85 | ND | 0.80 |
| 10 mM | 0.53 | 0.44 | ND | 0.46 | |
| 2-Chlorophenol | 0.25 mM | 0.91c | 0.99c | ND | ND |
| 0.5 mM | 0.86 | 0.90 | ND | ND | |
| 1.0 mM | 0.83 | 0.70 | ND | ND | |
| 1.5 mM | 0.71 | 0.51 | ND | ND | |
| Ethanol | 0.21 mM | 0.91c | 0.99c | ND | ND |
| 2,4-Dichlorophenol | 0.1 mM | 0.71 | 0.48 | ND | ND |
| 0.2 mM | 0.48 | 0.39 | ND | ND | |
Means of at least two experiments are shown. The results are expressed relative to the growth rate of the unstressed control.
ND, not determined (experiment not carried out).
These values are not significantly different (t test) from the control.
HtpG expression following heat shock to glucose-grown E. coli.
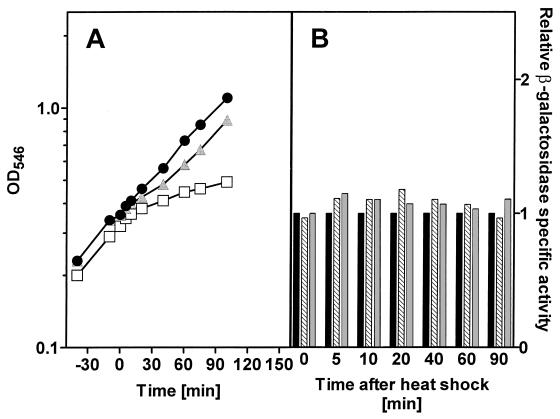
When E. coli JB23, growing in a mineral medium with glucose as the carbon source, was subjected to the same heat shock conditions, the growth rate decreased to 77% of that of the unstressed control following the transient (15 min) heat shock at 45°C (Fig. 2A). Growth in the culture which was maintained at 45°C slowed rapidly after 20 min to 50% of that of the control, while the culture transferred back to 37°C recovered its growth rate to approach that of the culture maintained at 37°C. Expression from the HtpG HSP promoter remained unaffected by any of the heat treatments despite the effects on the growth rate of the culture (Fig. 2B). Even in the culture that was maintained at 45°C, there was no enhanced expression of β-galactosidase from the htpG promoter when cells were subjected to a heat shock while growing in a glucose mineral medium.
FIG. 2.
Growth of and heat shock to E. coli JB23 growing in a glucose minimal medium (A) and HtpG expression (B). Symbols are the same as in Fig. 1.
Expression of HtpG following heat shock to E. coli grown on pyruvate.
Since expression of the gene encoding the sigma factor (ς32) required to recognize the heat shock gene promoters is partially affected by catabolite repression (14), it was considered possible that this caused the lack of enhanced HtpG synthesis following a heat shock in E. coli JB23 grown in a glucose mineral medium. In order to determine whether this was indeed caused by glucose catabolite repression, the same E. coli strain was grown on pyruvate as the sole carbon energy source in a mineral medium and subjected to the same heat shock regimens as described above. Heat stress caused a reduction in the growth rate following a transient (15 min) heat shock at 45°C, where it fell to 0.89 relative to the unstressed growth rate (Table 1). Following a return to 37°C, the culture recovered its pre-heat shock growth rate. When the temperature was maintained at 45°C, the relative growth rate decreased to 0.62 prior to eventually slowing down to approach zero.
In a manner analogous to that of glucose-grown cells, expression from the htpG promoter was unaffected by exposure to heat in any of these experiments with pyruvate as carbon source (Table 2). Identical results were also found in heat-shocked cultures of E. coli JB23 when glycerol was used as the sole carbon and energy source.
TABLE 2.
Effects of physical and chemical stress on β-galactosidase from the htpG promoter
| Stress | Condition(s) | Effect in growth
mediuma
|
|||
|---|---|---|---|---|---|
| LB | Glucose | Pyruvate | Glycerol | ||
| Heat shock | 37 → 45 → 37°C | + | 0 | 0 | 0 |
| 37 → 45°C | + | 0 | 0 | 0 | |
| Chemical stress: | |||||
| Phenol | 5 mM | + | − | ND | − |
| 10 mM | − (0) | − | ND | − | |
| 2-Chlorophenol | 0.25 mM | + | 0 (+) | ND | ND |
| 0.5 mM | + | − (0) | ND | ND | |
| 1.0 mM | + | − | ND | ND | |
| 1.5 mM | + | − | ND | ND | |
| Ethanol | 0.21 mM | 0 | 0 | ND | ND |
| 2,4-Dichlorophenol | 0.1 mM | 0 | 0 | ND | ND |
| 0.2 mM | 0 | 0 | ND | ND | |
+, increase in expression, −, decrease in expression; 0, no change; ND, not determined (experiment not performed). A symbol in parentheses indicates behavior following prolonged exposure.
Effect of chemical stress on expression of HtpG in E. coli JB23: phenol.
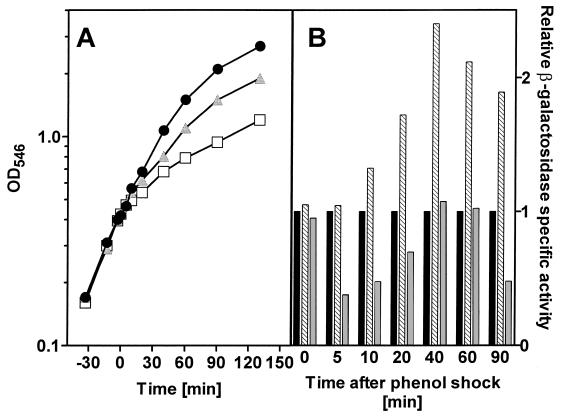
Since there was a strong dependence of growth conditions on the subsequent expression of the E. coli HSP HtpG, and it is known that many of the HSPs are expressed under a variety of other stress conditions (1, 5, 23–26, 53), htpG expression was examined following chemical stress during growth in rich and in minimal media. Unsubstituted and mono- and dichloro-substituted phenols were used as the chemical stressing agents, and expression of htpG was determined based on the level of β-galactosidase protein activity, as with the heat shock experiments. In Fig. 3, the results of addition of various concentrations of phenol to E. coli JB23 growing in a rich medium (LB broth) are shown. Following the addition of phenol, there was an immediate decrease in the rate of growth of the bacterial culture, the extent of the decrease being dependent on the concentration of phenol added. When the final concentration of phenol was 5 mM, the growth rate decreased to 81% of that of the untreated control while addition of 10 mM phenol resulted in a reduction in the relative growth rate to 0.53. In both instances, growth was inhibited but not repressed and continued for at least 2 h following the addition of the chemical stressing agent. Expression from the HtpG HSP promoter was 2.4 times that of the unstressed control following exposure to 5 mM phenol. The level of expression increased and reached a maximum at 40 min after exposure. In contrast, exposure to 10 mM phenol resulted in a decrease in the level of expression from the htpG promoter. The level declined immediately following the stress to a level ca. one-third of that in the unstressed control culture.
FIG. 3.
(A) Growth of E. coli JB23 in complex medium exposed to different concentrations of phenol. Cultures were grown to an OD546 of 0.4, at which time (0 min) the chemical shock was applied. ●, control; , 5 mM phenol; □, 10 mM phenol. (B) Expression of the htpG gene was determined as specific β-galactosidase activity of the htpG-lacZ fusion protein. ■, control; ▧, 0.5 mM phenol; , 10 mM phenol.
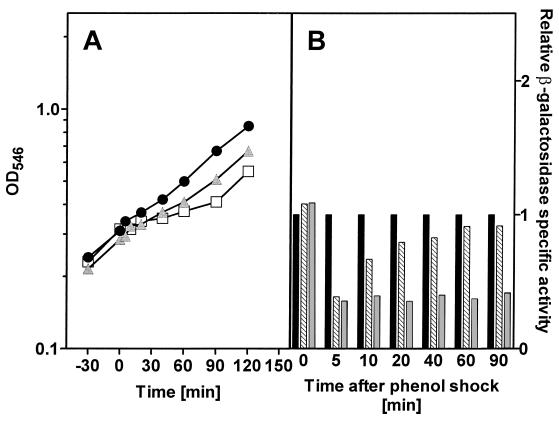
Exposure to the same phenol concentration during growth in a glucose mineral medium resulted in a decrease in the relative growth rates of the cultures to 0.85 for 5 mM phenol and to 0.44 for 10 mM phenol (Fig. 4). Expression of β-galactosidase was almost completely repressed during exposure to phenol in glucose-grown E. coli, both at 5 mM and at 10 mM phenol, with no recovery seen after 90 min.
FIG. 4.
(A) Growth of E. coli JB23 in glucose minimal medium exposed to different concentrations of phenol. Cultures were grown to an OD546 of 0.4, at which time (0 min) the chemical shock was applied. ●, control; , 5 mM phenol; □, 10 mM phenol. (B) Expression of the htpG gene was determined as specific β-galactosidase activity of the htpG-lacZ fusion protein. ■, control; ▧, 0.5 mM phenol; , 10 mM phenol.
As with glucose-grown E. coli, addition of phenol to glycerol-grown cultures of E. coli JB23 resulted in suppression of β-galactosidase expression both at 5 mM and at 10 mM stress concentrations (Table 2). At the lower concentration, the suppression was temporary, and 90 min after addition, the level of β-galactosidase approached that found in the control culture. Growth rate was suppressed at both concentrations of phenol (0.80 with 5 mM and 0.46 with 10 mM phenol). The growth rates in both phenol-stressed cultures increased about 1 h after addition. This recovery was also seen in the β-galactosidase levels in the culture treated with 5 mM phenol but not with the higher concentration.
Chemical stress with 2-chlorophenol.
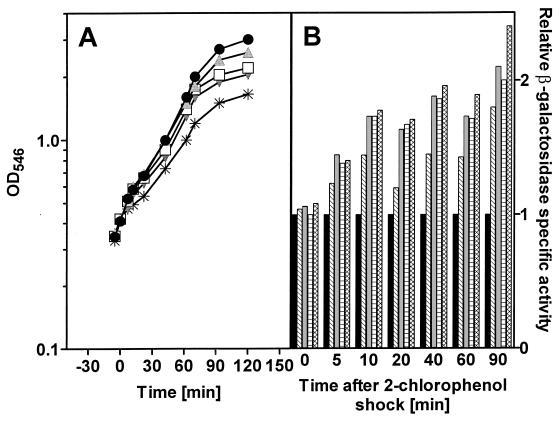
2-Chlorophenol also resulted in a reduction in the growth rate of E. coli JB23 growing on LB broth (Fig. 5A). The extent of growth rate inhibition was a direct function of the concentration of 2-chlorophenol to which the bacteria were exposed. When cells were stressed with 0.25 mM 2-chlorophenol, growth rate was unaffected, while at higher concentrations, there was a measurable reduction in the growth rate of the bacteria (Table 1). For all concentrations, following the chemical stress with 2-chlorophenol, expression from the htpG promoter increased (Fig. 5B). This increase was immediate, i.e., within 5 min following the shock. The increase in the level of expression of β-galactosidase was the same and independent of the concentration of 2-chlorophenol used. Even 90 min after the initial shock with 2-chlorophenol, the level of expression from the htpG promoter was continuing to increase.
FIG. 5.
(A) Growth of E. coli JB23 in complex medium exposed to different concentrations of 2-chlorophenol. Cultures were grown to an OD546 of 0.4, at which time (0 min) the chemical shock was applied. ●, control; , 0.25 mM 2-chlorophenol; □, 0.5 mM 2-chlorophenol; , 1 mM 2-chlorophenol; ✳, 1.5 mM 2-chlorophenol. (B) Expression of the htpG gene was determined as specific β-galactosidase activity of the htpG-lacZ fusion protein. ■, 0 mM 2-chlorophenol; ▧, 0.25 mM 2-chlorophenol; , 0.5 mM 2-chlorophenol; ▤, 1.0 mM 2-chlorophenol; , 1.5 mM 2-chlorophenol.
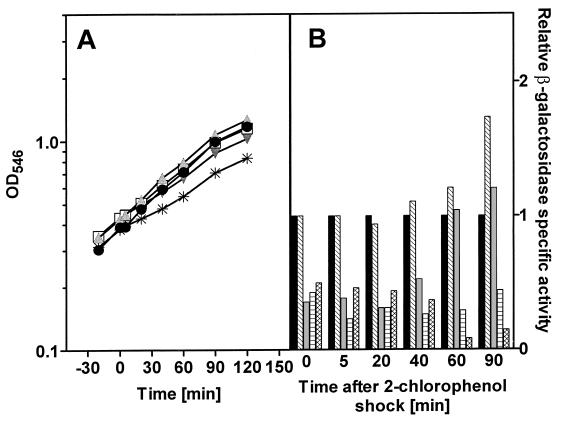
Growth on a glucose mineral medium also resulted in a similar reduction in growth rates during the exponential phase when 2-chlorophenol was added as a chemical stressing agent (Fig. 6A). The extent of growth rate reduction was also dependent on the concentration of 2-chlorophenol used, with no noticeable change in the relative growth rate with 0.25 mM 2-chlorophenol and an increasing level of repression for the higher concentrations (Table 1). In contrast to the increase in expression from the htpG promoter seen for 2-chlorophenol chemical stress in E. coli JB23 growing in LB medium, there was a temporary repression of β-galactosidase expression when the bacteria were subjected to a chemical shock with 2-chlorophenol while they were growing in glucose mineral medium (Fig. 6B). The extent of repression was a function of the concentration of the chemical stressing agent used. For the culture stressed with 0.25 mM 2-chlorophenol, there was no apparent repression of β-galactosidase synthesis. After 90 min following the shock, the level of β-galactosidase actually increased and was overexpressed by a factor of 1.6-fold. Expression from the htpG promoter following addition of 2-chlorophenol at higher concentrations resulted in repression of β-galactosidase synthesis. Recovery from this repression occurred with the 0.5 mM 2-chlorophenol-stressed culture in a manner similar to that observed for 0.25 mM but temporally delayed. No recovery occurred with either the 1.0 mM 2-chlorophenol culture or the 1.5 mM culture even at 90 min after the shock.
FIG. 6.
(A) Growth of E. coli JB23 in glucose minimal medium exposed to different concentrations of 2-chlorophenol. Cultures were grown to an OD546 of 0.4, at which time (0 min) the chemical shock was applied. ●, control; , 0.25 mM 2-chlorophenol; □, 0.5 mM 2-chlorophenol; , 1 mM 2-chlorophenol; ✳, 1.5 mM 2-chlorophenol. (B) Expression of the htpG gene was determined as specific β-galactosidase activity of the htpG-lacZ fusion protein. ■, 0 mM 2-chlorophenol; ▧, 0.25 mM 2-chlorophenol; , 0.5 mM 2-chlorophenol; ▤, 1.0 mM 2-chlorophenol; , 1.5 mM 2-chlorophenol.
Expression of HtpG following chemical stress with 2,4-dichlorophenol.
Since 2,4-dichlorophenol is very poorly soluble in water, it was added to the cells together with ethanol as a solvent. However, since ethanol itself is known to result in induction of HSPs such as GrpE (36), it is necessary to differentiate the effect of ethanol alone from that of 2,4-dichlorophenol in the presence of ethanol. When cells were grown in complex medium, addition of 2,4-dichlorophenol resulted in a decrease in the growth rate of E. coli JB23. The extent of inhibition was concentration dependent. A control culture was also challenged with 0.21 M ethanol. This concentration corresponded to that present together with the 0.2 M 2,4-dichlorophenol. As seen in Table 1, the growth rate reduction was more substantial for cultures grown in the complex medium than for those grown with glucose, while ethanol addition had no significant effect on growth rate reduction in either LB broth or glucose minimal medium. At the higher 2,4-dichlorophenol concentration, the growth rate was measured following addition of 2,4-dichlorophenol, and subsequently the growth rate declined further as the experiment progressed. When cells were grown in complex medium, no difference in the expression of the HtpG reporter, β-galactosidase, was detected with either glucose mineral medium-grown or rich nutrient medium-grown E. coli (Table 2).
DISCUSSION
This study demonstrated that the changes in the level of expression of the HSP HtpG following either a heat shock or a chemical shock were dependent on the growth conditions prior to and during the stress. Similar results were found previously for HtpG following a change in temperature from 37 to 42°C in a continuously growing culture of E. coli (17–19). For steady-state cultures, the extent of increase in the level of expression of htpG was dependent on the nature of the growth medium (19). In this study, we have shown that in response to heat stress (increase from 37 to 45°C), the HSP HtpG is overexpressed when cells are grown in complex medium while its expression remains unchanged during growth of cells on glucose, glycerol, or pyruvate (Table 2). Similarly, it was previously shown that in batch culture the level of HtpG remained unchanged following a shift from 37 to 45°C when cells were grown in glucose minimal medium, while in complex medium HtpG was induced to a ca. 40%-higher level than that at 37°C (17).
HtpG is thought to act as a chaperone in stressed cells, maintaining partially folded proteins in a configuration that facilitates their reactivation by interaction with other chaperones (43). The lack of HtpG in E. coli JB23 has no effect on growth at up to 46°C (43), and two-dimensional gel analysis suggests that no induction of synthesis of other proteins occurs to compensate for the absence of HtpG (3). Furthermore, no differences were observed in the sensitivities of the wild type or the htpG deletion strain to 256 chemicals, to UV irradiation, or to lambda phage infection (3).
Both htpG expression and growth rate were influenced by the pre-stress growth conditions. Temperature shock resulted in a change in the growth rate of E. coli JB23 in glucose, glycerol, and pyruvate mineral media but had no noticeable effect on the overall growth rate in complex medium. In contrast, chemical shocks resulted in a reduction in growth rates to approximately the same extent in both minimal medium and complex medium. Complex medium contains a wide range of proteins and amino acids from the hydrolysates of yeast extract and tryptone. Their presence in the growth medium alleviates the need for anabolic synthetic pathways. On the other hand, there is also a larger pool of molecules that can potentially be damaged by exposure to adverse environmental conditions, such as following a temperature shock or as a result of chemical interactions. Thus, changes which are seen in the level of HtpG following a heat shock in complex medium could be a result of multiple sites of damage to the pool of macromolecules. Repair mechanisms have been characterized previously for l-isoaspartyl residues that arise from spontaneous damage to aspartyl or asparagyl residues (48). This pool is not present in cells grown in glucose minimal medium, so that changes in the physical or chemical environmental conditions can be compensated for by changing growth rate rather than by overexpressing particular stress proteins. This suggested model does not exclude the possibility that other heat shock or stress proteins react differently.
One of the hypotheses considered was that catabolite repression might be important in the regulation of expression of the heat shock gene htpG. This was considered since induction of htpG occurred following a heat shock in complex medium while expression appeared to be unaltered following a heat shock in glucose mineral medium. One of the promoters of rpoH, P5, requires activation by the cyclic AMP-catabolite activator protein complex. Control of P5 activity is by catabolite repression and results in a two- to threefold-higher expression of the rpoH gene in glucose-free medium (14). However, the results with the glycerol- and pyruvate-grown cultures, which showed a response similar to that of the glucose-grown culture to a heat shock indicated that the differences in expression of HtpG between cells grown in complex medium and those grown in glucose mineral medium did not involve catabolite repression.
Stress proteins are modulators of metabolism, enabling growth to occur unperturbed by changes in environmental conditions or enhancing protection against damage by adverse conditions. These results suggest that studies of stress protein regulation need to be carried out under conditions more akin to real environmental conditions rather than under the ideal conditions often used in many such stress studies. In a separate study, we have also seen a difference in the induction of the katF gene, which encodes the ςS subunit of RNA polymerase and is responsible for induction of the stationary-phase proteins, as a function of the medium in which the cells are grown (data not shown). Furthermore, a recent report on the ςS-regulated gene uspB also described differences in expression in minimal medium and in complex medium (9).
REFERENCES
- 1.Ananthan J, Goldberg A L, Voellmy R. Abnormal proteins serve as eucaryotic stress signals and trigger the activation of heat shock genes. Science. 1986;232:522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 2.Bardwell J C, Craig E A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci USA. 1987;84:5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardwell J C, Craig E A. Ancient heat shock gene is dispensable. J Bacteriol. 1988;170:2977–2983. doi: 10.1128/jb.170.7.2977-2983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkin S, Smulski D R, Vollmer A C, Van Dyk T K, LaRossa R A. Oxidative stress detection with Escherichia coli harboring a katG′::luxfusion. Appl Environ Microbiol. 1996;62:2252–2256. doi: 10.1128/aem.62.7.2252-2256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blom A, Harder W, Matin A. Unique and overlapping pollutant stress proteins of Escherichia coli. Appl Environ Microbiol. 1992;58:331–334. doi: 10.1128/aem.58.1.331-334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukau B. Regulation of the Escherichia coliheat-shock response. Mol Microbiol. 1993;9:671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 7.Craig E A. The heat shock response. Crit Rev Biochem. 1985;18:239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- 8.Evans C G T, Herbert D, Tempest D W. The continuous cultivation of micro-organisms. 2. Construction of a chemostat. Methods Microbiol. 1970;2:277–327. [Google Scholar]
- 9.Farewell A, Kvint K, Nystrom T. uspB, a new ςS-regulated gene in Escherichia coliwhich is required for stationary-phase resistance to ethanol. J Bacteriol. 1998;180:6140–6147. doi: 10.1128/jb.180.23.6140-6147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferianc P, Farewell A, Nystrom T. The cadmium-stress stimulon of Escherichia coliK-12. Microbiology. 1998;144:1045–1050. doi: 10.1099/00221287-144-4-1045. [DOI] [PubMed] [Google Scholar]
- 11.Gage D J, Neidhardt F C. Adaptation of Escherichia colito the uncoupler of oxidative phosphorylation 2,4-dinitrophenol. J Bacteriol. 1993;175:7105–7108. doi: 10.1128/jb.175.21.7105-7108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry D R, Hernandez V J, Nguyen L H, Jensen D B, Cashel M. Synthesis of the stationary-phase sigma factor ςSis positively regulated by ppGpp. J Bacteriol. 1993;175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Givskov M, Eberl L, Molin S. Responses to nutrient starvation in Pseudomonas putidaKT2442: two-dimensional electrophoretic analysis of starvation- and stress-induced proteins. J Bacteriol. 1994;176:4816–4824. doi: 10.1128/jb.176.16.4816-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groat R G, Schultz J E, Zychlinsky E, Bockman A, Matin A. Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol. 1986;168:486–493. doi: 10.1128/jb.168.2.486-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman A D, Straus D B, Walter W A, Gross C A. ς32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987;1:179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Gu M B, Dhurjati S, Van Dyk T K, LaRossa R A. A miniature bioreactor for sensing toxicity using recombinant bioluminescent Escherichia colicells. Biotechnol Prog. 1996;12:393–397. doi: 10.1021/bp9600142. [DOI] [PubMed] [Google Scholar]
- 17.Heitzer A. Kinetic and physiological aspects of bacterial growth at superoptimum temperatures. Ph.D. thesis. Zürich, Switzerland: ETH-Zürich; 1990. [Google Scholar]
- 18.Heitzer A, Mason C A, Hamer G. Heat shock gene expression in continuous cultures of Escherichia coli. J Biotechnol. 1992;22:153–169. doi: 10.1016/0168-1656(92)90139-z. [DOI] [PubMed] [Google Scholar]
- 19.Heitzer A, Mason C A, Snozzi M, Hamer G. Some effects of growth conditions on steady state and heat shock induced htpG gene expression in continuous cultures of Escherichia coli. Arch Microbiol. 1990;155:7–12. doi: 10.1007/BF00291266. [DOI] [PubMed] [Google Scholar]
- 20.Herendeen S L, VanBogelen R A, Neidhardt F C. Levels of major proteins of Escherichia coliduring growth at different temperatures. J Bacteriol. 1979;139:185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hightower L E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 22.Jakob U, Meyer I, Bugl H, Andre S, Bardwell J C, Buchner J. Structural organization of procaryotic and eucaryotic Hsp90. Influence of divalent cations on structure and function. J Biol Chem. 1995;270:14412–14419. doi: 10.1074/jbc.270.24.14412. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins D E, Auger E A, Matin A. Role of RpoH, a heat shock regulator protein, in Escherichia colicarbon starvation protein synthesis and survival. J Bacteriol. 1991;173:1992–1996. doi: 10.1128/jb.173.6.1992-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins D E, Schultz J E, Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones P G, VanBogelen R A, Neidhardt F C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanemori M, Mori H, Yura T. Induction of heat shock proteins by abnormal proteins results from stabilization and not increased synthesis of ς32 in Escherichia coli. J Bacteriol. 1994;176:5648–5653. doi: 10.1128/jb.176.18.5648-5653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Watrud L S, Matin A. A carbon starvation survival gene of Pseudomonas putida is regulated by ς54. J Bacteriol. 1995;177:1850–1859. doi: 10.1128/jb.177.7.1850-1859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kjelleberg S, Albertson N, Flardh K, Holmquist L, Jouper-Jaan A, Marouga R, Ostling J, Svenblad B, Weichart D. How do non-differentiating bacteria adapt to starvation? Antonie Leeuwenhoek Int J Gen Mol Microbiol. 1993;63:333–341. doi: 10.1007/BF00871228. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H, Yamamoto M, Aono R. Appearance of a stress-response protein, phage-shock protein A, in Escherichia coliexposed to hydrophobic organic solvents. Microbiology. 1998;144:353–359. doi: 10.1099/00221287-144-2-353. [DOI] [PubMed] [Google Scholar]
- 30.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 31.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS(KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 32.Lupi C G, Colangelo T, Mason C A. Two-dimensional gel electrophoresis analysis of the response of Pseudomonas putidaKT2442 to 2-chlorophenol. Appl Environ Microbiol. 1995;61:2863–2872. doi: 10.1128/aem.61.8.2863-2872.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matin A. Starvation promoters of Escherichia coli. Their function, regulation, and use in bioprocessing and bioremediation. Ann N Y Acad Sci. 1994;721:277–291. doi: 10.1111/j.1749-6632.1994.tb47401.x. [DOI] [PubMed] [Google Scholar]
- 34.Matin A, Little C D, Fraley C D, Keyhan M. Use of starvation promoters to limit growth and select for trichloroethylene and phenol transformation activity in recombinant Escherichia coli. Appl Environ Microbiol. 1995;61:3323–3328. doi: 10.1128/aem.61.9.3323-3328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCann M P, Fraley C D, Matin A. The putative sigma factor KatF is regulated posttranscriptionally during carbon starvation. J Bacteriol. 1993;175:2143–2149. doi: 10.1128/jb.175.7.2143-2149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mecsas J, Rouviere P, Erickson J, Donohue T, Gross C. The activity of ςE, an Escherichia coliheat inducible sigma factor, is modulated by expression of outer membrane proteins. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 37.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 38.Nystrom T, Neidhardt F C. Cloning, mapping and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol Microbiol. 1992;6:3187–3198. doi: 10.1111/j.1365-2958.1992.tb01774.x. [DOI] [PubMed] [Google Scholar]
- 39.O’Neal C, Gabriel W M, Turk A K, Libby S J, Fang F C, Spector M P. RpoS is necessary for both the positive and negative regulation of starvation survival genes during phosphate, carbon, and nitrogen starvation in Salmonella typhimurium. J Bacteriol. 1994;176:4610–4616. doi: 10.1128/jb.176.15.4610-4616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouviere P E, De Las Penas A, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat-shock sigma factor, ςE, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirai Y, Akiyama Y, Ito K. Suppression of ftsHmutant phenotypes by overproduction of molecular chaperones. J Bacteriol. 1996;178:1141–1145. doi: 10.1128/jb.178.4.1141-1145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straus D B, Walter W A, Gross C A. The heat shock response of E. coli is regulated by changes in the concentration of ς32. Nature. 1987;329:348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 43.Thomas J G, Baneyx F. Roles of the Escherichia colismall heat shock proteins IbpA and IbpB in thermal stress management: comparison with ClpA, ClpB, and HtpG in vivo. J Bacteriol. 1998;180:5165–5172. doi: 10.1128/jb.180.19.5165-5172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tissieres A, Mitchell H K, Tracy U M. Protein synthesis in salivary glands of Drosophila melanogaster. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 45.Ueguchi C, Ito K. Multicopy suppression: an approach to understanding intracellular functioning of the protein export system. J Bacteriol. 1992;174:1454–1461. doi: 10.1128/jb.174.5.1454-1461.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.VanBogelen R A, Kelley P M, Neidhardt F C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987;169:26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Dyk T K, Majarian W R, Konstantinov K B, Young R M, Dhurjati P S, LaRossa R A. Rapid and sensitive pollutant detection by induction of heat shock gene-bioluminescence gene fusions. Appl Environ Microbiol. 1994;60:1414–1420. doi: 10.1128/aem.60.5.1414-1420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visick J E, Cai H, Clarke S. The l-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia colisubjected to secondary environmental stresses. J Bacteriol. 1998;180:2623–2629. doi: 10.1128/jb.180.10.2623-2629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vollmer A, Belkin S, Smulski D R, Van Dyk T K, LaRossa R A. Detection of DNA damage by use of Escherichia coli carrying recA′::lux, uvrA′::lux, or alkA′::luxreporter plasmids. Appl Environ Microbiol. 1997;63:2566–2571. doi: 10.1128/aem.63.7.2566-2571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner L, Brissette J L, Model P. Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on ς54and modulated by positive and negative feedback mechanisms. Genes Dev. 1991;5:1912–1923. doi: 10.1101/gad.5.10.1912. [DOI] [PubMed] [Google Scholar]
- 51.Weiner L, Brissette J L, Ramani N, Model P. Analysis of the proteins and cis-acting elements regulating the stress-induced phage shock protein operon. Nucleic Acids Res. 1995;23:2030–2036. doi: 10.1093/nar/23.11.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner L, Model P. Role of an Escherichia colistress-response operon in stationary-phase survival. Proc Natl Acad Sci USA. 1994;91:2191–2195. doi: 10.1073/pnas.91.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welch T J, Farewell A, Neidhardt F C, Bartlett D H. Stress response of Escherichia colito elevated hydrostatic pressure. J Bacteriol. 1993;175:7170–7177. doi: 10.1128/jb.175.22.7170-7177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yura T, Kawasaki Y, Kusukawa N, Nagai H, Wada C, Yano R. Roles and regulation of the heat shock sigma factor ς32 in Escherichia coli. Antonie Leeuwenhoek Int J Gen Mol Microbiol. 1990;58:187–190. doi: 10.1007/BF00548931. [DOI] [PubMed] [Google Scholar]