Highlights
-
•
Regulation of pro-inflammatory cytokines and immune cells by exosomes derived from SLE.
-
•
Abnormal expression of exosome composition in SLE.
-
•
Diagnostic role of miRNAs in Exosomes for SLE.
-
•
Feasibility of exosomes in the treatment of SLE.
Keywords: Systemic lupus erythematosus, Exosomes, Microribonucleic acid, Immunomodulation, Diagnostic role, Therapeutic potential
Abbreviations: SLE, Systemic lupus erythematosus; PAMPs, Pathogen-associated molecular patterns; PDCs, Plasmacytoid dendritic cells; TLR, Recombinant Toll Like Receptor; Treg, Regulatory T cells; HMGB1, High mobility group box 1; CfDNA, Circulating free DNA; MiRNAs, Microribonucleic acids; LN, Lupus nephritis; MSC, Mesenchymal stem cells (MSC)
Abstract
Systemic lupus erythematosus (SLE) is a global chronic autoimmune disease that invades most organs of the body, with kidney injury being the most prominent feature. Exosomes are extracellular vesicles that carry a variety of proteins, lipids and genetic material, participate in the exchange of local and intersystem information, and play an important immunoregulatory role in a variety of autoimmune diseases. At the same time, the use of exosomes as disease biomarkers and drug delivery carriers also shows great application prospects. This article reviews current progress in the application of exosomes in the pathogenesis, diagnosis and treatment of SLE.
Introduction
Systemic lupus erythematosus (SLE) is a chronic relapsing and remitting disease that results in a spectrum of mild to life-threatening symptoms. The survival statistics of SLE patients in China in the period from 1995 to 2013 showed that the 5-year survival rate was 94% and the 10-year survival rate was 89% [1]. The pathogenesis of SLE involves various manifestations, such as the hyperfunction of T and B cells, excessive activation of the complement system, excessive production of inflammatory cytokines, and defective macrophage function, but its aetiology is not yet fully understood [2], [3], [4]. Clinically, the conventional treatment for SLE is glucocorticoids, antimalarials, and immunosuppressive drugs. Combining these three types of drugs improves patient prognosis, but they exhibit poor targeting, are needed in high doses, and have long administration times and high recurrence rates [5], [6], [7]. Studies have shown that infection (33.2%), renal degeneration (18.7%), encephalopathy (13.8%), and cardiovascular disease (11.5%) are the main causes of death in SLE patients. However, due to the widespread use of hormonal drugs, infection has now become the number one cause of death in SLE patients in China [1], [8]. Long-term drug maintenance also places great economic pressure on patients, so it is urgent to seek new treatment options. In recent years, exosomes have been studied more extensively, and their source, composition and function have been gradually uncovered. Therefore, this article will review the research progress on the correlation, diagnostic role, and therapeutic potential between exosomes and SLE pathogenesis to provide a new direction for the study of the aetiological mechanism of SLE and the targeting of SLE treatment.
Production and composition of exosomes
Exosomes were first identified in sheep reticulocytes in 1983 and were named “exosomes” by Johnstone in 1987. Intracellular lysosomal microparticles invaginate to form vesicles, which are then released into the extracellular space as 30–100 nm membrane-bound vesicles after fusion of the extracorporeal membrane with the cell membrane [9]. Exosomes are widely sourced, and almost all types of cells in the human body can release exosomes under various physiopathological conditions. Their size, shape, and density are all highly variable, depending on the physiological state of the cell of origin [10]. Approximately 103–104 exosomes are produced per cell, and cancer cells can produce a greater number of exosomes than normal cells from the same or other tissues [11]. Exosomes are ubiquitous in body fluids, such as cerebrospinal fluid, saliva, milk, urine, blood, and semen and reach all parts of the body through blood circulation [12].The processes by which cells produce and absorb exosomes are interconnected, extending the complexity of exosomes in cellular communication [13].
Exosomes are complex in composition and encapsulated by sphingomyelin, cholesterol, and ceramide-rich phospholipid bilayers. A variety of transmembrane proteins and transporters are highly enriched in exosomes, of which CD9, CD63, CD81, and TSG101 are often used as marker proteins for exosomes [14], [15]. However, the recipient cell type is the key factor in determining whether exosomes can be taken up, and it does not depend on the expression of marker proteins [16]. It has been reported in the literature that there are abundant peptide-MHC complexes on the surface of exosomes, as well as autoantigens, such as DNA–protein and RNA–protein complexes, and chemically stimulated substances, such as bacterial DNA, viral antigens, and cell wall phospholipids. These are processed into peptides by professional antigen presenting cells (APCs), and the antigenic peptides produced intracellularly can be expressed on the surface of the exosome after binding to MHC molecules [17]. Although the antigen presentation rate of peptide-MHC complexes on the surface of exosomes is lower than that between cells, long-term cellular stimulation or cellular memory can be maintained in the circulation in vivo [18], [19]. In inflammatory or infectious states such as SLE, the density of MHC/peptides on the surface of exosomes is markedly increased, enhancing immune cell responses [20], [21]. In addition, DC-derived exosomes can transfer preformed peptide-MHC-class II complexes into MHC-class II-deficient dendritic cells, thus enabling them to activate antigen-specific CD4+T cells [22]. Bioactive molecules such as DNA, RNA, mRNA and cytokines are also important components of exosomes and participate in information transmission between exosomes and cells [14]. Studies have found that mRNA from the plasma exosomes of patients with SLE immune thrombocytopenia can regulate the tight binding proteins of endothelial cells, leading to impaired permeability of endothelial cells and promoting the progression of atherosclerosis, tumours and other diseases [23], [24].
Initially, exosome contents were thought to be a by-product of body discharge, and there is now increasing evidence that not all the material in the cell enters the exosome [25]. Agilent microRNA microarray analysis showed that some miRNAs (e.g., miR-760, miR-632, miR-654-5p, and miR-671-5p) were present in the exosomes of all cell types, whereas miR-335 was only present in the exosomes derived from primary dendritic cells [26]. In SLE patients, miR-451a was overexpressed in T and B cells, but the expression was significantly lower in T and B cell-derived exosomes. The entry of substances into the exosome is a selective process, and certain substances are selectively retained in the cell or exported to the exosome, representing a unique method of self-regulation in the body [27], [28]. The contents of exosomes affect their size, while the source of cells and the microenvironment in which they are located affect their function, and the mutual combination of different characteristics increases the heterogeneity and complexity of exosome groups. There are many mysteries about exosomes that deserve our exploration.
Regulatory effect of exosomes on proinflammatory cytokines
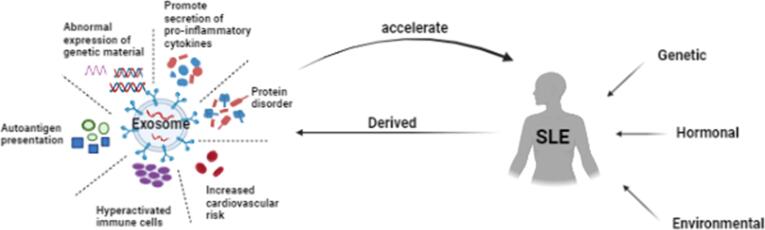
Some studies have found that the exosome volume in SLE patients is higher than that in normal individuals, which seems to be related to excessive cell activation and immune balance disorders in these patients. Overactivated immune cells in SLE patients play a very important role in the pathogenesis of SLE by secreting inflammatory cytokines and expressing abnormal adhesion molecules to promote the occurrence and development of inflammation. Similarly, exosomes derived from SLE patients can markedly induce the production of proinflammatory molecules by means of pathogen-associated molecular patterns (PAMPs) and the activation of downstream transcription factors [29]. Exosomes isolated from the plasma of SLE patients can bind to TLR7 to promote IFN-α production by plasmacytoid dendritic cells (pDCs) [30]. The use of TLR1/2, TLR7, and TLR9 antagonists can inhibit IFN-α production; after the use of TLR4 antagonists, the contents of TNF-α, IL-1β, and IL-6 are significantly reduced, which implies an important role for TLR receptors in the mechanism of the exosome proinflammatory effect in SLE patients [29].
SLE disease activity is clearly associated with the number of circulating exosomes and their proinflammatory potential, implying that high disease activity is closely related to the number and nature of exosomes produced [31]. These exosomes reduce cellular skeletal protein content, have higher concentrations of complement (Clq, Cls, C3, C4b, and C9) and immunoglobulin (IgG, IgM, and IgA) components, are more likely to induce the production of cytokines (BLyS, IFN-α, TNF-α, IL-6, IL-10, and IL-1) that are positively correlated with disease activity and are widely involved in complement activation and a series of subsequent abnormal immune responses [29], [32]. The large amount of IFN-α has been shown to be one of the main hallmarks and pathogenic mechanisms of SLE. Compared with those of healthy controls, SLE patients had increased levels of platelet-derived microparticles, T cell-derived microparticles, monocyte-derived microparticles, and endothelial cell-derived microparticles and were more pronounced in patients with higher SLEDIA scores [31], [33]. However, these particles (1–1000 nm) are a heterogeneous population within which exosomes (<100 nm) and microvesicles (MVs) (100–1000 nm) should be included [12], [34]. Therefore, the number of exosomes produced by specific cells is indeed increased in SLE patients [29], and it is uncertain whether all exosomes are increased.
The presence of senescent lymphocytes is another distinct feature of SLE, and these senescent lymphocytes can express more cytotoxic molecules and inflammatory cytokines, counteracting Treg suppression and apoptosis. It can be speculated that exosomes derived from senescent cells will present a proinflammatory effect [35]. Circulating exosomes in SLE patients have a proinflammatory effect and have a significant correlation with disease activity, so, circulating exosomes can also be used as novel markers of disease activity in SLE patients. In addition, the structural integrity of exosomes is important for the immune response, and proinflammatory cytokine production is not clearly induced in sera from SLE patients lacking exosomes or by mechanically disrupting the structure of exosomes [29]. Exosomes derived from SLE patients and treated with DNase 1-like molecule 3 (DNASE1L3) or IgG-specific degrading enzymes had significantly reduced stimulatory responsiveness to dendritic cells and macrophages, producing only low levels of cytokines such as MIP-1a, MIP-1b, IFN-α, IL-4, IL-1β, and IL-2 [36].
Regulation of immune cells by exosomes
Exosomes in SLE patients act as an important source of autoantigens in vivo, which further leads to lymphocyte activation while causing the release of proinflammatory cytokines and the formation of immune complexes. Exosomes control the inflammatory process by mediating immune stimulation and immune regulation, affecting the activation, differentiation and apoptosis of cells [9], [37].
Under physiological conditions, exosomes are independently or negatively correlated with the activation of cells and play a role in maintaining homeostasis and anti-inflammation states [35]. However, “SLE-specific” exosomes derived from SLE patients have been described as promoters of the inflammatory response [29]. In the SLE disease state, monocytes and T cells produce exosomes with higher numbers and surface protein expression than those in the healthy physiological state. These exosomes can activate immune cells such as lymphocytes, monocytes and neutrophils through blood circulation, increase the expression of IFNR1 and BLyS on the surface of these cells, change their normal function, and keep these cells in a higher activation state. In addition, overactivated immune cells produce large numbers of exosomes, forming a vicious cycle that accelerates disease progression [35]. Moreover, excessive exosomes in the patient's plasma may lead to thrombosis and increase cardiovascular risk [38].
Regulatory T cells (Tregs) inhibit the activation, proliferation, and cytokine secretion of immune cells such as B cells, T cells, NK cells, macrophages, and dendritic cells in a non-MHC-restricted manner [39], [40]. CD4+CD25+ and CD8+CD25+ regulatory T cells are T cells that negatively regulate autoimmune responses in vivo and play an important protective role in autoimmune diseases such as SLE, and exosomes derived from these cells have also been shown to have immunosuppressive functions. Treg-derived exosomes express molecules such as CTLA4, CD25, and CD73, which regulate the secretion of cytokines and the proliferation of effector T cells [41]. In in vitro and in vivo experiments, the exosomes released by Treg cells were found to transfer let-7d, which are normally carried by Treg cells, to Th1 cells and inhibit Th1 cell proliferation and IFN-γ secretion, thereby preventing and inhibiting systemic diseases. Exosome miRNA-mediated gene silencing is necessary for Treg cell-mediated suppression [42]. Therefore, exosomes derived from cells with immunosuppressive effects may become a new research direction in SLE, which provides a new strategy for the treatment of SLE.
Exosomes can affect the function of immune cells, and similarly, they are associated with lymphoid organs containing these cells. Using in vivo near-infrared (NIR) imaging techniques, Srinivasan et al. demonstrated that exosomes can be rapidly transported within minutes from the periphery to lymph nodes via lymphatic vessels [43]. They interact with dendritic cells, B lymphocytes, stromal cells, and subcapsular sinus macrophages within lymph nodes [44] (see Fig. 1).
Fig. 1.
Promoting effect of exosomes derived from patients with systemic lupus erythematosus on disease progression. The picture was created with BioRender.
Abnormal expression of exosome composition
Abnormal expression of proteins in exosomes of SLE
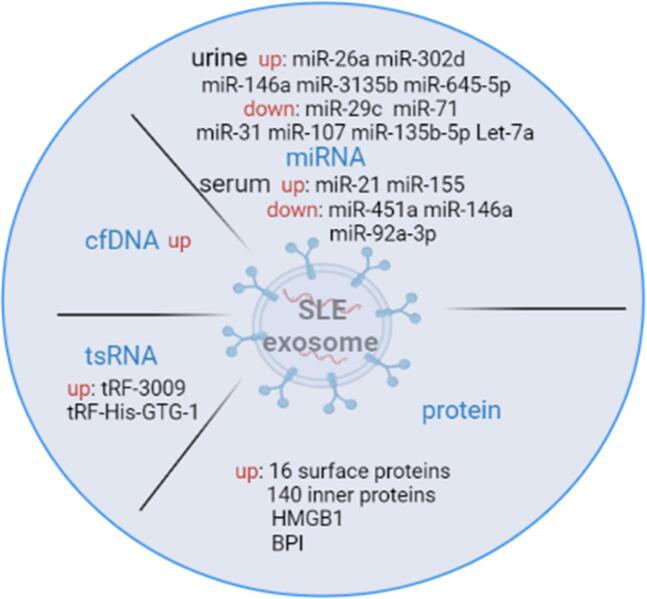
By comparing T cell-derived exosomes from healthy subjects with those from SLE patients, we found that 16 surface proteins and 140 inner proteins (protein phosphatases, protein kinases, and metabolic enzymes, among others) were overexpressed in the exosomes from SLE patients [45]. Chuang et al. found that eosinophil cationic protein (ECP) was overexpressed in the T cell-derived exosomes from SLE patients. In animal experiments, exosomes loaded with ECP were infused into normal mice, and it was observed that the exosomes were transferred to multiple tissues of the recipient mice. This had the effect of increasing the proportion of plasma B cells and inducing the production of autoantibodies and high levels of IFN-γ, leading to inflammation in various organs, such as nephritis, hepatitis and arthritis [45]. Chuang et al. demonstrated the overexpression of bactericidal/permeability increasing protein (BPI) in T cell-derived exosomes from SLE patients. BPI is not only a negative regulator of Treg differentiation but also induces a variety of inflammatory responses, enhances autoantibody rheumatoid factor, and stimulates IL-1β production by macrophages [46].
In SLE patients, due to macrophage apoptosis and the impaired macrophage clearance of apoptotic cells, apoptotic cells release more proinflammatory exosomes and membrane vesicles containing nuclear antigens as well as high mobility group Box 1 (HMGB1) into the blood while continuously forming large numbers of autoimmune complexes [34], [47]. Unlike cytokines, such as IL-1 and TNF-α, which are rapidly released at the initial stage of inflammation, HMGBl often appears in the late stage of the inflammatory response and lasts longer, further aggravating the inflammatory response and playing a key role in the pathogenesis of systemic autoimmune diseases, especially SLE. In addition, apoptotic cell-derived exosomes can induce B cell responses in naive and transplanted mice, aggravating vascular rejection and C4d deposition and leading to autoantibody production [48]. The role of exosome surface proteins and exosome-containing proteins in the disease process of SLE patients is not fully understood. However, SLE, as a typical immune imbalance disease, is often a concentrated manifestation of multiple pathway abnormalities, and the cumulative effect of several pathways can significantly increase the risk of the disease. Thus, abnormal protein expression in exosomes plays an important role in the pathogenesis of SLE.
Aberrant expression of transfer RNA (tRNA) -derived small noncoding RNAs in SLE patients
Transfer RNA (tRNA)-derived small noncoding RNA (tsRNA) has the characteristics of a more stable presence and more sensitive changes under stress conditions than other small noncoding RNA contents do [49]. Xu et al. found that a total of 355 tsRNAs were differentially expressed in SLE compared with normal controls [50]. Recently, Yang et al. proposed, for the first time, that the expression level of tsRNA in the serum of SLE patients can be used as a diagnostic indicator in SLE patients with LN. The most significant differences in serum tRF-His-GTG-1 can affect GTPase activity, thyroid T-cell differentiation, protein homodimerization, the mitogen-activated protein kinase (MAPK) signalling pathway, Epstein-Barr virus (EBV) infection, the tumour necrosis factor (TNF) signalling pathway and other signalling molecules that play an important role in regulating the immune system [51]. Geng et al. also showed that TRF-3009 is upregulated in CD4+T cells from SLE patients, regulates IFN-α-induced oxidative phosphorylation of CD4+T cells (OXPHOS) and is positively correlated with lupus nephritis and the disease activity index [52]. These aberrantly expressed tsRNAs are mainly secreted through exosomes, but the relationship between tsRNAs and exosomes is still unclear, providing an impetus for further exploration of the potential clinical application of tsRNAs in exosomes and insight into their biological functions in the future.
Abnormal expression of circulating free DNA (cfDNA) in SLE exosomes
In the healthy state, plasma circulating free DNA (cfDNA) mainly comes from apoptosis, inflammatory infection, and tumour tissue necrosis [53]. Persistent tissue inflammation and immune responses in SLE patients greatly impair the body's ability to clear apoptotic material, and therefore, many DNA fragments generated by apoptosis enter the exosome or exist on its surface [54]. Studies have also confirmed the overexpression of cfDNA in plasma exosomes of SLE patients, which is caused by the excessive production of cfDNA in vivo and minimal degradation in exosomes. Exosomes containing large amounts of cfDNA have a stimulatory activity on dendritic cells and macrophages, which in turn produce more cytokines, chemokines, and immune complexes [36].
Aberrant expression of miRNAs in SLE exosomes
Microribonucleic acids (miRNAs) are small noncoding single-stranded RNAs of approximately 18–25 nucleotides in length that use a variety of methods to regulate the expression of posttranscriptional genes, affect cell development, metabolism, proliferation, and apoptosis and maintain body homeostasis [55]. The aberrant expression or dysfunction of miRNAs can cause abnormal pathological phenotypes and serious biological consequences in different diseases. Epigenetic regulation, genetic susceptibility, environmental factors, and hormone levels can all contribute to the aberrant expression of miRNAs in SLE [56]. Multiple miRNA disorders exist in the clinical parameters of SLE patients and play a key role in SLE pathogenesis. Many extracellular miRNAs were found to be present in exosomes, and differential miRNA expression in exosomes and cells from patient serum involves cellular communication between exosome-derived blasts and other cells [57]. When exosomes undergo membrane fusion with recipient cells, receptor–ligand specific binding, or pinocytosis by specific cells, their miRNAs then enter recipient cells, which changes the genetic information of the recipient cell and induces abnormal immune response mechanisms, which are involved in the development and progression of SLE [58]. For example, miR-21 can affect the expression of the Programmed Cell Death 4 gene and modulate aberrant T cell responses in SLE patients. miR-21 derived from the exosomes of SLE patients was found to induce type I interferon production by human plasmacytoid dendritic cells [30] (see Fig. 2 and Table).
Fig. 2.
Abnormal composition of exosomes in SLE patients. The picture was created with BioRender.
Diagnostic role of miRNAs in exosomes for SLE
Diagnostic role of miRNAs in urine exosomes for SLE
An increasing number of studies have been conducted on exosomal miRNAs as biomarkers of disease activity. Lin Li et al. found markedly abnormal expression levels of miRNAs in the urinary exosomes of patients with chronic kidney disease compared to those of healthy individuals [59]. miRNAs in urinary exosomes are simple and easy to collect noninvasively, avoid various complications that may be brought about by renal biopsy, and have the potential to be novel markers of kidney injury in SLE [60], [61]. A very small number of miRNAs found in the urinary exosomes, which are filtered out during circulation, are mostly actively secreted by nephron cells. Based on different renal pathological changes, miRNAs selectively enter the exosomes and are concentrated in them, so miRNAs in the exosomes can reflect the physiological status of nephron cells [55].
miR-146a and miR-26a are significantly increased in the urinary exosomes of patients with active lupus nephritis and can be used as biomarkers of active lupus nephritis (LN) and renal podocyte injury [55], [62]. miR-146a in the exosomes of SLE patients is inversely correlated with chronic inflammatory parameters such as proteinuria and complement C3 and C4 activation. And in vitro experiments have demonstrated that miR-146a in exosomes negatively regulates inflammation by inhibiting TRAF6 and IRAK1 [63]. Similarly, miR-302d expression was significantly enhanced in the urinary exosomes of LN patients, which reduced the effects of TGF-β in terms of mesangial expansion and fibrosis [64]. The level of miR-29c in the urinary exosomes of LN patients was significantly lower than that of healthy controls and correlated with the degree of renal fibrosis, which has the potential to predict the degree of renal tissue fibrosis early [65]. When LN is in the active stage of the disease, the contents of let-7a and miR-21 in the exosome are significantly reduced and negatively correlated with glomerular filtration rate and protein leakage, but their contents gradually increase during concomitant treatment. This can serve as diagnostic criteria for the clinical staging of LN patients [66], [67]. miR-654-5p and miR-3135b may serve as noninvasive diagnostic markers for crescent-shaped LNIV [68]. It has also been experimentally shown that miR-31, miR-107, and miR-135b-5p levels are significantly increased in the urinary exosomes of LN patients after effective treatment, and these miRNAs are potential clinical prognostic markers that inhibit HIF1A and reduce the production of intercellular proliferation and chemokines in endothelial cells, thereby restoring renal function Table 1 [69].
Table 1.
Dysregulated expression of miRNAs in urine exosomes from patients with lupus and its significance in the pathogenesis of SLE.
| SLE exosomal miRNAs | Expression levels | Target | Clinical parameters | Disease market | References |
|---|---|---|---|---|---|
| miR-26a | up | mRNAs encoding podocyte proteins | Podocyte differentiation and cytoskeletal integrity | Injured podocytes in antoimmune glomerulonephritis | [62] |
| miR-302d | up | TGF-β |
Reduce the effects of mesangial expansion and fibrogenesis |
Inactive LN where normalized renal function | [55], [64] |
| miR-146a | up | TRAF6/IRAK1 | Associated with proteinuria, Lupus activity and histological features | Albuminuria, activity changes and disease flares in LN | [63], [66] |
| miR-29c | down | Smad3/MMP2 |
Correlated with degree of renal chronicity |
Early renal fibrosis in LN | [65] |
| Let-7a miR-21 | down | Interleukin expressions | Play a role in epigenetic regulation of the kidney | Clinical stage of LN | [67] |
| miR-3135b miR-654-5p |
up | / | Involved in the kidney disease | Potential biomarker of LNIV-CC | [68] |
| miR-31 miR-107 miR-135b-5p | down | HIF1A | Associated with mesangial proliferation and immune regulation | Clinical recovery in LN | [69] |
Diagnostic role of miRNAs in serum exosomes for SLE
It has been reported that 89 miRNAs were changed in the plasma of healthy individuals and SLE patients, and 17 miRNAs were different in the serum of SLE patients with or without LN. The sensitivity of detecting lupus nephritis with miR-221-5P, miR-380-3P, miR-556-5P, miR-758-3P, and miR-3074-3P in the plasma was 97%, the positive prediction was 82.5%, and the diagnostic rate of renal injury was 87.9% [70]. Most miRNAs in the serum are deposited in exosomes, and many researchers have found that multiple miRNA disorders also exist in the serum exosomes of SLE patients.
Under normal conditions, exosomes containing miR-146a can target the TRAF6-NF-κB signalling pathway to inhibit the senescence of MSCs. While miR-146a expression is significantly downregulated in the serum exosomes of SLE patients, accelerating the senescence of BM-MSCs, it is negatively correlated with anti-dsDNA antibodies [71]. miR-21 and miR-155 are upregulated in exosomes, are significantly positively correlated with the severity of urinary protein levels in patients and have diagnostic value for SLE and LN [60]. miR-451a expression was downregulated in CD4+T or B cell-derived exosomes from active SLE patients and was inversely correlated with 24 h urinary protein levels and SLEDAI scores. After treatment with conventional drugs, such as hydroxychloroquine or glucocorticoids, the content of miR-451a in serum exosomes was significantly increased. Experiments have also shown that the expression level of miR-451a is most significantly decreased in proliferative LN and can be used as a potential biomarker to determine renal injury in SLE and the different pathological types of lupus nephritis (LN) [27].
Exosomes in the sera of SLE patients are derived from platelets, leukocytes, and endothelial cells and are serologically and clinically relevant [72]. Changes in the number and content of exosomes have gradually become the active diagnostic criteria for various autoimmune diseases, such as SLE, rheumatoid arthritis (RA), multiple sclerosis (MS), and Sjogren's syndrome (SS) [14]. Murakami et al. demonstrated that miRNAs in serum exosomes can be used as biomarkers for the diagnostic stage of liver disease [73]. Zhang et al. demonstrated that hsa-miR-135b-5p in serum exosomes may be a promising seminal biomarker for the diagnosis of steroid-induced femoral osteonecrosis [74]. By detecting miRNAs in exosomes, it has been shown that exosomes have a certain specificity in different tissues and different disease states and are a new method of biomarker detection and disease monitoring. The exact mechanism of miRNA deregulation in SLE has not been clarified, which gives us much inspiration and is the focus of future research (see Table 2).
Table 2.
Dysregulated expression of miRNAs in serum exosomes from patients with lupus and its significance in the pathogenesis of SLE.
| SLE exosomal miRNAs | Expression levels | Target | Clinical parameters | Disease market | References |
|---|---|---|---|---|---|
| miR-451a | down | MIF | Associated with disease activity, renal damage and intercellular communication | Determination of renal damage in SLE and different pathological types of LN | [27] |
| miR-21 miR-155 |
up | / | Correlated with proteinuria | Diagnosis SLE with and without LN | [60] |
| miR-146a | down | TRAF6/NF-κB | Regulate the senescence of MSC | / | [71] |
Feasibility of exosomes in the treatment of SLE
Feasibility of exosomes as carriers
Exosomes have been shown to transport a variety of substances and molecules, such as endogenous and exogenous miRNAs, small interfering RNAs, long noncoding RNAs, DNA, proteins, and synthetic drugs [75]. miR-150 molecules can downregulate the expression of cellular kinase signalling 1 inhibitor (SOCS1) and promote renal fibrosis. It has been experimentally demonstrated that exosomes carrying anti-miR-150 molecules released by B lymphocytes can be effectively absorbed by CD8+ T cells, thereby reducing the damage caused by miR-150 to the kidney [76], [77], [78]. Thus, exosomes can be used to carry multiple therapeutic agents.
Numerous studies have shown that exosomes also show great promise as drug or gene delivery carriers for the treatment of SLE [74]. Curcumin has anti-inflammatory, antioxidant, and immunoregulatory effects and can effectively reduce macrophage activation and the macrophage secretion of B cell activator (BAFF), thus alleviating acute and chronic immune nephritis, but its rapid clearance in vivo and low bioavailability limit its clinical use [79]. Sun et al. reported that encapsulating curcumin with exosomes enhanced its therapeutic ability and effectively treated lipopolysaccharide-induced septic shock in mice [80]. The lipid bilayer of the exosomes prevents curcumin from being degraded and facilitates the drug’s exertion of long-acting therapeutic effects. In addition, exosomes are membrane vesicles produced by the secretion of various cells in the body, are decomposed by enzymes after inactivation and do not accumulate in large numbers for a long time, thus possessing few toxic side effects [81]. The anti-inflammatory effects of the drug, in combination with the functional properties of exosomes, can be used to treat inflammatory SLE and other diseases [75], [82]. Moreover, the nanoscale volume allows exosomes to easily cross many barriers to reach the brain and kidneys (that may be damaged in SLE) and do not easily cause immune rejection in the body [83], [84]. In addition, the diverse route of administration of exosomes is another advantage of their wide application, and appropriate administration methods can be selected according to the lesion site, such as subcutaneous injection, intravenous injection, intraperitoneal injection, intranasal injection, and oral administration [85], [86].
Therapeutic effects of exosomes derived from stem cells
Bone marrow mesenchymal stem cells (MSCs) have the ability of self-renewal and multilineage differentiation, play a key role in maintaining immune tolerance, immunosuppression, and bone marrow microenvironment stability, and can produce a greater number of exosomes than other cells [87]. In recent years, the MSC deficiency theory has become a research hotspot in SLE. It has been experimentally shown that exosomes in SLE can break down the cytoskeleton, reduce the growth rate, and promote the senescence of bone marrow stem cells through the NF-κB signalling pathway [63]. Liu et al. demonstrated in MRL/lpr mice that exosomes derived after stem cell transplantation can transfer the Fas gene to recipient bone marrow stem cells and restore the function of bone marrow stem cells by regulating the miR-29b/Dnmt1/Notch epigenetic cascade. In addition, they can also reduce the inflammatory response in vivo by regulating innate and adaptive immune responses, reducing B cell proliferation and activation, mediating T cell apoptosis, and reducing lymph node and spleen weights in mice [88]. In SLE, M1 macrophages are largely activated, M2 macrophage expression is decreased, and the imbalance of M1/M2 is an important cause of the disease process [89], [90]. A study by Dou's team showed that MSC-derived exosomes could significantly change tRNA-derived fragments (tRFs) in macrophages after coculture. Of those tRFs, tsRNA-21109 may inhibit macrophage polarization to M1 and correct the imbalance between M1/M2 through signalling pathways, such as Rap1, Ras, Hippo, Wnt, MAPK and TGF-beta, providing a promising potential therapeutic strategy for the treatment of SLE [49], [91]. Song et al. showed that human umbilical cord-derived bone marrow stem cells pretreated with IL-1β significantly upregulated the expression of miR146a in their derived exosomes, induced macrophage polarization to the M2 phenotype, and ultimately prolonged the survival time of septic mice [92], [93]. Interestingly, olfactory extrinsic stem cell-derived exosomes (OE-MSC-EXOs) also enhance the immunosuppressive function of myeloid-derived suppressor cells (MDSCs), increase ROS and NO levels, and upregulate arginase expression [94]. These findings all provide promising potential therapeutic strategies for the treatment of SLE.
Discussion
Early treatment and disease control are essential for patients with systemic lupus erythematosus. The 2020 Chinese Guidelines for the Diagnosis and Treatment of Systemic Lupus Erythematosus recommend that stable SLE patients should be assessed for disease activity every 3–6 months and that patients with active SLE should be checked at least monthly. Patients and physicians should work together to provide regular assessment and administer and comply with rational individualized medication plans. Exosomes, as substance reservoirs and information transmitters, can have their contents analysed to roughly assess the status of SLE patients. This can provide ideas for targeted therapy with the help of drug-carrying exosomes while tracking their traces.
T cells play an important role in the occurrence and development of SLE and participate in the disease process by expressing abnormal adhesion molecules, activating other immune cells, secreting cytokines, and disrupting the balance of T cells. Aberrantly expressed proteins in T cell-derived exosomes from SLE patients promote autoimmune responses through both intrinsic (regulatory gene expression levels) and extrinsic (induction of inflammatory exosome migration) pathways. However, whether the expression of these abnormal proteins in exosomes is the cause, consequence, or both of increased SLE activity requires further investigation. Research on exosomes is in the active stage, and the progress of experiments and technological innovation will expand the value of exosomes and improve their potential in diagnosis and treatment. Many studies have focused on the relationship between exosomes and inflammation and tumours, and there are limited studies on exosomes in autoimmune diseases. Exosomes have great application prospects in the treatment of SLE, but we must also objectively recognize that the body is a complex regulatory system. The exact mechanism of exosomes in the process of SLE and the realization of large-scale production and high purity separation of exosomes so that exosome drugs have precise targeting should be further studied. In conclusion, the application of exosomes as diagnostic and therapeutic tools in the field of clinical medicine will be a promising tool that can provide solutions to numerous medical problems.
Author contributions
JS and MZ collected most of the review materials and wrote the main part of the review. MP proposed content and writing checks for review articles. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China through grant No. 81972695, and Innovation Team of Immune Microenvironment and Inflammatory Diseases Research of Introduction and Education Program for young Talents in Shandong Colleges and Universities.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wang Z., Wang Y., Zhu R., Tian X., Xu D., Wang Q., Wu C., Zhang S., Zhao J., Zhao Y., Li M., Zeng X. Long-term survival and death causes of systemic lupus erythematosus in China: a systemic review of observational studies. Medicine (Baltimore) 2015;94(17):e794. doi: 10.1097/MD.0000000000000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong G., Yang Y., Li X., Yao X., Zhu Y., Zhang H., Wang H., Ma Q., Zhang J., Shi H., Ning Z., Yan F., Zhai W., Dai J., Li Z., Li C., Ming J., Xue Q., Meng X., Si C., Xiong H. Granulocytic myeloid-derived suppressor cells contribute to IFN-I signaling activation of B cells and disease progression through the lncRNA NEAT1-BAFF axis in systemic lupus erythematosus. Biochim Biophys Acta, Mol Basis Dis. 2020;1866(1):165554. doi: 10.1016/j.bbadis.2019.165554. [DOI] [PubMed] [Google Scholar]
- 3.Tsai C.Y., Li K.J., Hsieh S.C., Liao H.T., Yu C.L. What's wrong with neutrophils in lupus? Clin Exp Rheumatol. 2019;37(4):684–693. [PubMed] [Google Scholar]
- 4.Tsai C.Y., Hsieh S.C., Lu C.S., Wu T.H., Liao H.T., Wu C.H., Li K.J., Kuo Y.M., Lee H.T., Shen C.Y., Yu C.L. Cross-Talk between Mitochondrial Dysfunction-Provoked Oxidative Stress and Aberrant Noncoding RNA Expression in the Pathogenesis and Pathophysiology of SLE. Int J Mol Sci. 2019;20(20) doi: 10.3390/ijms20205183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J.W., Kim Y.Y., Lee H., Park S.H., Kim S.K., Choe J.Y. Risk of Retinal Toxicity in Longterm Users of Hydroxychloroquine. J Rheumatol. 2017;44(11):1674–1679. doi: 10.3899/jrheum.170158. [DOI] [PubMed] [Google Scholar]
- 6.Chen H.L., Shen L.J., Hsu P.N., Shen C.Y., Hall S.A., Hsiao F.Y. Cumulative Burden of Glucocorticoid-related Adverse Events in Patients with Systemic Lupus Erythematosus: Findings from a 12-year Longitudinal Study. J Rheumatol. 2018;45(1):8389. doi: 10.3899/jrheum.160214. [DOI] [PubMed] [Google Scholar]
- 7.Li M., Zhao Y., Zhang Z., Huang C., Liu Y., Gu J., Zhang X., Xu H., Li X., Wu L., Song Y., Li X., Jin H., Lei J., Chen Y., Zeng X. 2020 Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus. Rheumatol Immunol Res. 2020;1(1):5–23. doi: 10.2478/rir-2020-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanouriakis A., Kostopoulou M., Alunno A., Aringer M., Bajema I., Boletis J.N., Cervera R., Doria A., Gordon C., Govoni M., Houssiau F., Jayne D., Kouloumas M., Kuhn A., Larsen J.L., Lerstrom K., Moroni G., Mosca M., Schneider M., Smolen J.S., Svenungsson E., Tesar V., Tincani A., Troldborg A., van Vollenhoven R., Wenzel J., Bertsias G., Boumpas D.T. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 9.Tan L., Wu H., Liu Y., Zhao M., Li D., Lu Q. Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity. 2016;49(6):357–365. doi: 10.1080/08916934.2016.1191477. [DOI] [PubMed] [Google Scholar]
- 10.Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr Biol. 2018;28(8):R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. doi: 10.1172/jci81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pegtel D.M., Gould S.J. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 15.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 16.Horibe S., Tanahashi T., Kawauchi S., Murakami Y., Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18(1):47. doi: 10.1186/s12885-017-3958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mok C.C., Lau C.S. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56(7):481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., Geuze H.J. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvo V., Izquierdo M. Inducible Polarized Secretion of Exosomes in T and B Lymphocytes. Int J Mol Sci. 2020;21(7) doi: 10.3390/ijms21072631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Niel G., Mallegol J., Bevilacqua C., Candalh C., Brugiere S., Tomaskovic-Crook E., Heath J.K., Cerf-Bensussan N., Heyman M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52(12):1690–1697. doi: 10.1136/gut.52.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschow S.I., Nolte-'t Hoen E.N., van Niel G., Pols M.S., ten Broeke T., Lauwen M., Ossendorp F., Melief C.J., Raposo G., Wubbolts R., Wauben M.H., Stoorvogel W. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 22.Thery C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 23.Dong J., Wang L., Zhao L., Pan L., Zhang Y. Effect of plasma exosomes on endothelial cell tight junction proteins in SLE patients with immune thrombocytopenia. Clin Rheumatol. 2021;40(8):3273–3278. doi: 10.1007/s10067-021-05651-5. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y., Zhang C., Xiang P., Shen J., Sun W., Yu H. Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells. J Extracell Vesicles. 2020;9(1):1722385. doi: 10.1080/20013078.2020.1722385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natasha G., Gundogan B., Tan A., Farhatnia Y., Wu W., Rajadas J., Seifalian A.M. Exosomes as immunotheranostic nanoparticles. Clin Ther. 2014;36(6):820–829. doi: 10.1016/j.clinthera.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Mittelbrunn M., Gutierrez-Vazquez C., Villarroya-Beltri C., Gonzalez S., Sanchez-Cabo F., Gonzalez M.A., Bernad A., Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan L., Zhao M., Wu H., Zhang Y., Tong X., Gao L., Zhou L., Lu Q., Zeng J. Downregulated Serum Exosomal miR-451a Expression Correlates With Renal Damage and Its Intercellular Communication Role in Systemic Lupus Erythematosus. Front Immunol. 2021;12:630112. doi: 10.3389/fimmu.2021.630112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guduric-Fuchs J., O'Connor A., Camp B., O'Neill C.L., Medina R.J., Simpson D.A. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J.Y., Park J.K., Lee E.Y., Lee E.B., Song Y.W. Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res Ther. 2016;18(1):264. doi: 10.1186/s13075-016-1159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvi V., Gianello V., Busatto S., Bergese P., Andreoli L., D'Oro U., Zingoni A., Tincani A., Sozzani S., Bosisio D. Exosome-delivered microRNAs promote IFN-alpha secretion by human plasmacytoid DCs via TLR7. JCI Insight. 2018;3(10) doi: 10.1172/jci.insight.98204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez P., Rodriguez-Carrio J., Martinez-Zapico A., Caminal-Montero L., Suarez A. Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity. Int J Cardiol. 2017;236:138–144. doi: 10.1016/j.ijcard.2017.02.107. [DOI] [PubMed] [Google Scholar]
- 32.Ostergaard O., Nielsen C.T., Iversen L.V., Tanassi J.T., Knudsen S., Jacobsen S., Heegaard N.H. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. 2013;65(10):2680–2690. doi: 10.1002/art.38065. [DOI] [PubMed] [Google Scholar]
- 33.Cicarini W.B., Ferreira K.S., Loures C.M.G., Consoli R.V., Neiva C.L.S., Padua P.M., Nunes F.F.C., Alves L.C.V., Reis E.A., Toledo V., Lwaleed B.A., Carvalho M.G. Systemic lupus erythematosus: disease activity may influence the release of endothelial microparticles? Blood Coagul Fibrinolysis. 2018;29(2):189–195. doi: 10.1097/MBC.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y., Wei W., Liu M.L. Extracellular vesicles and lupus nephritis - New insights into pathophysiology and clinical implications. J Autoimmun. 2020;115:102540. doi: 10.1016/j.jaut.2020.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez P., Rodriguez-Carrio J., Caminal-Montero L., Suarez A. Relationship Between T-Cell Exosomes and Cellular Subsets in SLE According to Type I IFN-Signaling. Front Med (Lausanne) 2020;7:604098. doi: 10.3389/fmed.2020.604098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J., et al. Comparison of plasma levels and immunoactivities of different forms of circulating-free DNA in systemic lupus erythematosus patients. Chin J Cell Mol Immunol. 2021;37(07):633–640. [PubMed] [Google Scholar]
- 37.Zhang B., Yin Y., Lai R.C., Lim S.K. Immunotherapeutic potential of extracellular vesicles. Front Immunol. 2014;5:518. doi: 10.3389/fimmu.2014.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sellam J., Proulle V., Jungel A., Ittah M., Miceli R.C., Gottenberg J.E., Toti F., Benessiano J., Gay S., Freyssinet J.M., Mariette X. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11(5):R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheinecker C., Goschl L., Bonelli M. Treg cells in health and autoimmune diseases: New insights from single cell analysis. J Autoimmun. 2020;110:102376. doi: 10.1016/j.jaut.2019.102376. [DOI] [PubMed] [Google Scholar]
- 40.Dall'Era M., Pauli M.L., Remedios K., Taravati K., Sandova P.M., Putnam A.L., Lares A., Haemel A., Tang Q., Hellerstein M., Fitch M., McNamara J., Welch B., Bluestone J.A., Wofsy D., Rosenblum M.D. Autoimmunity Centers of E. Adoptive Treg Cell Therapy in a Patient With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71(3):431–440. doi: 10.1002/art.40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal A., Fanelli G., Letizia M., Tung S.L., Boardman D., Lechler R., Lombardi G., Smyth L.A. Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol. 2014;5:555. doi: 10.3389/fimmu.2014.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okoye I.S., Coomes S.M., Pelly V.S., Czieso S., Papayannopoulos V., Tolmachova T., Seabra M.C., Wilson M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41(1):89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivasan S., Vannberg F.O., Dixon J.B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep. 2016;6:24436. doi: 10.1038/srep24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hood J.L. The association of exosomes with lymph nodes. Semin Cell Dev Biol. 2017;67:29–38. doi: 10.1016/j.semcdb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Chuang H.C., Chen M.H., Chen Y.M., Ciou Y.R., Hsueh C.H., Tsai C.Y., Tan T.H. ECP Overexpression in T Cells and Exosomes Induces IFN-gamma Production and Tissue Inflammation. Arthritis Rheumatol. 2021 doi: 10.1002/art.41920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuang H.C., Chen M.H., Chen Y.M., Yang H.Y., Ciou Y.R., Hsueh C.H., Tsai C.Y., Tan T.H. BPI overexpression suppresses Treg differentiation and induces exosome-mediated inflammation in systemic lupus erythematosus. Theranostics. 2021;11(20):9953–9966. doi: 10.7150/thno.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiller M., Heyder P., Ziegler S., Niessen A., Classen L., Lauffer A., Lorenz H.M. During apoptosis HMGB1 is translocated into apoptotic cell-derived membranous vesicles. Autoimmunity. 2013;46(5):342–346. doi: 10.3109/08916934.2012.750302. [DOI] [PubMed] [Google Scholar]
- 48.Dieudé M., Bell C., Turgeon J., Beillevaire D., Pomerleau L., Yang B., Hamelin K., Qi S., Pallet N., Béland C., Dhahri W., Cailhier J.-F., Rousseau M., Duchez A.-C., Lévesque T., Lau A., Rondeau C., Gingras D., Muruve D., Rivard A., Cardinal H., Perreault C., Desjardins M., Boilard É., Thibault P., Hébert M.-J. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med. 2015;7(318) doi: 10.1126/scitranslmed.aac9816. [DOI] [PubMed] [Google Scholar]
- 49.Liu B., Cao J., Wang X., Guo C., Liu Y., Wang T. Deciphering the tRNA-derived small RNAs: origin, development, and future. Cell Death Dis. 2021;13(1):24. doi: 10.1038/s41419-021-04472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H., Chen W., Zheng F., Tang D., Dai W., Huang S., Zhang C., Zeng J., Wang G., Dai Y. The potential role of tRNAs and small RNAs derived from tRNAs in the occurrence and development of systemic lupus erythematosus. Biochem Biophys Res Commun. 2020;527(2):561–567. doi: 10.1016/j.bbrc.2020.04.114. [DOI] [PubMed] [Google Scholar]
- 51.Yang P., Zhang X., Chen S., Tao Y., Ning M., Zhu Y., Liang J., Kong W., Shi B., Li Z., Shen H., Wang Y. A Novel Serum tsRNA for Diagnosis and Prediction of Nephritis in SLE. Front Immunol. 2021;12:735105. doi: 10.3389/fimmu.2021.735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng G., Wang H., Xin W., Liu Z., Chen J., Danting Z., Han F., Ye S. tRNA derived fragment (tRF)-3009 participates in modulation of IFN-alpha-induced CD4(+) T cell oxidative phosphorylation in lupus patients. J Transl Med. 2021;19(1):305. doi: 10.1186/s12967-021-02967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Truszewska A., Wirkowska A., Gala K., Truszewski P., Krzemien-Ojak L., Perkowska-Ptasinska A., Mucha K., Paczek L., Foroncewicz B. Cell-free DNA profiling in patients with lupus nephritis. Lupus. 2020;29(13):1759–1772. doi: 10.1177/0961203320957717. [DOI] [PubMed] [Google Scholar]
- 54.Hartl J., Serpas L., Wang Y., Rashidfarrokhi A., Perez O.A., Sally B., Sisirak V., Soni C., Khodadadi-Jamayran A., Tsirigos A., Caiello I., Bracaglia C., Volpi S., Ghiggeri G.M., Chida A.S., Sanz I., Kim M.Y., Belmont H.M., Silverman G.J., Clancy R.M., Izmirly P.M., Buyon J.P., Reizis B. Autoantibody-mediated impairment of DNASE1L3 activity in sporadic systemic lupus erythematosus. J Exp Med. 2021;218(5) doi: 10.1084/jem.20201138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez-Hernandez J., Forner M.J., Pinto C., Chaves F.J., Cortes R., Redon J. Increased Urinary Exosomal MicroRNAs in Patients with Systemic Lupus Erythematosus. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long H., Wang X., Chen Y., Wang L., Zhao M., Lu Q. Dysregulation of microRNAs in autoimmune diseases: Pathogenesis, biomarkers and potential therapeutic targets. Cancer Lett. 2018;428:90–103. doi: 10.1016/j.canlet.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Turchinovich A., Cho W.C. The origin, function and diagnostic potential of extracellular microRNA in human body fluids. Front Genet. 2014;5:30. doi: 10.3389/fgene.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Hernandez J., Redon J., Cortes R. Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lv L.L., Cao Y., Liu D., Xu M., Liu H., Tang R.N., Ma K.L., Liu B.C. Isolation and quantification of microRNAs from urinary exosomes/microvesicles for biomarker discovery. Int J Biol Sci. 2013;9(10):1021–1031. doi: 10.7150/ijbs.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W., Liu S., Chen Y., Weng R., Zhang K., He X., He C. Circulating Exosomal microRNAs as Biomarkers of Systemic Lupus Erythematosus. Clinics (Sao Paulo) 2020;75:e1528. doi: 10.6061/clinics/2020/e1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramachandran K., Saikumar J., Bijol V., Koyner J.L., Qian J., Betensky R.A., Waikar S.S., Vaidya V.S. Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clin Chem. 2013;59(12):1742–1752. doi: 10.1373/clinchem.2013.210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ichii O., Otsuka-Kanazawa S., Horino T., Kimura J., Nakamura T., Matsumoto M., Toi M., Kon Y., Rastaldi M.P. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS ONE. 2014;9(10):e110383. doi: 10.1371/journal.pone.0110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Hernandez J., Martinez-Arroyo O., Ortega A., Galera M., Solis-Salguero M.A., Chaves F.J., Redon J., Forner M.J., Cortes R. Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J Nephrol. 2021;34(4):1157–1167. doi: 10.1007/s40620-020-00832-y. [DOI] [PubMed] [Google Scholar]
- 64.Mirzaei R., Zamani F., Hajibaba M., Rasouli-Saravani A., Noroozbeygi M., Gorgani M., Hosseini-Fard S.R., Jalalifar S., Ajdarkosh H., Abedi S.H., Keyvani H., Karampoor S. The pathogenic, therapeutic and diagnostic role of exosomal microRNA in the autoimmune diseases. J Neuroimmunol. 2021;358:577640. doi: 10.1016/j.jneuroim.2021.577640. [DOI] [PubMed] [Google Scholar]
- 65.Sole C., Cortes-Hernandez J., Felip M.L., Vidal M., Ordi-Ros J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol Dial Transplant. 2015;30(9):1488–1496. doi: 10.1093/ndt/gfv128. [DOI] [PubMed] [Google Scholar]
- 66.Miao C., Wang X., Zhou W., Huang J. The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol Res. 2021;169:105680. doi: 10.1016/j.phrs.2021.105680. [DOI] [PubMed] [Google Scholar]
- 67.Tangtanatakul P., Klinchanhom S., Sodsai P., Sutichet T., Promjeen C., Avihingsanon Y., Hirankarn N. Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac J Allergy Immunol. 2019;37(4):189–197. doi: 10.12932/AP-130318-0280. [DOI] [PubMed] [Google Scholar]
- 68.Li Y., Xu X., Tang X., Bian X., Shen B., Zhao H., Luo S., Chen Z., Zhang K. MicroRNA expression profile of urinary exosomes in Type IV lupus nephritis complicated by cellular crescent. J Biol Res (Thessalon) 2018;25:16. doi: 10.1186/s40709-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Vives E., Sole C., Moline T., Vidal M., Agraz I., Ordi-Ros J., Cortes-Hernandez J. The Urinary Exosomal miRNA Expression Profile is Predictive of Clinical Response in Lupus Nephritis. Int J Mol Sci. 2020;21(4) doi: 10.3390/ijms21041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Navarro-Quiroz E., Pacheco-Lugo L., Lorenzi H., Díaz-Olmos Y., Almendrales L., Rico E., Navarro R., España-Puccini P., Iglesias A., Egea E., Aroca G., Zhou X.-J. High-Throughput Sequencing Reveals Circulating miRNAs as Potential Biomarkers of Kidney Damage in Patients with Systemic Lupus Erythematosus. PLoS ONE. 2016;11(11):e0166202. doi: 10.1371/journal.pone.0166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dong C., Zhou Q., Fu T., Zhao R., Yang J., Kong X., Zhang Z., Sun C., Bao Y., Ge X., Zhang Z., Lu Z., Li J., Zheng W., Gu Z., Ji J. Circulating Exosomes Derived-miR-146a from Systemic Lupus Erythematosus Patients Regulates Senescence of Mesenchymal Stem Cells. Biomed Res Int. 2019;2019:6071308. doi: 10.1155/2019/6071308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perez-Hernandez J., Cortes R. Extracellular Vesicles as Biomarkers of Systemic Lupus Erythematosus. Dis Markers. 2015;201 doi: 10.1155/2015/613536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murakami Y., Toyoda H., Tanahashi T., Tanaka J., Kumada T., Yoshioka Y., Kosaka N., Ochiya T., Taguchi Y.-h., Miao X.-P. Comprehensive miRNA expression analysis in peripheral blood can diagnose liver disease. PLoS ONE. 2012;7(10):e48366. doi: 10.1371/journal.pone.0048366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang M., Chen D., Zhang F., Zhang G., Wang Y., Zhang Q., He W., Wang H., Chen P. Serum exosomal hsa-miR-135b-5p serves as a potential diagnostic biomarker in steroid-induced osteonecrosis of femoral head. Am J Transl Res. 2020;12(5):2136–2154. [PMC free article] [PubMed] [Google Scholar]
- 75.Ortega A., Martinez-Arroyo O., Forner M.J., Cortes R. Exosomes as Drug Delivery Systems: Endogenous Nanovehicles for Treatment of Systemic Lupus Erythematosus. Pharmaceutics. 2020;13(1) doi: 10.3390/pharmaceutics13010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Classen L., Tykocinski L.O., Wiedmann F., Birr C., Schiller P., Tucher C., Krienke S., Raab M.S., Blank N., Lorenz H.M., Schiller M. Extracellular vesicles mediate intercellular communication: Transfer of functionally active microRNAs by microvesicles into phagocytes. Eur J Immunol. 2017;47(9):1535–1549. doi: 10.1002/eji.201646595. [DOI] [PubMed] [Google Scholar]
- 77.Luan J., Fu J., Chen C., Jiao C., Kong W., Zhang Y., Chang Q., Wang Y., Li D., Illei G.G., Kopp J.B., Pi J., Zhou H. LNA-anti-miR-150 ameliorated kidney injury of lupus nephritis by inhibiting renal fibrosis and macrophage infiltration. Arthritis Res Ther. 2019;21(1):276. doi: 10.1186/s13075-019-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou H., Hasni S.A., Perez P., Tandon M., Jang S.I., Zheng C., Kopp J.B., Austin H., 3rd, Balow J.E., Alevizos I., Illei G.G. miR-150 promotes renal fibrosis in lupus nephritis by downregulating SOCS1. J Am Soc Nephrol. 2013;24(7):1073–1087. doi: 10.1681/ASN.2012080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oskouie M.N., Aghili Moghaddam N.S., Butler A.E., Zamani P., Sahebkar A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J Cell Physiol. 2019;234(6):8182–8191. doi: 10.1002/jcp.27615. [DOI] [PubMed] [Google Scholar]
- 80.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C., Barnes S., Grizzle W., Miller D., Zhang H.G. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng H., Ji W., Zhao R., Yang J., Lu Z., Li Y., Zhang X. Exosome: a significant nano-scale drug delivery carrier. J Mater Chem B. 2020;8(34):7591–7608. doi: 10.1039/d0tb01499k. [DOI] [PubMed] [Google Scholar]
- 82.Li Q., Tan S., Xu K., Fu X., Yu J., Yang H., Wang H. Curcumin attenuates lupus nephritis in MRL/lpr mice by suppressing macrophage-secreted B cell activating factor (BAFF) Int J Clin Exp Pathol. 2019;12(6):2075–2083. [PMC free article] [PubMed] [Google Scholar]
- 83.Armstrong J.P., Holme M.N., Stevens M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano. 2017;11(1):69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lara P., Chan A.B., Cruz L.J., Quest A.F.G., Kogan M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics. 2020;12(11) doi: 10.3390/pharmaceutics12111022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., Batrakova E.V. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brossa A., Fonsato V., Grange C., Tritta S., Tapparo M., Calvetti R., Cedrino M., Fallo S., Gontero P., Camussi G., Bussolati B. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int J Cancer. 2020;147(6):1694–1706. doi: 10.1002/ijc.32925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang C., Sun J., Tian Y., Li H., Zhang L., Yang J., Wang J., Zhang J., Yan S., Xu D. Immunomodulatory Effect of MSCs and MSCs-Derived Extracellular Vesicles in Systemic Lupus Erythematosus. Front Immunol. 2021;12:714832. doi: 10.3389/fimmu.2021.714832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu S., Liu D., Chen C., Hamamura K., Moshaverinia A., Yang R., Liu Y., Jin Y., Shi S. MSC Transplantation Improves Osteopenia via Epigenetic Regulation of Notch Signaling in Lupus. Cell Metab. 2015;22(4):606–618. doi: 10.1016/j.cmet.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohammadi S., Saghaeian-Jazi M., Sedighi S., Memarian A. Sodium valproate modulates immune response by alternative activation of monocyte-derived macrophages in systemic lupus erythematosus. Clin Rheumatol. 2018;37(3):719–727. doi: 10.1007/s10067-017-3922-0. [DOI] [PubMed] [Google Scholar]
- 90.Mohammadi S., Saghaeian-Jazi M., Sedighi S., Memarian A. Immunomodulation in systemic lupus erythematosus: induction of M2 population in monocyte-derived macrophages by pioglitazone. Lupus. 2017;26(12):1318–1327. doi: 10.1177/0961203317701842. [DOI] [PubMed] [Google Scholar]
- 91.Dou R., Zhang X., Xu X., Wang P., Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol Immunol. 2021;139:106–114. doi: 10.1016/j.molimm.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 92.Song Y., Dou H., Li X., Zhao X., Li Y., Liu D., Ji J., Liu F., Ding L., Ni Y., Hou Y. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1beta-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells. 2017;35(5):1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 93.Shen Z., Huang W., Liu J., Tian J., Wang S., Rui K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front Immunol. 2021;12:749192. doi: 10.3389/fimmu.2021.749192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rui K., Hong Y., Zhu Q., Shi X., Xiao F., Fu H., Yin Q., Xing Y., Wu X., Kong X., Xu H., Tian J., Wang S., Lu L. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate murine Sjogren's syndrome by modulating the function of myeloid-derived suppressor cells. Cell Mol Immunol. 2021;18(2):440–451. doi: 10.1038/s41423-020-00587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]