SUMMARY
Despite infection control measures, an important increase in the extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae incidence density occurred in our hospital from 2006 onwards. This study, focusing on the 2005–2007 period, was performed in an attempt to explain this increase. ESBLs were characterized, isolates were typed by ERIC2-PCR, and sequence type (ST) of clustered isolates was determined. Temporal-spatial relationships of patients were analysed to assess possible cross-contamination. Of the 74 ESBL-producing isolates, 30 (40%) were detected at admission, 53 (71·5%) produced CTX-M enzymes, 40 displayed unique ERIC2-PCR profiles and 34 were assigned into six clusters: ST16 (n = 21), ST101, ST48, ST35, ST13, and ST436. Relationships were identified in 22 of the 34 patients harbouring clustered isolates. This study highlights the complex epidemiology of ESBL-producing K. pneumoniae in the mid-2000s with potential cross-contamination for only 30% of the 74 patients in our hospital, and the emergence of clones that are currently spreading worldwide.
Key words: Antibiotic resistance, hospital-acquired (nosocomial) infections, infectious disease epidemiology, Klebsiella, molecular epidemiology
INTRODUCTION
Klebsiella pneumoniae is a major nosocomial pathogen. Isolates producing extended-spectrum β-lactamase (ESBL) appeared in the early 1980s and were involved in numerous outbreaks, especially in intensive-care units (ICUs) [1–3]. While hygiene measures permitted the control of ESBL-producing K. pneumoniae nosocomial infections in the 1990s, ESBL-producing Escherichia coli emerged in the early 2000s, not only in hospitals but also in the community [1]. Whereas K. pneumoniae isolates were in the main producers of TEM and SHV derivatives, E. coli isolates were shown to produce mainly CTX-M enzymes [1]. In the mid-2000s, public health concern increased due not only to the worldwide dissemination of CTX-M-producing E. coli, but also to the local emergence of carbapenamase-producing K. pneumoniae [4].
β-lactamase gene dissemination results from a combination of plasmid dissemination in genetically distinct strains and clonal spread of plasmid-harbouring resistant strains [1, 4, 5]. Indeed, within E. coli species, clone sequence type (ST)131 isolates producing in particular CTX-M-15, have been identified globally [6]. Similarly, within K. pneumoniae, clones ST11 and ST258 grouped into clonal group (CG) 258 [7], and clone ST14 grouped into CG15 [7], have been involved in several outbreaks on different continents [5, 7].
In our hospital, measures to control transmission of ESBL-producing organisms have been implemented since late 2004. These are based essentially on (i) notification of ESBL-producing Enterobacteriaceae carriers (identified from clinical samples or rectal swabs performed at admission for patients with previous history of hospitalization or at admission, and once a week for patients being hospitalized in ICUs), and (ii) implementation of precaution barriers.
In spite of these measures, the annual incidence density of ESBL-producing K. pneumoniae, [expressed as the annual number of patients carrying an ESBL-producing K. pneumoniae per 1000 hospitalization days (HD)], evolved as follows: 0·07 in 2005, 0·21 in 2006, 0·32 in 2007, 0·31 in 2008 and 0·27 in 2009 (our unpublished data).
In order to better characterize the determinants of the sharp increase in incidence density observed in 2006, we undertook a retrospective study focusing on the molecular features of isolates and epidemiological links of patients during the 2005–2007 period.
METHODS
Hospital setting
Beaujon Hospital is a 461-bed teaching hospital of Assistance Publique-Hôpitaux de Paris (AP-HP) that comprises seven surgical wards, seven medical wards, two ICUs [ICU(A) and ICU(B)], a maternity unit, and an emergency unit. The hospital specializes in chronic digestive diseases (n = 161 beds).
Bacterial isolates
The first ESBL-producing K. pneumoniae isolate recovered from faecal or clinical samples in each patient hospitalized between January 2005 and December 2007 was included in the study (one isolate per patient). Faecal isolates had been selected on Drigalski agar plates containing 1 mg/l cefotaxime. Isolate identification was performed by the API System (bioMérieux, France) and ESBL production was assessed by using the double disk synergy test [2].
ESBL characterization
Genes encoding ESBL enzymes (blaCTX-M, blaTEM, blaSHV types) were characterized by polymerase chain reaction (PCR) and DNA sequencing with specific primers, as described previously [8].
ERIC2-PCR typing
Isolates were typed using the ERIC2-PCR method as described previously [6]. Briefly, 1 mm of primer (5′-AAG TAA GTG ACT GGG GTG AGC G-3′) and amplification conditions including 44 cycles and a 36 °C hybridization temperature were used. PCR products were further submitted to 100 V electrophoresis for 1·5 h in 1·5% agarose gels.
Two isolates were considered to be genetically related (cluster) when they exhibited a strictly identical ERIC2-PCR profile.
Epidemiological data
Hospitalization dates and wards were obtained from the hospital database. ESBL-producing K. pneumoniae isolated after 48 h of hospitalization were deemed hospital acquired whereas isolates recovered within the 48 h following admission were considered as imported. The imported isolates were further grouped as healthcare associated, when patients had undergone hospitalization during the previous 3 months, or as community acquired, when patients had no contact with any healthcare setting during the previous 3 months.
As recovery of genetically related isolates (i.e. from the same ERIC2-PCR cluster) in different patients could suggest cross-transmission, we looked for temporal-spatial relationships of patients (patients being hospitalized during overlapping days in the same ward) over the study period.
Multi-locus sequence typing (MLST)
The international MLST scheme (www.pasteur.fr/mlst) [9] was used to determine the ST of clustered isolates including, for each cluster, representative isolates from patients without a temporal and spatial link.
Statistical analysis
Categorical variables were compared by Fisher's exact test while Student's t test was used to compare continuous data. All statistical analyses were performed by Epi Info (CDC, USA). A P value of ⩽0·05 was considered as statistically significant.
RESULTS
Bacterial isolates
From January 2005 to December 2007, 74 patients were detected as carriers (from rectal swab or clinical sample) of ESBL-producing K. pneumoniae (10 in 2005, 26 in 2006, 38 in 2007) (Table 1). Rectal swabs provided 39 isolates whereas 35 were obtained from clinical samples [urine (16), blood culture (6), drainage liquids (6), surgical sites (2), abscess (1), ascites (1), catheter (1), genital (1) and neonatal (1)]. Whatever the year, isolates recovered from rectal swabs numbered about the same as those recovered from clinical samples (Table 1).
Table 1.
Distribution of the carriers of an ESBL-positive Klebsiella pneumoniae isolate according to ward and sample type, and number of patients admitted with this isolate from 2005 to 2007
| Ward/sample | Number of patients (%) | |||
|---|---|---|---|---|
| 2005 | 2006 | 2007 | Total | |
| Total | 10 | 26 | 38 | 74 |
| Imported | 5 (50) | 9 (35) | 16 (42) | 30 (40·5) |
| Rectal | 2 | 2 | 8 | 12 |
| Clinical | 3 | 7 | 8 | 18 |
| Hospital acquired | 5 (50) | 17 (65) | 22 (58) | 44 (59·5) |
| Rectal | 3 | 12 | 12 | 27 |
| Clinical | 2 | 5 | 10 | 17 |
| Intensive-care unit | 5 | 9 | 16 | 30 |
| Imported | 2 (40) | 0 (0) | 2 (12·5) | 4 (13) |
| Rectal | 1 | 0 | 1 | 2 |
| Clinical | 1 | 0 | 1 | 2 |
| Hospital acquired | 3 (60) | 9 (100) | 14 (87·5) | 26 (87) |
| Rectal | 3 | 7 | 11 | 21 |
| Clinical | 0 | 2 | 3 | 5 |
| Medicine | 2 | 12 | 11 | 25 |
| Imported | 0 (0) | 6 (50) | 6 (54·5) | 12 (48) |
| Rectal | 0 | 2 | 4 | 6 |
| Clinical | 0 | 4 | 2 | 6 |
| Hospital acquired | 2 (100) | 6 (50) | 5 (45·5) | 13 (52) |
| Rectal | 0 | 4 | 0 | 4 |
| Clinical | 2 | 2 | 5 | 9 |
| Surgery | 2 | 2 | 5 | 9 |
| Imported | 2 (100) | 0 (0) | 2 (40) | 4 (44) |
| Rectal | 1 | 0 | 2 | 3 |
| Clinical | 1 | 0 | 0 | 1 |
| Hospital acquired | 0 (0) | 2 (100) | 3 (60) | 5 (66) |
| Rectal | 0 | 1 | 1 | 2 |
| Clinical | 0 | 1 | 2 | 3 |
| Others | 1 | 3 | 6 | 10 |
| Imported | 1 (0) | 3 (100) | 6 (100) | 10 (100) |
| Rectal | 0 | 0 | 1 | 1 |
| Clinical | 1 | 3 | 5 | 9 |
ESBL, Extended-spectrum β-lactamase.
ESBL characterization
CTX-M-type enzymes were identified in 53 (71·5%) of the 74 isolates whereas TEM and SHV derivatives were identified in 15% and 13·5%, respectively (Table 2). These three types of ESBLs were present each year. CTX-M-15, SHV-12 and TEM-3 were the most commonly identified ESBLs (Table 2). The number of CTX-M-producing isolates (especially CTX-M-15) increased over the 3 years, whereas the number of TEM and SHV ESBL-producing isolates remained stable (Table 2). A new variant of CTX-M was identified from a rectal swab isolate obtained from a patient hospitalized in ICU(A) in January 2005. This enzyme showed serine at position 167 (as in CTX-M-19) and arginine at position 274 (as in CTX-M-24). This enzyme was designated CTX-M-99 and its sequence has been registered in public sequence databases under accession number HM803271.
Table 2.
ESBL types of Klebsiella pneumoniae isolates over the study period
| ESBL type | Number (%) of isolates | |||
|---|---|---|---|---|
| 2005 | 2006 | 2007 | Total | |
| TEM | 5 (50) | 3 (12) | 3 (8) | 11 (15) |
| 3 | 4 | 2 | 1 | 7 |
| 12 | 0 | 1 | 1 | 2 |
| 21 | 1 | 0 | 0 | 1 |
| 167 | 0 | 0 | 1 | 1 |
| SHV | 1 (10) | 4 (15) | 5 (13) | 10 (13·5) |
| 2 | 0 | 1 | 3 | 4 |
| 12 | 1 | 3 | 2 | 6 |
| CTX-M | 4 (40) | 19 (73) | 30 (79) | 53 (71·5) |
| 3 | 1 | 0 | 0 | 1 |
| 15 | 2 | 19 | 27 | 48 |
| 14 | 0 | 0 | 2 | 2 |
| 27 | 0 | 0 | 1 | 1 |
| 99 | 1 | 0 | 0 | 1 |
| Total | 10 (100) | 26 (100) | 38 (100) | 74 (100) |
ESBL, Extended-spectrum β-lactamase.
Epidemiological data
The 74 patients colonized or infected with ESBL-producing K. pneumoniae were hospitalized in 15 of the 17 clinical wards of our hospital. Many (40%) of these patients were in ICUs, whereas 34% and 12% were in medical and surgical wards, respectively (Table 1). For 44 (60%) patients, the isolate was hospital acquired. The remaining 30 (40%) patients carried the ESBL-producing K. pneumoniae isolate at admission. For 18 of them, the isolate was healthcare associated and for 12, it was community acquired. The proportion of carriers at admission was not significantly different each year (P = 0·6). However, carriers at admission were significantly less frequent in ICUs than in the other clinical wards (13% vs. 64%, P < 10−4) (Table 1).
ERIC2-PCR typing
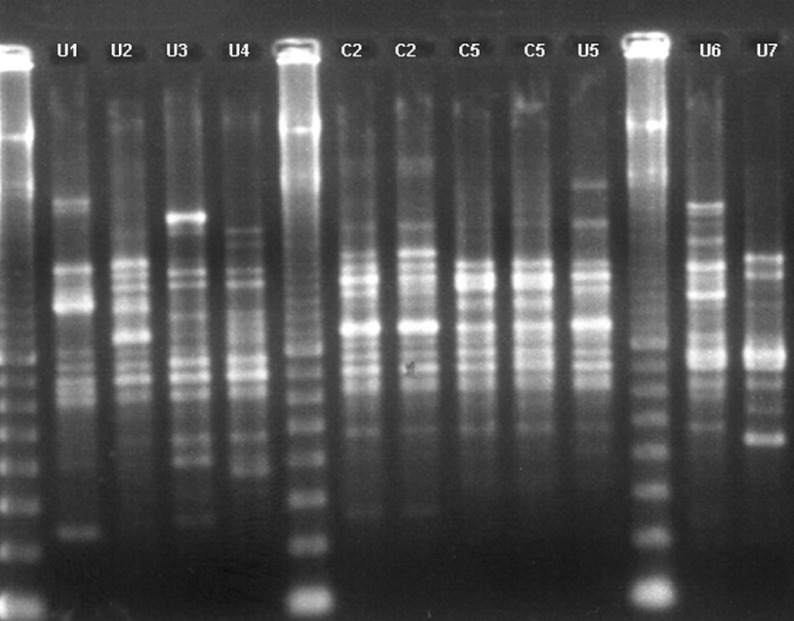
The 74 isolates showed 46 ERIC2-PCR profiles (Fig. 1) corresponding to 40 unique profiles and six clusters of 2–21 isolates. Of the 30 imported isolates, 23 displayed unique ERIC2-PCR profiles whereas seven were classified into clusters. Of the 44 hospital-acquired isolates, 17 displayed unique ERIC2-PCR profiles and 27 belonged to clusters. Unique profiles were significantly more frequent in imported isolates than in hospital-acquired isolates (P = 0·002), consistent with the increased risk of cross-transmission within the hospital.
Fig. 1.
Examples of ERIC2-PCR profiles from ESBL-producing Klebsiella pneumoniae. Seven unique profiles (U1, U2, U3, U4, U6, U7), and two representative isolates of cluster 2 (C2) and cluster 5 (C5) are presented. Lanes 1, 6 and 12 contain molecular-weight marker.
Temporal-spatial relationships in patients and STs of genetically related strains
One strain producing SHV-2 was transmitted in 2007 by a mother to her newborn during delivery. Both isolates which grouped into cluster 1 and were of ST35 were considered as imported (Table 3).
Table 3.
Epidemiology and sequence type of six ERIC2-PCR-defined clusters of ESBL-producing Klebsiella pneumoniae
| Cluster | No. of isolates | Year of isolation | No. of imported isolates | No. of wards | ESBL type | Sequence Type (ST) | No. of patients with temporal-spatial link |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 2007 | 2 | 1a | SHV-2 | ST35 | 2 |
| 2 | 21 | 2006–2007 | 1 | 7b | CTX-M-15 | ST16 | 14 |
| 3 | 4 | 2006–2007 | 2 | 1c | CTX-M-15 | ST101 | 3 |
| 4 | 2 | 2006–2007 | 1 | 1d | CTX-M-15 | ST436 | 0 |
| 5 | 2 | 2006–2007 | 1 | 2e | TEM-3 | ST13 | 0 |
| 6 | 3 | 2005 | 1 | 2f | TEM-3 | ST48 | 3 |
ESBL, Extended-spectrum β-lactamase.
Maternity.
ICU(A), ICU(B), neurosurgery, digestive system surgery, colorectal surgery, gastroenterology and internal medicine.
Hepatology.
Internal medicine.
Gastroenterology and neurosurgery.
ICU(B) and digestive system surgery.
Cluster 2 comprised 21 isolates, of which one was imported by a patient who had stayed in ICU(A) during a previous hospitalization at the same time as another cluster 2 carrier. The 21 isolates were recovered from 15 rectal swabs and six clinical samples in 2006 and 2007. They all produced CTX-M-15 and displayed ST16 according to the MLST method applied to eight representative isolates. The 21 patients were hospitalized in seven different wards but 14 of these patients, including the index case, were cared for in ICU(A) when their ESBL-producing isolate was detected. Hospital stay overlaps were observed in 12 of the 14 patients hospitalized in ICU(A), clearly suggesting cross-contamination in this ward. Three other patients with cluster 2 isolates, hospitalized in three different wards (neurosurgery, gastroenterology, internal medicine) at the time of detection of being an ESBL carrier, had previously stayed in ICU(A). However, for two of these patients, ICU(A) hospitalization occurred during a period with no cluster 2 carrier detection. Of the four remaining patients, one was hospitalized in a surgery ward at the same time as a patient known to have contracted the epidemic strain in ICU(A) some weeks ago, and the three others were hospitalized in wards where other patients with cluster 2 had never stayed [ICU(B) and digestive system surgery] or had stayed 3 months previously (internal medicine). Overall, of the 21 patients carrying cluster 2, 14 had both temporal and spatial relationships whereas two patients had neither temporal nor spatial relationships with other carriers. For the five remaining patients, there was a spatial link (same ward) with other cluster 2 carriers, but without temporal overlap. As the study was retrospective, it was not possible to look for environmental strains of cluster 2.
Similar features were observed for the other clusters (Table 3): temporal-spatial relationships between three of the four patients harbouring cluster 3, spatial but not temporal relationships between the two patients harbouring cluster 4, no temporal-spatial relationship between the two patients harbouring cluster 5, and temporal-spatial relationships between the three patients harbouring cluster 6. The STs determined for two representative isolates of clusters 3, 4 and 5, and one of cluster 6 were ST101, ST436, ST13 and ST48, respectively.
Overall, for the 34 patients carrying clustered isolates, hospital stay overlaps with other patients harbouring the same cluster were documented in 22 cases (Table 3).
DISCUSSION
Based on 57 participating hospitals, the rate of K. pneumoniae blood isolates resistant to extended-spectrum cephalosporins increased from 4% in 2005 to 10% in 2007 in France (EARS-net, http://www.rivm.nl.earss/). Such an evolution has also been observed in other parts of the world [10–12]. The multiplication by 4·4 of the incidence density of ESBL-producing K. pneumoniae in our hospital between 2005 and 2007 is, thus, in accordance with the evolution observed elsewhere. Therefore, we were interested in determining which factors could explain this increase in spite of the implementation of infection control measures.
Compared with the epidemiological features of ESBL-producing K. pneumoniae identified in the 1980s, the present study provides noticeable differences. In the past, ESBL-producing K. pneumoniae were essentially observed in the hospital setting, as is currently the case for carbapenamase-producing K. pneumoniae [4]. In contrast, between 2005 and 2007, 40% of strains were imported in our hospital and 16% were apparently community acquired. Our results are in accord with those from recent studies from Spain [13] and Canada [11]. In the past, ESBL-producing K. pneumoniae isolates were essentially found in ICUs [1, 3], whereas in our study the 74 patients carrying ESBL-producing K. pneumoniae were hospitalized in 15 of the 17 wards. We identified, as largely documented, nosocomial cross-transmission especially in ICUs, but the number of patients involved in cross-contamination in ICUs as well as in the other wards was low (22/74, 30%). We found that the number of K. pneumoniae isolates producing CTX-M increased each year and that this enzyme was predominant, whereas the number of isolates producing TEM and SHV enzymes was stable. A shift from TEM and SHV to CTX-M type enzymes in K. pneumoniae has recently been reported in different studies [11, 12, 14]. Nevertheless, in the present study we identified two enzymes (TEM-3, SHV-2) that were commonly produced by K. pneumoniae in the past [3].
Hence, our findings concur to suggest that a change occurred in the epidemiology of ESBL-producing K. pneumoniae in the mid-2000s, similar to the shift observed for E. coli in the early 2000s [1] when this species became the predominant CTX-M-producing Enterobacteriaceae.
The 74 ESBL-producing K. pneumoniae isolates displayed a polyclonal structure. However, this polyclonal structure was dominated by a few clusters over the study period, one of which (cluster 2, ST16) was highly predominant as it included 21 persons. We showed that this predominance was not related to cross-transmission in all of the cases. Three hypotheses could explain the recurrence of clusters in the same ward: (i) contamination from patients whose colonization was not detected, (ii) persistence of the strain in the hospital environment [15, 16], and (iii) the repeated introduction into the hospital of globally disseminated clones. The latter phenomenon has been well described for E. coli ST131 [17].
ST16, the dominant clone in our study, has already been described in Paris in the early 2000s in a non-ESBL producer [9]. Next, ST16 has been shown to be involved in outbreaks of CTX-M-15-producing K. pneumoniae in Sweden [18] and Denmark [19, 20], respectively, in 2005 and 2008, and reported as a KPC producer in Brazil in 2008 [21, 22]. It should be noted that ST16 is a single locus variant (SLV) of ST17, which has been reported as an ESBL producer in Spain (2008) [23], Denmark (2008) [19], Canada (2002) [11] and Asia (2008) [12], as a DHA-1 producer in Spain (2007) [24], and as a KPC producer in Greece (2009) [25]. Therefore, it is possible that isolates of ST16 and ST17 are part of a clone disseminated worldwide. Previously [7], we defined clonal groups as groups including a chosen ST (central genotype), its SLVs (first circle SLVs), and the SLVs of the first circle SLVs (second circle SLVs). However, current MLST diversity (based on the 971 STs in the K. pneumoniae database at www.pasteur.fr/mlst) does not allow defining a meaningful clonal group that would encompass ST16 and ST17, as the application of our previous definition to ST16 or ST17 as central genotypes would include a large proportion of all K. pneumoniae STs. The difficulty in defining clonal groups in K. pneumoniae is related to the high rate of recombination between STs [26].
ST101, identified in this study in four patients, is another widespread clone harbouring various β-lactamases: CTX-M-15 in 2003 in Tunisia [27]; both CTX-M-15 and OXA-48 in 2009 in France, from a patient transferred from Tunisia [28], and in Barcelona where it caused a hospital outbreak [29]; OXA-48 in Israel from a Palestinian patient in 2011 [30]; SHV-12 in 2006 in Eastern European countries [31]; CTX-M-15 in 2008 in Asia [12]; and, finally, KPC in 2007 in the USA [32] and in 2009 in Brazil [21].
ST48, producing TEM-3 and identified in three patients with temporal-spatial relationships, has already been described as producing SHV-12 in 2003 in Tunisia [27] and CTX-M-15 in 2006 and 2008 in Korea [33, 34].
ST35 producing SHV-2 was recovered in a mother and her newborn. ST35 has also been reported in Tunisia (SHV-12 in 2002) [27], in Spain (CTX-M-15 in 2008) [23], and in Denmark (without ESBL in 2008) [19].
ST13 identified in two patients in our study without an epidemiological link, was reported in Curaçao during an ESBL-producing K. pneumoniae outbreak in 2005 [9] and in Spain during a DHA-1-producing K. pneumoniae outbreak in 2007 [24]. Moreover, ST13 is a SLV of ST327 that has been reported to harbour CTX-M-15 in Spain [35] and KPC in Israel [36] and Brazil [22].
The last cluster, producing CTX-M-15 and identified in this study in two patients without a link, displayed ST436, which is unrelated to any another ST and, to our knowledge, has not been reported previously.
In conclusion, this study shows the complex epidemiology of ESBL-producing K. pneumoniae over the 2005–2007 period, during when a sharp increase in ESBL-producing K. pneumoniae incidence density was observed in our hospital and elsewhere. On the one hand, the polyclonal structure of ESBL-producing K. pneumoniae detected over the 2005–2007 period, together with the analysis of the temporal-spatial relationships of patients, strongly suggest that cross-contamination is not the major cause of the increased incidence density. On the other hand, we observed that a few clonal groups isolated from both epidemiologically and non-epidemiologically linked patients, corresponded in fact to clones that have been reported in other countries, occasionally producing distinct enzymes. These observations suggest that these clones might have emerged as early as 2006, as observed in our hospital, and had diffused widely since then as was documented for ST258/KPC-2 [22]. Alternately, the fact that distinct enzymes are harboured by these clones in different countries suggests independent β-lactamase gene acquisition, perhaps because these clones are frequent in human commensal flora and subsequently frequently exposed to antibiotics, as described for ST131 E. coli [37]. Better knowledge of the distribution of these STs/clonal groups in digestive tract carriage and other sources will be important to understand better the global epidemiology of ESBL-producing K. pneumoniae.
ACKNOWLEDGEMENTS
This study was supported by a grant from la Direction de la Recherche Clinique Ile-de-France (project PAS07010).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Cantón R, et al. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clinical Microbiology and Infection 2008; 14 (Suppl. 1): 144–153. [DOI] [PubMed] [Google Scholar]
- 2.Jarlier V, et al. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Reviews of Infectious Diseases 1988; 10: 867–878. [DOI] [PubMed] [Google Scholar]
- 3.Brun-Buisson C, et al. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet 1987; 2: 302–306. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TR. Clinically significant carbapenemases: an update. Current Opinion in Infectious Diseases 2008; 21: 367–371. [DOI] [PubMed] [Google Scholar]
- 5.Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiology Reviews 2011; 35: 736–755. [DOI] [PubMed] [Google Scholar]
- 6.Nicolas-Chanoine M-H, et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. Journal of Antimicrobial Chemotherapy 2008; 61: 273–281. [DOI] [PubMed] [Google Scholar]
- 7.Breurec S, et al. Klebsiella pneumoniae resistant to third-generation cephalosporins in five African and two Vietnamese major towns: multiclonal population structure with two major international clonal groups, CG15 and CG258. Clinical Microbiology and Infection. Published online: 15 February 2012. doi: 10.1111/j.1469-0691.2012..03805.x. [DOI] [PubMed] [Google Scholar]
- 8.Leflon-Guibout V, et al. Emergence and spread of three clonally related virulent isolates of CTX-M-15-producing Escherichia coli with variable resistance to aminoglycosides and tetracycline in a French geriatric hospital. Antimicrobial Agents and Chemotherapy 2004; 48: 3736–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diancourt L, et al. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. Journal of Clinical Microbiology 2005; 43: 4178–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damjanova I, et al. Expansion and countrywide dissemination of ST11, ST15 and ST147 ciprofloxacin-resistant CTX-M-15-type beta-lactamase-producing Klebsiella pneumoniae epidemic clones in Hungary in 2005-the new ‘MRSAs’? Journal of Antimicrobial Chemotherapy 2008; 62: 978–985. [DOI] [PubMed] [Google Scholar]
- 11.Peirano G, et al. Molecular epidemiology of extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae over a 10 year period in Calgary, Canada. Journal of Antimicrobial Chemotherapy 2012; 67: 1114–1120. [DOI] [PubMed] [Google Scholar]
- 12.Lee MY, et al. High prevalence of CTX-M-15-producing Klebsiella pneumoniae isolates in Asian countries: diverse clones and clonal dissemination. International Journal of Antimicrobial Agents 2011; 38: 160–163. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz de Alegría C, et al. Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases in Spain: microbiological and clinical features. Journal of Clinical Microbiology 2011; 49: 1134–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valverde A, et al. Complex molecular epidemiology of extended-spectrum beta-lactamases in Klebsiella pneumoniae: a long-term perspective from a single institution in Madrid. Journal of Antimicrobial Chemotherapy 2008; 61: 64–72. [DOI] [PubMed] [Google Scholar]
- 15.Gaillot O, et al. Nosocomial outbreak of Klebsiella pneumoniae producing SHV-5 extended-spectrum beta-lactamase, originating from a contaminated ultrasonography coupling gel. Journal of Clinical Microbiology 1998; 36: 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gastmeier P, et al. A cluster of nosocomial Klebsiella pneumoniae bloodstream infections in a neonatal intensive care department: identification of transmission and intervention. American Journal of Infection Control 2003; 31: 424–430. [DOI] [PubMed] [Google Scholar]
- 17.Blanco M, et al. Molecular epidemiology of Escherichia coli producing extended-spectrum beta-lactamases in Lugo (Spain): dissemination of clone O25b:H4-ST131 producing CTX-M-15. Journal of Antimicrobial Chemotherapy 2009; 63: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 18.Lytsy B, et al. The first major extended-spectrum beta-lactamase outbreak in Scandinavia was caused by clonal spread of a multiresistant Klebsiella pneumoniae producing CTX-M-15. Acta Pathologica, Microbiologica, et Immunologica Scandinavica 2008; 116: 302–308. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen JB, et al. Identification of CTX-M15-, SHV-28-producing Klebsiella pneumoniae ST15 as an epidemic clone in the Copenhagen area using a semi-automated Rep-PCR typing assay. European Journal of Clinical Microbiology & Infectious Diseases 2011; 30: 773–778. [DOI] [PubMed] [Google Scholar]
- 20.Lester CH, et al. Emergence of extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae in Danish hospitals; this is in part explained by spread of two CTX-M-15 clones with multilocus sequence types 15 and 16 in Zealand. International Journal of Antimicrobial Agents 2011; 38: 180–182. [DOI] [PubMed] [Google Scholar]
- 21.Seki LM, et al. Molecular epidemiology of KPC-2- producing Klebsiella pneumoniae isolates in Brazil: the predominance of sequence type 437. Diagnostic Microbiology and Infectious Disease 2011; 70: 274–277. [DOI] [PubMed] [Google Scholar]
- 22.Andrade LN, et al. Dissemination of blaKPC-2 by the spread of Klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among Enterobacteriaceae species in Brazil. Antimicrobial Agents and Chemotherapy 2011; 55: 3579–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oteo J, et al. Emergence of CTX-M-15-producing Klebsiella pneumoniae of multilocus sequence types 1, 11, 14, 17, 20, 35 and 36 as pathogens and colonizers in newborns and adults. Journal of Antimicrobial Chemotherapy 2009; 64: 524–528. [DOI] [PubMed] [Google Scholar]
- 24.Diestra K, et al. Multiclonal epidemic of Klebsiella pneumoniae isolates producing DHA-1 in a Spanish hospital. Clinical Microbiology and Infection 2011; 17: 1032–1036. [DOI] [PubMed] [Google Scholar]
- 25.Giakkoupi P, et al. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009–10). Journal of Antimicrobial Chemotherapy 2011; 66: 1510–1513. [DOI] [PubMed] [Google Scholar]
- 26.Brisse S, et al. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 2009; 4: e4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elhani D, et al. Molecular epidemiology of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae strains in a university hospital in Tunis, Tunisia, 1999-2005. Clinical Microbiology and Infection 2010; 16: 157–164. [DOI] [PubMed] [Google Scholar]
- 28.Cuzon G, et al. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrobial Agents and Chemotherapy 2011; 55: 2420–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitart C, et al. First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 beta-lactamase in Klebsiella pneumoniae in Spain. Antimicrobial Agents and Chemotherapy 2011; 55: 4398–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler A, et al. Introduction of OXA-48-producing Enterobacteriaceae to Israeli hospitals by medical tourism. Journal of Antimicrobial Chemotherapy 2011; 66: 2763–2766. [DOI] [PubMed] [Google Scholar]
- 31.Hrabák J, et al. International clones of Klebsiella pneumoniae and Escherichia coli with extended-spectrum beta-lactamases in a Czech hospital. Journal of Clinical Microbiology 2009; 47: 3353–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitchel B, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrobial Agents and Chemotherapy 2009; 53: 3365–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko KS, et al. Predominance of an ST11 extended-spectrum beta-lactamase-producing Klebsiella pneumoniae clone causing bacteraemia and urinary tract infections in Korea. Journal of Medical Microbiology 2010; 59: 822–828. [DOI] [PubMed] [Google Scholar]
- 34.Shin J, Kim DH, Ko KS. Comparison of CTX-M-14- and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae isolates from patients with bacteremia. Journal of Infection 2011; 63: 39–47. [DOI] [PubMed] [Google Scholar]
- 35.Coelho A, et al. Detection of three stable genetic clones of CTX-M-15-producing Klebsiella pneumoniae in the Barcelona metropolitan area, Spain. Journal of Antimicrobial Chemotherapy 2009; 64: 862–864. [DOI] [PubMed] [Google Scholar]
- 36.Leavitt A, et al. Molecular epidemiology, sequence types, and plasmid analyses of KPC-producing Klebsiella pneumoniae strains in Israel. Antimicrobial Agents and Chemotherapy 2010; 54: 3002–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leflon-Guibout V, et al. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. Journal of Clinical Microbiology 2008; 46: 3900–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]