Abstract
Climate and soil factors induce substantial controls over plant biodiversity in stressful ecosystems. Despite of some studies on plant biodiversity in extreme ecosystems including rocky outcrops, simultaneous effects of climate and soil factors have rarely been studied on different facets of biodiversity including taxonomic and functional diversity in these ecosystems. In addition, we know little about plant biodiversity variations in such extreme ecosystems compared to natural environments. It seems that environmental factors acting in different spatial scales specifically influence some facets of plant biodiversity. Therefore, we studied changes in taxonomic and functional diversity along precipitation and soil gradients in both landscapes (i) rocky outcrops and (ii) their nearby rangeland sites in northeast of Iran. In this regard, we considered six sites across precipitation and soil gradients in each landscape, and established 90 1m2 quadrates in them (i.e. 15 quadrats in each site; 15 × 6 = 90 in each landscape). Then, taxonomic and functional diversity were measured using RaoQ index, FDis and CWM indices. Finally, we assessed impacts of precipitation and soil factors on biodiversity indices in both landscapes by performing regression models and variation partitioning procedure. The patterns of taxonomic diversity similarly showed nonlinear changes along the precipitation and soil factors in both landscapes (i.e. outcrop and rangeland). However, we found a more negative and significant trends of variation in functional diversity indices (except for CWMSLA) across precipitation and soil factors in outcrops than their surrounding rangelands. Variations of plant biodiversity were more explained by precipitation factors in surrounding rangelands, whereas soil factors including organic carbon had more consistent and significant effects on plant biodiversity in outcrops. Therefore, our results represent important impacts of soil factors in structuring plant biodiversity facets in stressful ecosystems. While, environmental factors acting in regional and broad scales such as precipitation generally shape vegetation and plant biodiversity patterns in natural ecosystems. We can conclude that rocky outcrops provide suitable microenvironments to present plant species with similar yields that are less able to be present in rangeland ecosystems.
Subject terms: Biodiversity, Community ecology, Ecology
Introduction
Rocky outcrops and cliffs have been identified as one of the most important micro-habitats in stressful ecosystems1. These micro-habitats are known by inaccessible topography to humans and livestock, high environmental changes, and low levels of biotic interactions, which provided safe sites for the native, relic, and endemic species2–4. On the other hand, they are characterized by shallow soils, low water and nutrients, low night temperatures, high sunlight, and strong winds3,5,6. These fragmented and isolated natural habitats are characterized by spatial and ecological segregation that hinder dispersal and migration due to environmental and geographical conditions7–9. Despite the importance of these ecosystems, biodiversity studies have been conducted less in them and need further study10–14. Therefore, it is necessary to examine the impacts of different environmental factors and rocky outcrops on biodiversity to understand better the factors influencing biodiversity patterns.
Outcrops are recognized as biodiversity hotspots for plants with specific adaptive traits, such as tolerance to environmental stresses15. Certain studies have also shown a degree of floristic similarity between the outcrops16–19 with a low degree of genetic exchange rates between different rock outcrop populations20–24. Such features of outcrops have led to some differences in biodiversity between these specific ecosystems with their surrounding environments, in which environmental factors indirectly handle such differences25–28. However, relative importance of environmental factors acting in different spatial scales such as climatic factors in regional scale compared to soil factors in fine scales on shaping such differences between outcrops and their surrounding environments is unknown29.
Understanding the processes shaping the biodiversity in outcrops is necessary due to shed light in relative importance the environmental factors on biodiversity of outcrops30. Biodiversity in outcrops may be associated to variation in some environmental factors such as soil depth, soil fertility, topographic and climatic gradients31,32. Various studies have shown the effects of precipitation and soil fertility on taxonomic and functional diversity in some outcrops located in northeastern Iran and Spain25,29,33–39. Precipitation is much more pronounced in arid and semi-arid ecosystems than other climatic factors, with an important impact on shaping the biodiversity at regional spatial scales35,40. Whereas, soil factors indicated important effects on structuring biodiversity facets at fine and local spatial scales41–45.
Most of studies on the flora and biodiversity of rocks and outcrop communities have focused on taxonomic diversity3,46–51. Biodiversity is not just the diversity of species present in a community, but a multiple structure and concept calculated and interpreted through a wide range of genes, species, and functional traits in the ecosystem52,53. Taxonomic diversity may give us minor information about all dimensions of biodiversity. For example, plant diversity can have precisely the same taxonomic diversity but very different levels of functional diversity resulting in very different levels of biodiversity53,54. Furthermore, approaches based on functional traits have come out as a promising way to understand plant ecological strategies, plant interactions, and their linkages to ecosystem functioning55–59. Therefore, it is necessary to measure plant functional diversity simultaneous with taxonomic diversity for a mechanistic understanding the impacts of different environmental factors on structuring the biodiversity in outcrops and their surrounding environments60,61.
This study was conducted on limestone outcrops in northern Iran, along a cross transaction of Alborz Mountain Ranges from Shahroud (dry steppe rangeland) to Gorgan (temperate forest). Various geological and topographical situations, huge climatic contrasts, and a long history of evolution in this region make its rocky outcrops and surrounding rangelands as interesting and challenging environments for study62–68. However, studies on biodiversity of outcrops and their surrounding rangelands in Iran are limited to measuring taxonomic diversity69–73. In addition, relative importance of environmental factors on structuring of biodiversity facets and degree of correlation between outcrops and their surrounding rangelands with respect to biodiversity facets are not recognized so far. Therefore, we investigated the effects of precipitation and soil factors on taxonomic and functional diversity in the outcrops and their surrounding rangelands. In addition, we tested relative importance of these environmental factors to explain variations in taxonomic and functional diversity, and addressed following questions: (1) how the taxonomic and functional diversity vary across precipitation and soil factors with respect to ecosystem considered (i.e. outcrops vs surrounding rangelands)? (2) Do precipitation and soil factors exhibit significant effects on structuring taxonomic and functional diversity, and if so, by how much?
Results
Plant taxonomic and functional diversity across precipitation and soil factors
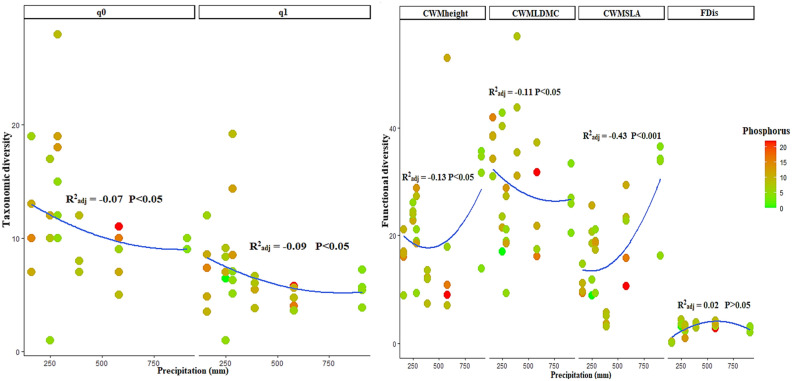
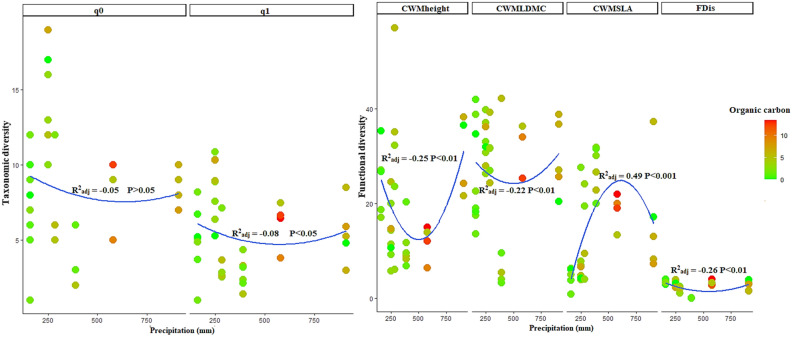
The taxonomic diversity similarly changed across precipitation and soil factors in both outcrops and their surrounding rangelands (Figs. 1, 2, Appendix S2). In this regard, we found a decrease in taxonomic diversity especially under intermediate levels of precipitation and soil factors (organic carbon in outcrops and phosphorus in rangelands) in both the environments (please see q0 and q1 panels in Figs. 1 and 2 and Appendix S2). Variations in taxonomic indices across environmental factors were not significant in outcrops (except for q1 index), whereas taxonomic indices significantly responded to precipitation and phosphorus in surrounding rangelands. In general, rangeland ecosystems had more species richness and diversity than outcrops (Fig. 1; q0 and q1 panels).
Figure 1.
Variation in taxonomic (q0 and q1 in left panels) and functional (CWMheight, CWMLDMC, CWMSLA and FDis in right panels) diversity in rangeland sites across precipitation and phosphorus (P) gradients.
Figure 2.
Variation in taxonomic (q0 and q1 in left panels) and functional (CWMheight, CWMLDMC, CWMSLA and FDis in right panels) diversity in rocky outcrops across precipitation and organic carbon factors.
Functional indices showed significant nonlinear variations across precipitation and soil factors in both outcrops and their surrounding rangelands (Figs. 1, 2). In rangelands, functional diversity indices including CWM-plant height (CWMHeight), CWMSLA and FDis significantly decreased under moderate levels of precipitation and phosphorus gradients (Fig. 1; CWMHeight, CWMSLA, FDis panels). In contrast, an increase for CWMLDMC was observed under intermediate levels of precipitation and phosphorus gradients (Fig. 1; CWMLDMC panel). In outcrops, CWMHeight, CWMLDMc and FDis indices significantly decreased under moderate levels of precipitation and organic carbonWhereas, we found a strong and significant increase for CWMSLA across organic carbon and precipitation gradients. Overall, our results indicated more consistent and significant variations for functional diversity indices than taxonomic indices in both outcrops and their surrounding rangelands. (Figs. 1, 2; R2 and P-value for FDis, CWMLDMC, CWMheight and CWMSLA in outcrops and rangelands). In addition, in outcrops, variations in functional diversity indices across environmental factors were stronger than rangelands, with more negative and significant effects of precipitation and organic carbon on FDis, CWMLDMC and CWMheight Whereas, CWMSLA under moderate levels of precipitation and organic carbon (Fig. 2). In rangeland sites, although all functional diversity indices studied exhibited negative trends across precipitation and phosphorus factors (except in FDis index), we found more negative and significant variations of CWMSLA across environmental gradients (Fig. 1).
Relative importance of precipitation and soil factors on taxonomic and functional diversity
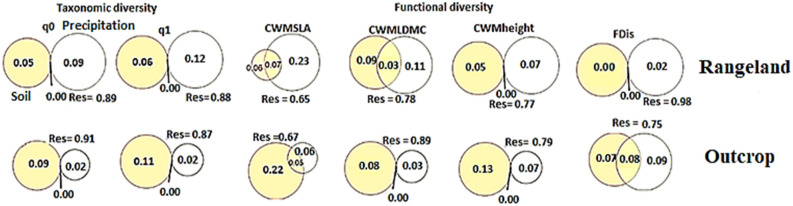
Our results indicated relative importance of precipitation and soil factors on structuring taxonomic and functional diversity in outcrops and their surrounding rangelands (Fig. 3). In outcrops, soil factors including organic carbon) Fig. 3; yellow circles) explained higher contributions of variation in taxonomic (q0 (9%), q1 (11%)) and functional (CWMSLA (22%), CWMLDMC (8%), CWMHeight (13%) and FDis (7%)) diversity than precipitation (Fig. 3; Outcrop panel, yellow circles). Indeed, Precipitation was not an appropriate factor in explaining the changes for biodiversity indices in outcrops. In contrast, precipitation explained a large contribution of plant biodiversity in surrounding rangelands. In this regard, precipitation (Fig. 3; white circles) explained higher proportion of variations in taxonomic (q0 (9%), q1 (12%)) and functional (CWMSLA (23%), CWMLDMC (11%), CWMHeight (7%) and FDis (2%)) diversity than soil factors such as phosphorus (yellow circles) (Fig. 3; Rangeland panel). Therefore, precipitation and soil factors including organic carbon had more consistent and significant effects on biodiversity indices in rangelands and outcrops, respectively (Fig. 3; Rangeland and Outcrop panels).
Figure 3.
The relative contribution of environment (in yellow; i.e. soil factors; organic carbon (OC) for rocky outcrops, Phosphorus (P) for surrounding rangelands) and precipitation (in white) to taxonomic (q0 and q1 indices) and functional (CWMALA, CWMLDMC, CWMheight and FDis) in surrounding rangelands (up) and rocky outcrops (down). Values represent the adjusted R2‐values.
Discussion
We found environmental factors such as precipitation and soil factors significantly influenced taxonomic and functional diversity in outcrops and their surrounding rangelands. However, there are differences in the trend and extend of such variations along the gradients.
Plant diversity indices along the precipitation and soil factors gradients
Throughout the system under study, hill numbers as taxonomic diversity indices showed in the rangeland a greater diversity of outcrops. Taxonomic variability showed similar changes and decreased along soil and precipitation gradient in outcrop and rangeland. In the outcrops and their surrounding rangelands, taxonomic diversity was decreased under moderate levels of variation across environmental factors. Taxonomic diversity generally can be affected by soil fertility, topographic factors, and even biotic factors74. However, variations in some soil factors can significantly influence presence of plant species based on their realized niches especially in more severe spatial scales44,75. Therefore, in outcrops with more severe environmental conditions than rangelands, we found a more significant effects of soil factors than climatic factors such as precipitation on structuring taxonomic diversity (see Fig. 3; Outcrop panel). While, in rangelands, important effects of climatic factors such as precipitation could determine shaping taxonomic diversity and presence of plant species in plant communities (see Fig. 3; Rangeland panel). On the other hands, more significant effects of phosphorus factors than other soil factors studied can be related to competition between plant species in absorption of phosphorus of deeper layers of soil76. However, absorption of phosphorus is correlated to the amount of precipitation, as a decrease in precipitation can lead to an increase in phosphorus (due to the death of some plant tissues under an increase in aridity index)77. In a study across the precipitation and soil fertility in northeastern Iran78, a decrease in taxonomic diversity was also observed by increasing mean annual precipitation. Huston79 believes variations in taxonomic diversity across precipitation gradient are associated to mechanisms regulating the species coexistence and variation in functional diversity80,81.
We found nonlinear changes in functional diversity in response to environmental factors, under harsh outcrop conditions and in their surrounding rangeland sites. More negative and significant trends of variation in functional diversity (except for CWMSLA) across environmental factors was observed in outcrops than rangeland sites. Such reduction in functional diversity can be due to presence of low-yielding species that are functionally similar12,82. Climatic factors coupled by variations in soil factors reduce biodiversity by providing different adaptations to deal with water and nutrient stress. Therefore, such adaptations may maintain the coexistence of species with different levels of functional traits80,81. In addition, spatial (resource niche) and temporal heterogeneity (temporal climate diversity) in arid conditions provide heterogeneous niches for presence of species with different levels of functional traits83. Changes in functional diversity have different trends in rangeland and outcrop with increasing precipitation. While is observed, an increase in the presence of species with different functions especially in the middle of soil and precipitation gradient in rangeland. This shows that the functional differentiation among species and species diversity can very rather independently to each other. It has been noted that environmental filters might limit species diversity and composition to a given range of functional characteristics35. The reason for the presence of species with high functional convergence is due to the micro-habitat role of the outcrops in facilitating functional groups with similar characteristics in the outcrops77. Functional facilitation of species can also occur indirectly under the influence of environmental factors. Functional divergence of species can be due to the prominent role of competition or equilibrium interaction between competition and facilitation, especially in the middle of soil and precipitation gradient in rangeland. Competition between similar functional groups due to having similar niches causes plant species with different functional domains to be present in a community12.
Changes in ecological and functional traits of plant community
Changes in community traits showed a similar trend across precipitation and soil factors gradients in outcrops and rangelands. However, analysis of the measured plant traits showed different functional syndromes of traits observed at the community level along the precipitation and soil factors gradients. The average specific leaf area and plants height were low in the drier sites but increased along the precipitation and Phosphorus gradients in rangelands. However, in outcrops community height and leaf dry matter content decreased and specific leaf area increased in the middle gradient to increasing precipitation and organic carbon.
In the arid ecosystems, harsh environmental conditions such as low precipitation and high temperatures induces carbon storage, which result in lower leaf area and specific leaf area as compared with plant growing under the more favorable environmental conditions84. Previous studies have also shown that SLA and LA are often positively correlated with photosynthesis and growth rate and evapotranspiration rate85, and plants with high SLA and LA strategies and high evapotranspiration rates are often unable to tolerate drought stress86,87. Our results also indicated plant height increase along the precipitation gradient from arid-steppe towards dense temperate forest. Plant height is positively related to the competitive ability in obtaining light in plant communities88. On the other hand, high leaf dry matter content that we found in drier areas indicate species adaptation and their increased resistance to environmental stresses89–91. like previous researches in our study also, soil factors (i.e. phosphorus) was more associated with plant traits92, leaf area, specific leaf area, plant height, and leaf dry matter content positively correlated with available soil phosphorus and nitrogen93, soil factors influenced plant traits and species diversity and richness94,95.
Role of environmental factors on plant diversity indices
The results of biodiversity analysis on variation partitioning showed that both precipitation and soil properties influenced taxonomic and functional diversity. However, the contribution of soil factors in outcrops was more than rainfall. Whereas, in the rangeland sites, precipitation was the most important factor in structuring of taxonomic and functional diversity.
Richness indices, taxonomic and functional diversity were affected by organic carbon in the outcrop. This shows that soil fertility can play an essential role in increasing these indices. Soil fertility has a significant effect on controlling the amount of moisture and nutrients available to plants, which have profound effects on vegetation composition changes96. The factors that directly affect taxonomic diversity, later on may affect other abiotic factors97. Similarly in granitic and gneissic outcrops of south-eastern Brazil, taxonomic and functional diversity were significantly affected by abiotic factors of soil factors and soil depth98.
In the rangeland (i.e. soil covered landscapes), Precipitation factor was the most important factor affecting richness, Hill diversity and functional dispersion. In general, Hill plant diversity was more affected by soil properties (organic carbon) in outcrop, while the average annual precipitation was more important in rangeland11,13,99. Nevertheless for functional dispersion, the contribution of precipitation factor (soil vs. precipitation) was more than phosphorus and organic carbon in rangeland and outcrops100.
Plant traits were more associated with organic carbon than climatic factors in outcrop. Indices of the community single traits (i.e. specific leaf area, plant height, and leaf dry matter content) were more explained by organic carbon than precipitation in outcrop101. Rocky outcrops showed more correlation to soil factors, and climatic factors such as precipitation had a negligible effect on explaining biodiversity and ecological traits. In rangeland plant traits were more affected with precipitation factors than soil phosphorus. Main factors affecting plant Leaf Dry Matter Content (LDMC) in the outcrop was organic carbon, but in the nearby covered lands they were mostly affects by phosphorus. The high observed changes in LDMC are likely related to an efficient nutrient conservation strategy102,103. In addition, leaves with a high dry matter content may maintain torque stress with relatively more minor water potential and increase drought resistance and freezing resistance104,105. In general, plant height and their growth period were decreased by increasing soil Na and K that salinity may be due to disturbances in nutrient uptake, disturbance of ionic balance or reduction of soil water potential and osmotic stress, or changes in enzymes affecting the plant photosynthetic activity apparatus106.
Conclusions
We investigated changes in plant taxonomic and functional diversity, along the precipitation gradients and soil properties in rocky outcrops and adjacent covered landscapes. According to our results, changes in soil properties (organic carbon) more affected taxonomic, functional diversity and functional traits in outcrop. Whereas, in the rangeland sites, precipitation was the most important factor in structuring of taxonomic and functional diversity. Whereas, similar environmental factors (Phosphorus and organic carbon and precipitation) regulated taxonomic diversity of rocky outcrops and nearby rangelands, functional diversity showed greater diversity of drought adapted traits at the community level of rocky outcrops. Therefore, our results highlight important role of micro-scale environmental factors such as presence of critical species (keystone species) and/or effects of microhabitats on plant community composition and diversity facet along the environmental gradients. For future researches, considering other climatic variables (seasonal precipitation and minimum and maximum annual temperatures, etc.) and topographic factors (height, aspect, and percentage of slope), and the effects of biotic interactions and their relative importance along abiotic factors, will bring more insights on plant diversity of the mountainous (here out-crop versus nearby soil covered landscapes) dryland ecosystems.
Methods
Study region
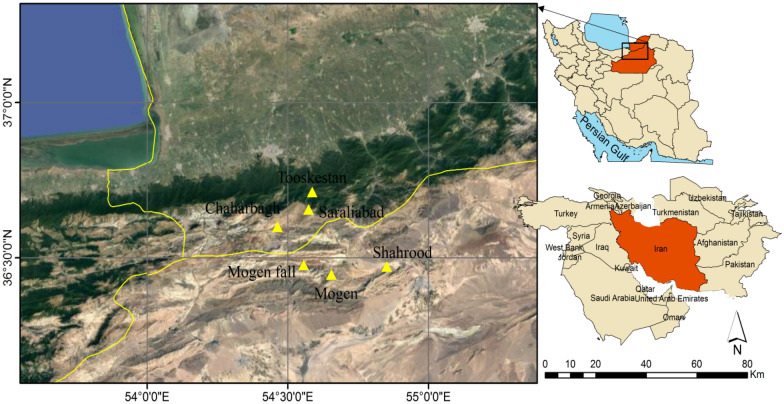
The study area is located in southwest Asia and north of Iran, crosses Alborz mountains from arid steppe rangeland in Shahroud to temperate forests in Gorgan. The study areas were selected on calcareous geological formation and north facing slopes. Six sites were selected (Fig. 4), with the annual precipitation (mm) from 160 (Shahroud), 250 (Mojen), 285 (Mojen waterfall), 390 (Chaharbagh), 580 (Sar Ali Abad), to 910 (Tooskestan) (Appendix S1). The selected homogeneous study sites. They were located in in terms north-facing slopes, calcareous geological formation, main land use as rangelands However, they were different depending in terms of dominant plant species (shrubs, perennial forbs and shrub, and large trees), average yearly precipitation [160 mm (Shahroud) − 910 mm (Tooskestan)], elevation [800 m (Shahroud) − 2700 m (Sar Ali Abad)] and soil factors including pH [7.9 (Tooskestan) − 8.7 (Shahroud)m], EC [74 (Mojen) − 338 (Tooskestan) (dS m−1)], K [216 (SarAliAbad) – 860 (Chaharbagh) (mg kg−1)], N [0.06 (Shahroud) − 0.69 (SarAliAbad) (mg kg−1)], Lime (%) [10.5 (Chaharbagh) − 26.27 (Shahroud)], Na [24 (Tooskestan) − 86 (Shahroud) (mg kg−1)], P [4 (Mojen) − 25 (Chaharbagh) (mg kg−1)], OC [0.4 (Shahroud) − 16 (Tooskestan) (%)], Clay [22 (Shahroud) − 60 (Chaharbagh) (%)], Silt [28(Shahroud) − 60 (Chaharbagh) (%)] and Sand [4 (Tooskestan) − 44 (Shahroud) (%)] (Appendix S1). We confirm that our study and sampling methods conducted comply with local and national regulations or guidelines.
Figure 4.
Map of the study area in Northeast of Iran, in which site locations are shown. The following map has been generated in َArcmap ver. 10.2 (https://www.esri.arcGIS.desktop.com).
Data collection
Vegetation sampling was done in a systematic randomized method in which six sites (i.e. Shahroud, Mojen, Mojen waterfall, Chaharbagh, Sar Aliabad, and Tooskestan) were systematically established along the precipitation and soil gradients and in two landscapes (i.e. rocky outcrops and their surrounding rangelands). Then, within each landscape in each site, 15 1 m2 quadrats were randomly established (15 × 6 = 90 quadrats in total for each landscape). Sampling was conducted in May–June 2017, but complementary sampling was accomplished as same months in 2018. The distance between sampling units were approximately 50–100 m. In each quadrat, the species abundance, canopy cover, climatic, soil and topographic information were recorded. Plant specimens were collected to identify and measure functional trait (10 individuals of each species) in each plot. They were immediately, packed in paper bags, sealed in plastic bags, and transferred to the laboratory.
Plant functional information
Functional diversity is assessed based on functional traits. Functional traits describe indicators of competition, growth, adaptation, establishment, and environmental variables. As a result, 10 qualitative and quantitative traits were selected based on the objectives of study. They were: Plant height, Seed mass, Leaf area (LA), Specific leaf area (SLA), Leaf dry-matter content (LDMC), Life form, Life span, Clonality, Spinences and Palatability107. To measure these traits, 10 individuals of each species were collected and placed in plastic bags to retain moisture and transferred to the laboratory. Plant height is the shortest distance between the upper foliage boundary and ground level108.
We determined the leaf area using a digital scanner and Leaf Area Measurement v1.3 software (Andrew Askew, University of Shefeld, UK). Leaf fresh matter content was obtained from saturated leaves, and leaf dry matter content was determined after drying for 72 h in an oven at 70 °C. For qualitative traits, plant life forms were coded into five classes: phanerophytes (Ph), chamaephytes (Ch), hemicryptophytes (He), geophyte (Ge), and therophytes (Th), using Raunkiaer’s109 classification. Clonality was expressed as the presence/absence of clonal reproduction of individual species via assessing of rhizomes or runners. Life span was also divided into annual and perennial. Thorns were also classified based on the presence or absence of thorns.
Soil collection and processing
Soil samples (~ 500 g) were taken at a 0–20 cm depth, placed in a polyethylene bag, labeled, and transported to the laboratory. The following properties were measured in each plot in the outcrop (39 samples) and their surrounding rangelands (30 samples): pH, electrical conductivity (EC), organic carbon (OC), Sodium (Na), total nitrogen (N), Potassium (K), phosphorus (P) and soil texture components including lime, silt, sand, and clay percentage. Bykas hydrometric method110 was used to determine soil texture. Total nitrogen (N) was determined by the Kjeldahl method111. Organic carbon (OC) was analyzed by the Walkley and Black112 method113. Soil electrical conductivity (EC) and acidity (pH) were determined using pH and EC meters. Total potassium (K) and sodium (Na) were analyzed by flame atomic absorption spectrophotometer114. Absorbable phosphorus was analyzed by the Olsen method. The percentage of total lime was measured by titration method with 0.01 N NaOH115. Finally, we prepared a matrix of 12 independent variables (i.e. annual precipitation, sodium (Na), potassium (K), pH, electrical conductivity (EC), lime, total nitrogen (N), phosphorus (P), organic carbon (OC), clay, sand, and silt ) and used in further analyses.
Statistical analysis
Measures of taxonomic and functional diversity
We measured the taxonomic diversity using RaoQ index. In this regard, the first three Hill numbers of RaoQ index were selected to estimate species richness (q = 0), the exponential of Shannon's entropy (q = 1; referring to Shannon diversity) and the inverse of Simpson's concentration (q = 2; referring to Simpson diversity). This analyze was computed using R package hillR116.
Functional diversity was calculated using the community weighted means index (CWM) and multi-trait functional diversity indices such as FDis. CWM traits were calculated as mean trait values for each vegetation plot, weighted by the relative abundances of species with that particular trait values117,118. The community‐weighted means (CWM) for each trait and community sample were calculated as ΣPi × Trait i, where Pi is the relative abundance of species i in the community sample and j trait i is the trait value. Further, mean values of individual traits (height, seed mass, leaf area, clonality, annual–perennial life history) were calculated for each vegetation plot. Eventually, the mean trait values per plot (weighted by the relative abundances of species) were essential in the analyses. Functional dispersion (FDis) was calculated based on Laliberte and Legendre119 procedure. We chose FDis among the many functional diversity metrics because it describes the distribution of species in trait space, can be used for multiple traits, is not strongly influenced by outliers, and is independent of species richness. We calculated FDis using the “FD” function in R package FD119.
Statistical analyses
We analyzed variation in taxonomic and functional diversity relative to precipitation, soil factors and their interactions in both outcrops and their surrounding rangelands. Some of the environmental factors were highly correlated with each other and could induce multicollinearity in our models. To avoid this, environmental variables with |r|> 0.7 were considered highly correlated120 hence they were removed from analysis to to avoid model predictions induced by multicollinearity among environmental variables (Appendix S1 and S4). Further, we analyzed multicollinearity amongst the remaining variables using variance inflation factors (VIF) (function vif() in the package ‘car’121 and variables with VIF scores > 10 were considered to be highly collinear122 and removed from our environmental matrix. For outcrops, only seven variables (annual precipitation, OC, Clay, Silt, P, elevation and limestone were selected and used on subsequent analyses. Annual precipitation, pH, P, elevation, Silt, Sand and K were selected as non-correlated variables for surrounding rangelands. To further simplify our models, we used a forward selection procedure (function forward.sel () in the package “packfor”123), keeping only those environmental variables selected in the most parsimonious models for taxonomic and functional diversity with respect to the usual alpha significance level and the adjusted coefficient of multiple determination (R2adj) calculated using all explanatory variables124. Annual precipitation and Organic Carbon (OC) were selected as most important factors influencing the plant biodiversity indices in outcrops, whereas Phosphorus (P) and annual precipitation were the ones selected in surrounding rangelands.
With the final list of predictors, we developed linear regression models with precipitation and Phosphorus in outcrops and Annual precipitation and Limestone in surrounding rangelands as explanatory variables and q = 0, q = 1, FDis, CWM SLA, CWM LA, CWM LDMC and CWM plant height as response variables. In addition to the linear trend, we also tested for non-linear trends of biodiversity facets with our explanatory predictors by developing non-linear regression models. Then, we compared these models using second-order Akaike information criteria (AIC) and R2adj values in both the ecosystems (see details in Appendix S3 in Supporting Information). Finally, the best models were plotted and their R2adj values were obtained using the package ‘vegan”125.
To assess the impacts of precipitation, soil factors, and their interactions on biodiversity indices at rangeland and outcrop sites, we performed variation partitioning based on partial linear regression using the “varpart” function125. The total percentage of variation explained was divided into unique and shared contributions for two predictors: (1) precipitation (white fraction), (2) soil (i.e. phosphorus in rangeland and organic carbon in outcrop) (yellow fraction), and (3) shared contributions of both factors (shared area between yellow and white fractions). Res. Value indicated residuals (i.e. the part of plant biodiversity which was not explained by the studied explanatory variables). Analyses were conducted in R ver. 4.0.0, and figures were produced using the ggplot2 package126.
Supplementary Information
Acknowledgements
The authors wish to thank the Ferdowsi University of Mashhad for funding this research under the Grant No. 3/49472.
Author contributions
H.E. and M.F. conceived of the research idea; F.R. collected data; F.R. and M.B. performed statistical analyses; F.R. with contributions from M.B. and M. F. wrote the paper; all authors discussed the results and commented on the manuscript.
Funding
This paper is part of a dissertation for fulfillment of PhD requirements of the first author, supported by the Research Council of the Ferdowsi University of Mashhad (Grant No. 3/49472).
Data availability
All data generated or analyzed during this study are included in this published article [Appendix S5 and S6 in supporting information].
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Affiliation 3, which was incorrectly given as ‘Herbarium of Nowshahr Botanical Garden, Research Center of Agriculture and Natural Resources of Mazandaran, Mazandaran, Iran’. The correct affiliation is listed here. Botanical Garden of Nowshahr, Research Institute of Forests and Rangelands, Agricultural Research, Education and Extension Organization, AREEO, Tehran, Iran.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/22/2022
A Correction to this paper has been published: 10.1038/s41598-022-16723-0
Contributor Information
Hamid Ejtehadi, Email: hejtehadi@um.ac.ir.
Mohammad Farzam, Email: mjankju@um.ac.ir.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13123-2.
References
- 1.Larson DW, Matthes U, Kelly PE. Cliff Ecology. Cambridge University Press; 2000. [Google Scholar]
- 2.Cooper A. Plant species coexistence in cliff habitats. J. Biogeogr. 1997;24:483–494. [Google Scholar]
- 3.Davis PH. Cliff vegetation in the eastern Mediterranean. J. Ecol. 1951;39:63–93. [Google Scholar]
- 4.Snogerup S, et al. Evolutionary and plant geographical aspects of chasmophytic communities. In: Davis PH, et al., editors. Plant life of South-West Asia. Bot. Soc. Edinb; 1971. pp. 157–170. [Google Scholar]
- 5.Baskin JM, Baskin CC. Endemism in rock outcrop plant communities of unglaciated eastern United States: An evaluation of the roles of the edaphic, genetic and light factors. J. Biogeogr. 1988;15:829–840. [Google Scholar]
- 6.Medina BMO, Fernandes GW. The potential of natural regeneration of rocky outcrop vegetation on rupestrian field soils in Serra do Cipo, Brazil. Braz. J. Bot. 2007;30:665–678. [Google Scholar]
- 7.Alves RJV, Cardin L, Kropf MS. Angiosperm disjunction “Campos Rupestres-Restingas”: Are-evaluation. Acta Bot. Bras. 2007;2:675–685. [Google Scholar]
- 8.Harley, R. M. Introduction. In Flora of the Pico das Almas, Chapada Diamantina, Bahia, Brazil (eds Stannard, B. L., Harvey, Y. B. & Harley, R. M) 1–42 (Royal Botanic Gardens, 1995).
- 9.Hubbell SP. Neutral theory in ecology and the evolution of ecological equivalence. Ecology. 2006;87:1387–1398. doi: 10.1890/0012-9658(2006)87[1387:ntateo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Conceição AA, Pirani JR, Meirelles ST. Floristics, structure and soil of insular vegetation in four quartzite-sandstone outcrops of “Chapada Diamantina”, Northeast Brazil. Rev. Bras. Bot. 2007;30:641–656. [Google Scholar]
- 11.Le Stradic S, Buisson E, Wilson FG. Vegetation composition and structure of some Neotropical mountain grasslands in Brazil. J Mt Sci 12:864–77. An. Acad. Bras. Ciênc. 2015;87(4):2097–2110. [Google Scholar]
- 12.Nunes JA, Schaefer CEGR, Ferreira-Junior WG, Neri AV, Correa GR, Enright NJ. Soil–vegetation relationships on a banded ironstone ‘island’, Carajás Plateau, Brazilian Eastern Amazonia. An. Acad. Bras. Cienc. 2015;87(4):2097–2110. doi: 10.1590/0001-376520152014-0106. [DOI] [PubMed] [Google Scholar]
- 13.Silva, W. A. Gradiente vegetacional e pedológico em complexo rupestre de quartzito no Quadrilátero Ferrífero, Minas Gerais, Brasil. MSc Thesis. (Universidade Federal de Viçosa, 2013).
- 14.Vincent RC, Meguro M. Influence of soil properties on the abundance of plant species in ferruginous rocky soils vegetation, southeastern Brazil. Braz. J. Bot. 2008;31:377–388. [Google Scholar]
- 15.Porembski S. Tropical inselbergs: Habitat types, adaptive strategies and diversity patterns. Rev. Bras. de Bot. 2007;30:579–586. [Google Scholar]
- 16.De Paula LFA, Forzza RC, Neri AV, Bueno ML, Porembski S. Sugar Loaf Land in south-eastern Brazil: A center of diversity for mat-forming bromeliads on inselbergs. Bot. J. Linn. Soc. 2016;181:459–476. [Google Scholar]
- 17.Rezende MG, Elias RCL, Salimena FRG, Neto LM. Flora vascular da Serra da Pedra Branca, Caldas, Minas Gerais e relações florísticas com áreas de altitude da Região Sudeste do Brasil. Biota Neotrop. 2013;13:201–224. [Google Scholar]
- 18.Sarthou C, Villiers JF, Ponge JF. Shrub vegetation on tropical granitic inselbergs in French Guiana. J. Veg. Sci. 2003;14:645–652. [Google Scholar]
- 19.Tinti BV, et al. Plant diversity on granite/gneiss rock outcrop at Pedra do Pato, Serra do Brigadeiro State Park, Brazil. Check List. 2015;11:1780. [Google Scholar]
- 20.Barbara T, Martinelli G, Fay MF, Mayo SJ, Lexer C. Population differentiation and species cohesion in two closely related plants adapted to neotropical high-altitude “inselbergs”, Alcantarea imperialis and Alcantarea geniculata (Bromeliaceae) Mol. Ecol. 2007;16:1981–1992. doi: 10.1111/j.1365-294X.2007.03272.x. [DOI] [PubMed] [Google Scholar]
- 21.Boisselier-Dubayle MC, Leblois R, Samadi S, Lambourdière J, Sarthou C. Genetic structure of the xerophilous bromeliad Pitcairnia geyskesii on inselbergs in French Guiana—A test of the forest refuge hypothesis. Ecography. 2010;33:175–184. [Google Scholar]
- 22.Domingues R, et al. Genetic variability of an endangered Bromeliaceae species (Pitcairnia albiflos) from the Brazilian Atlantic rainforest. Genet. Mol. Res. 2011;10:2482–2491. doi: 10.4238/2011.October.13.5. [DOI] [PubMed] [Google Scholar]
- 23.Hmeljevski KV, et al. Conservation assessment of an extremely restricted bromeliad highlights the need for population-based conservation on granitic inselbergs of the Brazilian Atlantic Forest. Flora Morpho. Distribut. Funct. Ecolo. Plants. 2014;209:250–259. [Google Scholar]
- 24.Palma-Silva C, et al. Sympatric bromeliad species (Pitcairnia spp.) facilitate tests of mechanisms involved in species cohesion and reproductive isolation in Neotropical inselbergs. Mol. Ecol. 2011;20:3185–3201. doi: 10.1111/j.1365-294X.2011.05143.x. [DOI] [PubMed] [Google Scholar]
- 25.Gomes P, Alves M. Floristic diversity of two crystalline rocky outcrops in the Brazilian northeast semi-arid region. Rev. Bras. Bot. 2010;33(4):661–676. [Google Scholar]
- 26.Nunes JA, Villa PM, Neri AV, Silva WA, Schaefer CEGR. Seasonality drives herbaceous community beta diversity in lithologically different rocky outcrops in Brazil. Plant. Ecol. Evol. 2020;153(2):208–218. [Google Scholar]
- 27.Speziale KL, Ezcurra C. The role of outcrops in the diversity of Patagonian vegetation: Relicts of glacial palaeofloras? Flora Morphol. Distrib. Funct. Ecol. Plant. 2012;207:141–149. [Google Scholar]
- 28.Speziale KL, Ruggiero A, Ezcurra C. Plant species richness–environment relationships across the Subantarctic-Patagonian transition zone. J. Biogeogr. 2010;37:449–464. [Google Scholar]
- 29.Yates CJ, et al. High species diversity and turnover in granite inselberg floras highlight the need for a conservation strategy protecting many outcrops. Ecol. Evol. 2019;9:7660–7675. doi: 10.1002/ece3.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaston KJ. Geographic range limits: Achieving synthesis. Proc. R. Soc. B Biol. Sci. 2009;276:1395–1406. doi: 10.1098/rspb.2008.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGann TD. How insular are ecological ‘islands’? An example from the granitic outcrops of the New England Batholith of Australia. Proc. R. Soc. Queensland. 2002;110:1–13. [Google Scholar]
- 32.Parmentier I, Stévart T, Hardy OJ. The inselberg flora of Atlantic Central Africa. I. Determinants of species assemblages. J. Biogeogr. 2005;32:685–696. [Google Scholar]
- 33.Changwe K, Balkwill K. Floristics of the Dunbar Valley serpentinite site, Songimvelo Game Reserve, South Africa. Bot. J. Linn. Soc. 2003;143:271–285. [Google Scholar]
- 34.Clarke PJ. Habitat islands in fire-prone vegetation: Do landscape features influence community composition? J. Biogeogr. 2002;29:677–684. [Google Scholar]
- 35.De Bello F, Leps J, Sebastia MT. Variations in species and functional plant diversity along climatic and grazing gradients. Ecography. 2006;29(6):801–810. [Google Scholar]
- 36.Porembski S, Martinelli G, Ohlemüller R, Barthlott W. Diversity and ecology of saxicolous vegetation mats on inselbergs in the Brazilian Atlantic rainforest. Divers. Distrib. 1998;4:107–119. [Google Scholar]
- 37.Porembski S, Szarzynski J, Mund JP, Barthlott W. Biodiversity and vegetation of small-sized inselbergs in a West African rain forest (Taï, Ivory Coast) J. Biogeogr. 1996;23:47–55. [Google Scholar]
- 38.Rahmanian S, et al. Effects of livestock grazing on soil, plant functional diversity, and ecological traits vary between regions with different climates in northeastern Iran. Ecol. Evol. 2019;9:8225–8237. doi: 10.1002/ece3.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speziale KL, Ezcurra C. Patterns of alien plant invasions in northwestern Patagonia, Argentina. J. Arid Environ. 2011;75:890–897. [Google Scholar]
- 40.Qian H, Chen SH, Zhang JL. Disentangling environmental and spatial effects on phylogenetic structure of angiosperm tree communities in China. Sci. Rep. 2017;7:5864. doi: 10.1038/s41598-017-04679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farzam M, Ejtehadi H. Effects of drought and canopy facilitation on plant diversity and abundance in a semiarid mountainous rangeland. J. Plant. Ecol. 2016;10(4):626–633. [Google Scholar]
- 42.Heino J, Tolonen KT. Ecological drivers of multiple facets of beta diversity in a lentic macroinvertebrate metacommunity. Limnol. Oceanogr. 2017;62:2431–2444. doi: 10.1002/lno.10577. [DOI] [Google Scholar]
- 43.Miranda JD, Armas C, Padilla FM, Pugnaire FI. Climatic change and rainfall patterns: Effects on semi-arid plant communities of the Iberian Southeast. J. Arid. Environ. 2011;75:1302–1309. [Google Scholar]
- 44.Pashirzad M, Ejtehadi H, Vaezi J, Shefferson RP. Multiple processes at different spatial scales determine beta diversity patterns in a mountainous semi-arid rangeland of Khorassan-Kopet Dagh floristic province, NE Iran. Plant. Ecol. 2019;220(9):829–844. [Google Scholar]
- 45.Victorero L, Robert K, Robinson LF, Taylor ML, Huvenne VAI. Species replacement dominates megabenthos beta diversity in a remote seamount setting. Sci. Rep. 2018;8:4152. doi: 10.1038/s41598-018-22296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deil U. Rock communities in tropical Arabia. Flora et Vegetation Mundi. 1991;9:175–187. [Google Scholar]
- 47.Dimopoulos P, Sýkora KV, Mucina L, Georgiadis T. The high-rank syntaxa of the rock-cliff and scree vegetation of the mainland Greece and Crete. Folia Geobot. 1997;32:313–334. [Google Scholar]
- 48.Hein P, Kürschner H, Parolly G. Phytosociological studies on high mountain plant communities of the Taurus Mountains (Turkey) 2. Rock communities. Phytocoenologia. 1998;28:465–563. [Google Scholar]
- 49.Nowak A, Nowak S, Nobis M, Nobis A. Vegetation of rock clefts and ledges in the Pamir Alai Mts, Tajikistan (Middle Asia) Cent. Eur. J. Biol. 2014;9:444–460. [Google Scholar]
- 50.Urbis A, Blazyca B. Rock vascular plant species of the Kraków-Częstochowa, Uplands. Thaiszia J. Bot. 2011;21:207–214. [Google Scholar]
- 51.Wiser SK, Peet RK, White PS. High-elevation rock outcrop vegetation of the Southern Appalachian Mountains. J. Veg. Sci. 1996;7:703–722. [Google Scholar]
- 52.Cadotte MW. Experimental evidence that evolutionarily diverse assemblages result in higher productivity. PNAS. 2013;110(22):8996–9000. doi: 10.1073/pnas.1301685110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swenson, G.N. Functional and Phylogenetic Ecology in R (Use R!) Kindle Edition (2014).
- 54.Cadotte MW, Davies PR. Why phylogenies do not always predict ecological differences. Ecol. Monogr. 2016;87(4):535–551. [Google Scholar]
- 55.De Bello F, LepŠ JAN, Sebastià MT. Predictive value of plant traits to grazing along a climatic gradient in the Mediterranean. J. Appl. Ecol. 2005;42(5):824–833. [Google Scholar]
- 56.Funk J, et al. Revisiting the Holy Grail: Using plant functional traits to understand ecologica processes. Biol. Rev. 2017;92(2):1156–1173. doi: 10.1111/brv.12275. [DOI] [PubMed] [Google Scholar]
- 57.Lavorel S, Garnier É. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002;16(5):545–556. [Google Scholar]
- 58.Violle C, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- 59.Zheng S, Li W, Lan Z, Ren H, Wang K. Functional trait responses to grazing are mediated by soil moisture and plant functional group identity. Sci. Rep. 2015;5:18163. doi: 10.1038/srep18163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gillison AN. Plant functional types and traits at the community, ecosystem and world level. In: van der Maarel E, Franklin J, editors. Vegetation Ecology. Wiley; 2013. pp. 347–386. [Google Scholar]
- 61.Loreau M. Biodiversity and ecosystem functioning: Recent theoretical advances. Oikos. 2000;91:3–17. [Google Scholar]
- 62.Akhani H, Djamali M, Ghorbanalizadeh A, Ramezani E. Plant biodiversity of Hyrcanian relict forests, N Iran: An overview of the flora, vegetation, paleoecology and conservation. Pak. J. Bot. 2010;42:231–258. [Google Scholar]
- 63.Hamzehee B, et al. Phytosociological survey of remnant Alnus glutinosa ssp. barbata communities in the lowland Caspian forests of northern Iran. Pytocoenologia. 2008;38:117–132. [Google Scholar]
- 64.Moradi H, et al. Elevational gradient and vegetation-environmental relationships in the central Hyrcanian forests of northern Iran. Nord. J. Bot. 2016;34:1–14. [Google Scholar]
- 65.Naqinezhad A, Esmailpoor A, Jafari N. A new record of Pyrola minor (Pyrolaceae) for the flora of Iran as well as a description of its surrounding habitats. Taxon. Biosyst. 2015;22:71–80. [Google Scholar]
- 66.Naqinezhad A, Zare-Maivan H, Gholizadeh H. A floristic survey of the Hyrcanian forests in Northern Iran, using two lowland-mountain transects. J. For. Res. 2015;26:187–199. [Google Scholar]
- 67.Sagheb-Talebi K, Sajedi T, Pourhashemi M. Forests of Iran. Springer Sci; 2014. [Google Scholar]
- 68.Siadati S, et al. Botanical diversity of Hyrcanian forests; a case study of a transect in the Kheyrud protected lowland mountain forests in northern Iran. Phytotaxa. 2010;7:1–18. [Google Scholar]
- 69.Akhani H, Ziegler H. Photosynthetic pathways and habitats of grasses in Golestan National Park (NE Iran), with an emphasis on the C 4-grass dominated rock communities. Phytocoenologia. 2002;32:455–501. [Google Scholar]
- 70.Akhani H, Mahdavi P, Noroozi J, Zarrinpour V. Vegetation patterns of the Irano-Turanian steppe along a 3,000 m altitudinal gradient in the Alborz Mountains of Northern Iran. Folia Geobot. 2013;48:229–255. [Google Scholar]
- 71.Klein JC. The altitudinal vegetation Alborez The Central (Iran) between the Iranian-Turanian and Euro-Siberian regions (French) Institut Français de Recherche en Iran; 2001. [Google Scholar]
- 72.Noroozi, J. Case study: High Mountain Regions in Iran 255–260. of Chapter 7 (Endemism in mainland regions-case studies). In Endemism in Vascular plants. Plant. Veg. (ed Hobohm, C.) 9. (Springer, 2014).
- 73.Noroozi J, Akhani H, Willner W. Phytosociological and ecological study of the high alpine vegetation of Tuchal Mountains (Central Alborz, Iran) Phytocoenologia. 2010;40:293–321. [Google Scholar]
- 74.Do Carmo FF, Jacobi CM. Diversity and plant trait-soil relationships among rock outcrops in the Brazilian Atlantic rainforest. Plant Soil. 2015;403:7–20. [Google Scholar]
- 75.Cavender-Bares J, Kozak KH, Fine PVA, Kembel S. The merging of community ecology and phylogenetic biology. Ecol Lett. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 76.Heydari M, Poorbabaei H, Esmailzadeh O, Salehi A, EshaghiRad J. Indicator plant species in monitoring forest soil conditions using logistic regression model in Zagros Oak (Quercus brantii var. persica) forest ecosystems. Ilam city. J. Plant Res. 2014;27(5):811–828. [Google Scholar]
- 77.Speziale KL, Ezcurra C. Rock outcrops as potential biodiversity refugia under climate change in North Patagonia. Plant Ecol. Diver. 2014;8:353–361. [Google Scholar]
- 78.Rahmanian S, et al. Effects of livestock grazing on plant species diversity vary along a climatic gradient in northeastern Iran. Appl. Veg. Sci. 2020;23:551–561. [Google Scholar]
- 79.Huston MA. Biological Diversity: The Coexistence of Species in Changing Landscape. Cambridge University; 1994. [Google Scholar]
- 80.Mason NW, Mouillot D, Lee WG. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos. 2005;111:112–118. [Google Scholar]
- 81.Stubbs WJ, Wilson JB. Evidence for limiting similarity in a sand dune community. J. Ecol. 2004;92:557567. [Google Scholar]
- 82.Stanisci A, et al. Functional composition and diversity of leaf traits in subalpine versus alpine vegetation in the Apennines. Ann. Bot. Comp. plants. 2020;12:plaa004. doi: 10.1093/aobpla/plaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chesson P, et al. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia. 2004;141:236–253. doi: 10.1007/s00442-004-1551-1. [DOI] [PubMed] [Google Scholar]
- 84.Rosbakh S, et al. Contrasting effects of extreme drought and snowmelt patterns on mountain plants along an elevation gradient. Front. Plant Sci. 2017;8:1478. doi: 10.3389/fpls.2017.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korner C. Alpine Treelines: Functional Ecology of the Global High Elevation tree Limits. Springer Sci. & Business Media; 2012. [Google Scholar]
- 86.Reich PB, et al. Generality of leaf trait relationships: A test across six biomes. Ecology. 1999;80:1955–1969. [Google Scholar]
- 87.Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: Some leading dimensions of variation between species. Ann. Rev. Ecol. Syst. 2002;33:125–159. [Google Scholar]
- 88.Hautier Y, Niklaus PA, Hector A. Competition for light causes plant biodiversity loss after eutrophication. Science. 2009;324:636–638. doi: 10.1126/science.1169640. [DOI] [PubMed] [Google Scholar]
- 89.De Bello FD, et al. Hierarchical effects of environmental filters on the functional structure of plant communities: A case study in the French Alps. Ecography. 2013;36:393–402. [Google Scholar]
- 90.Korner C, Neumayer M, Menendez-Riedl SP, Smeets-Scheel A. Functional morphology of mountain plants. Flora. 1989;182:353–383. [Google Scholar]
- 91.Rosbakh S, Römermann C, Poschlod P. Specific leaf area correlates with temperature new evidence of trait variation at the population, species and community levels. Alp. Bot. 2015;125:79–86. [Google Scholar]
- 92.Ordonez JC, et al. Global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009;18:137–149. [Google Scholar]
- 93.Li W, et al. Community-weighted mean traits but not functional diversity determine the changes in soil properties during wetland drying on the Tibetan Plateau. Solid Earth. 2017;8:137–147. [Google Scholar]
- 94.Bardgett RD, Mommer L, De Vries FT. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014;29:692–699. doi: 10.1016/j.tree.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 95.Lane DR, Coffin DP, Lauenroth WK. Effects of soil texture and precipitation on above-ground net primary productivity and vegetation structure across the Central Grassland region of the United States. J. Veg. Sci. 1998;9:239–250. [Google Scholar]
- 96.Noy-Meir I. Multivariate analysis of the semi-arid vegetation of southern Australia. II. Vegetation catenae an environmental gradients. Aust. J. Bot. 1973;22:40–115. [Google Scholar]
- 97.Moura MR, Villalobos F, Costa GC, Garcia PCA. Disentangling the role of climate, topography and vegetation in species richness gradients. PLoS ONE. 2016;11(3):0152468. doi: 10.1371/journal.pone.0152468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neri AV, et al. Soil and altitude drives diversity and functioning of Brazilian Páramos (Campo de Altitude) J. plant. Ecol. 2016;10(5):771–779. [Google Scholar]
- 99.Benites VM, Schaefer CEGR, Simas FNB, Santos HG, Mendonca BAF. Soils associated to rock outcrops in the Brazilian mountain ranges Mantiqueira and Espinhaço. Rev. Bras. Bot. 2007;30:569–577. [Google Scholar]
- 100.Flynn DFB, et al. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009;12:22–33. doi: 10.1111/j.1461-0248.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 101.Zuo XA, et al. Testing associations of plant functional diversity with along a restoration gradient of sandy grassland. Front. Plant. Sci. 2016;7:1–11. doi: 10.3389/fpls.2016.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers-Smith IH, et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 2011;6:045509. [Google Scholar]
- 103.Vankoughnett MR, Grogan P. Nitrogen isotope tracer acquisition in low and tall birch tundra plant communities: A 2-year test of the snow–shrub hypothesis. Biogeochemistry. 2014;118:291–306. [Google Scholar]
- 104.Pescador DS, de Bello F, Valladares F, Escudero A. Plant trait variation along an altitudinal gradient in Mediterranean high mountain grasslands: Controlling the species turnover effect. PLoS ONE. 2015;10:e0118876. doi: 10.1371/journal.pone.0118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pescador DS, Sierra-Almeida A, Torres PJ, Escudero A. Summer freezing resistance: A critical filter for plant community assemblies in Mediterranean high mountains. Front. Plant. Sci. 2016;7:194. doi: 10.3389/fpls.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heydarnejad S, Ranjbar A. Investigation of the effect of salinity stress on growth characteristic and ion accumulation in plants. J. Desert Ecos. Eng. 2013;3(4):1–10. [Google Scholar]
- 107.Perez-Harguindeguy N, et al. New handbook for standardized measurement of plant functional traits worldwide. Aust. J. Bot. 2013;61:167–234. [Google Scholar]
- 108.Cornelissen JHC, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003;51:335–380. [Google Scholar]
- 109.Raunkiaer C. The Life Forms of Plants and Statistical Plant Geography. Oxford University Press; 1934. [Google Scholar]
- 110.Gee, G. W. & Bauder, J. W. Particle size analysis. In Methods of Soil Analysis. Part 1, 2nd ed. (ed Klute, A.) Agronomy Monographs, Vol. 9, 383–409 (Am. Soc. Agr., 1986).
- 111.Bremner, J. M. In Nitrogen-Total Methods of Soil Analysis. (eds Sparks, D. L.) Soil Sci Soc Am J. 1085–1122 (Am Soc Agr. Inc, 1996).
- 112.Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38. [Google Scholar]
- 113.Nelson, D. W. & Sommers, L. Total carbon, organic carbon, and organic matter 1. Methods of soil analysis. Part 2. Chemical and microbi‐ological properties, (methodsofsoilan2), 539–579 (1982).
- 114.Miller RH, Keeney DR. Methods of soil analysis, 2nd ed. In: Page AL, Miller RH, Keeney DR, editors. Part 2. Chemical and Microbiological Properties. ASA, SSSA; 1982. pp. 1–129. [Google Scholar]
- 115.Food and Agriculture Organization-FAO. Management of gypsiferous soils. Soil Bulletin, 62, (FAO, 1990).
- 116.Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014;84:45–67. [Google Scholar]
- 117.Shipley B, Vile D, Garnier É. from plant traits to plant communities: A statistica mechanistic approach to biodiversity. Science. 2006;314(5800):812–814. doi: 10.1126/science.1131344. [DOI] [PubMed] [Google Scholar]
- 118.Zhu J, Jiang L, Zhang Y. Relationships between functional diversity and aboveground biomass production in the Northern Tibetan alpine grasslands. Sci. Rep. 2016;6:34105. doi: 10.1038/srep34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laliberte E, Legendre P. A distance-based framework for measuring functional diversity from multiple traits. Ecology. 2010;91(1):299–305. doi: 10.1890/08-2244.1. [DOI] [PubMed] [Google Scholar]
- 120.Wheeler D, Tiefelsdorf M. Multicollinearity and correlation among local regression coefficients in geographically weighted regression. J. Geogr. Syst. 2005;7:161–187. [Google Scholar]
- 121.Fox J, Weisberg S. A review of: an R companion to applied regression, second edition. J. Biopharm. Stat. 2011;22:418–419. [Google Scholar]
- 122.Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007;41:673–690. [Google Scholar]
- 123.Dray, S., Legendre, P. & Blanchet, F. G. packfor: forward selection with permutation (Canoco p. 46). (2011) http://R-Forge.R-project.org/projects/sedar (Accessed 7 Nov 2016).
- 124.Blanchet FG, Legendre P, Borcard D. Forward selection of explanatory variables. Ecology. 2008;89:2623–2632. doi: 10.1890/07-0986.1. [DOI] [PubMed] [Google Scholar]
- 125.Oksanen, J. et al.vegan: Community Ecology Package (2017).
- 126.Wickham H, et al. Ggplot2: Elegant Graphics for Data Analysis. 2. Springer International Publishing; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [Appendix S5 and S6 in supporting information].