Abstract
Refrigerated processed foods of extended durability such as cook-chill and sous-vide foods rely on a minimal heat treatment at 70 to 95°C and then storage at a refrigeration temperature for safety and preservation. These foods are not sterile and are intended to have an extended shelf life, often up to 42 days. The principal microbiological hazard in foods of this type is growth of and toxin production by nonproteolytic Clostridium botulinum. Lysozyme has been shown to increase the measured heat resistance of nonproteolytic C. botulinum spores. However, the heat treatment guidelines for prevention of risk of botulism in these products have not taken into consideration the effect of lysozyme, which can be present in many foods. In order to assess the botulism hazard, the effect of heat treatments at 70, 75, 80, 85, and 90°C combined with refrigerated storage for up to 90 days on growth from 106 spores of nonproteolytic C. botulinum (types B, E, and F) in an anaerobic meat medium containing 2,400 U of lysozyme per ml (50 μg per ml) was studied. Provided that the storage temperature was no higher than 8°C, the following heat treatments each prevented growth and toxin production during 90 days; 70°C for ≥2,545 min, 75°C for ≥463 min, 80°C for ≥230 min, 85°C for ≥84 min, and 90°C for ≥33.5 min. A factorial experimental design allowed development of a predictive model that described the incubation time required before the first sample showed growth, as a function of heating temperature (70 to 90°C), period of heat treatment (up to 2,545 min), and incubation temperature (5 to 25°C). Predictions from the model provided a valid description of the data used to generate the model and agreed with observations made previously.
Food-borne botulism is an intoxication involving the consumption of preformed botulinum neurotoxin. The consumption of as little as 0.1 g of food in which Clostridium botulinum has grown and produced the botulinum neurotoxin can result in severe illness (19). Two physiologically distinct clostridia, proteolytic C. botulinum and nonproteolytic C. botulinum, are responsible for food-borne botulism. Nonproteolytic C. botulinum is the principal microbiological consideration for the safe production of refrigerated processed foods of extended durability (REPFEDs) (18–20, 22, 32). REPFEDs have been developed in response to a demand for high-quality foods that are lightly processed, contain low levels of preservatives, and require minimal preparation time. REPFEDs include cook-chill and sous-vide foods. The heat process applied to REPFEDs is intended to retain maximum sensory and organoleptic quality and generally consists of heating at a maximum temperature in the range of 65 to 95°C, often for an extended period of time. After heat processing, the food is cooled rapidly and stored at a refrigeration temperature. REPFEDs are not sterile, and the product shelf life, which can typically be up to 42 days, is dependent upon the heat treatment applied and the storage temperature. There is a risk that spores of nonproteolytic C. botulinum may survive some of the milder heat treatments given to these foods. Although the foods are stored at refrigeration temperatures, these temperatures alone will not necessarily prevent growth of nonproteolytic C. botulinum, which can grow and produce toxin at 3.0 to 3.3°C (7, 8, 14, 33). The packaging of REPFEDs under a vacuum or an anaerobic atmosphere restricts the growth of aerobic bacteria but not growth of clostridia or other anaerobic bacteria. The extended shelf life of REPFEDs also provides additional time for growth and toxin formation. Guidelines to ensure the safety of these foods have been drawn up (1, 9).
The measured heat resistance of spores of nonproteolytic C. botulinum is increased by perhaps 2 orders of magnitude by the presence of hen egg white lysozyme and other factors (e.g., egg yolk emulsion, fruit and vegetable extracts, or other enzymes) in the medium used for enumeration of survivors (2, 20, 24, 28, 34, 35, 37). The germination system in spores of nonproteolytic C. botulinum is relatively easily inactivated by heating, and, in the absence of lysozyme, these heat-damaged spores may remain viable but unable to germinate. Lysozyme is able to induce germination of the subpopulation (0.1 to 1%) of heat-damaged spores that possess permeable coats (25). Lysozyme can diffuse through the coats of these spores, inducing germination by hydrolyzing peptidoglycan in the spore cortex (11, 23).
The widespread occurrence of lysozymes or other lytic enzymes in foods of all types (20, 28, 31, 37) at an activity higher than that required to increase the measured heat resistance of spores of nonproteolytic C. botulinum (20, 24) makes it essential from a safety point of view to consider the inclusion of lysozyme in model food studies. Studies on the heat resistance of hen egg white lysozyme indicate that it will survive some of the heat treatments given to REPFEDs (20, 21, 26, 27, 31). For example, after heating at 90°C for 20 min and at 95°C for 15 min, growth from an inoculum of 106 spores was observed at 25°C within 20 and 32 days, respectively, when lysozyme (480 to 625 U/ml) was added prior to heating, but growth was not observed within 93 days when no addition of lysozyme was made (26, 27). In view of the increased growth potential in foods that contain lysozyme, it is important to identify combinations of heat treatment, refrigerated storage, and limited shelf life that may be used to give a 106 reduction in the probability of growth of nonproteolytic C. botulinum in foods containing lysozyme.
The aim of the present work was to study the effect of extended heat treatment at 70 to 90°C and incubation temperatures of 5 to 25°C on growth and toxin production from 106 spores of nonproteolytic C. botulinum. This safety factor was selected on the basis of current guidelines (1, 9). An anaerobic model food system (meat slurry) was used, with hen egg white lysozyme added prior to heating, to represent a modified atmosphere or vacuum-packed product that contains lysozyme. The results were then used to produce a mathematical model that predicts the combined effect of heat treatment and incubation temperature on time to growth from 106 spores of nonproteolytic C. botulinum. This work complements an earlier study (10), in which lysozyme was not added to the model food system and a corresponding predictive model was developed.
MATERIALS AND METHODS
Bacteria.
Cultures of nonproteolytic C. botulinum types B (Eklund 2B, Eklund 17B, and Hobbs FT50), E (Beluga, Hazen 36208, and Foster B96), and F (Eklund 202F and Craig 610) were originally obtained from sources described previously (10). All were maintained as described previously (24). Tests were done to confirm that the concentration of toxin formed in peptone-yeast extract-glucose-starch medium (PYGS) (18) was greater than 1,000 mouse lethal doses/ml (27) and that all of the strains grew rapidly at 8°C.
Preparation of spores and assessment of spore heat resistance.
Spores were produced on a two-phase medium and washed as described previously (24). Tests were done to confirm that the spores had heat resistance similar to that described previously (25).
Preparation of meat medium.
An anaerobic meat medium was prepared as described previously (10) and contained the following: raw minced beef, 500 g; glucose, 10 g; NaCl, 10 g; soluble starch, 10 g; and glass-distilled water, to 1,000 g. This medium was dispensed under N2 by strict anaerobic techniques as 20-ml volumes into anaerobic culture tubes which were capped and sealed. The medium was sterilized by autoclaving at 121°C for 15 min, stored at 1°C, and used within 3 weeks of preparation. The pH of the medium after autoclaving was 6.4 to 6.6, and the final total extractable fat content was 8.8% (±0.1%) (wt/wt) when determined by extraction with petroleum ether (6). The water activity of the meat medium was measured with a CX-2 water activity system (Decagon Devices, Inc.), and the average value was 0.99.
Preparation of lysozyme and addition to the meat medium.
On the day prior to inoculation, sterile lysozyme (hen egg white lysozyme, 48,000 U/mg) (L6876; Sigma, Poole, United Kingdom) was added to all tubes. A solution of 5 mg of lysozyme/ml was made in distilled water prepared under N2 and sterilized by filtration (0.22-μm-pore-size Millex-GV filter; Millipore, Watford, United Kingdom), and a 0.2-ml volume was added to each tube to give a final concentration of 2,400 U/ml (50 μg/ml).
Preparation of spore suspension and inoculation of meat medium.
A suspension of spores (approximately 5 × 106/ml) containing an equal number of spores of the eight different strains of nonproteolytic C. botulinum (three type B strains, three type E strains, and two type F strains) was made in saline (0.85%, wt/vol) prepared under N2. With a syringe, 0.2-ml volumes of suspension were added to sealed tubes of medium that had been prewarmed at 45°C for 10 min to melt and mix the fat. The tubes were then shaken to disperse the added spores and cooled immediately in an ice-water bath. Five replicate tubes were used for each combination of heat treatment and incubation temperature. The inoculated tubes were then held in an ice-water bath and were heat treated within 1 h of the addition of spores.
Measurement of the heat treatments applied.
To monitor the temperature during thermal treatments, eight thermocouples (copper-copper nickel thermocouple probes [type T] in a 1.6-mm-diameter stainless steel probe [R.S. Components, Corby, United Kingdom]) were sealed singly into tubes of uninoculated meat medium. Each probe had been previously calibrated against certified precision mercury-in-glass thermometers in a range of 65 to 95°C. The temperature was recorded to the nearest 0.1°C. Temperatures were recorded with a Data Acquisition Unit (series 410; Anville Instruments, Camberley, United Kingdom), with the response of the probes monitored every 6 s for the shorter heat treatments and every 30 s for heat treatments longer than 600 min. The heat treatment was assessed by placing the temperature-monitoring tubes (those with thermocouples) towards the center of the rack containing tubes to be heat treated; this ensured that they received the lowest heat treatment of all tubes. An appropriate heat treatment was applied by immersing the rack of tubes rapidly in a large water bath (W38 bath; Grant Instruments, Cambridge, United Kingdom) set at the desired temperature, and the temperature of the bath was monitored with a precision mercury-in-glass thermometer. When the core temperature of the meat medium was within 0.1°C of that required, timing was started. After an appropriate period of time at the desired temperature, the rack of tubes was removed and plunged into a deep ice bath. The tubes were then shaken vigorously to effect a rapid cooling. When the core temperature of the meat medium had fallen below 10°C in all monitored tubes, the tubes were dried and transferred to an appropriate incubator. The lethality of the heat treatments was calculated as described previously (10).
Incubation of tubes.
After heat treatment and cooling, tubes of meat medium previously inoculated with spores of C. botulinum were incubated in low-temperature incubators (Astell-Hearson model MK III), the temperatures of which were monitored with platinum resistance thermometers connected to a data logger (Anville Instruments) and recorded at intervals of 30 min. The thermometers were placed in vials of water identical to those used with the meat medium. The target incubation temperatures were 5, 8, 12, 16, and 25°C for all of the heat treatments applied. The incubation temperatures represented typical refrigeration temperatures and temperatures included in legislation, as well as mild, moderate, and severe abuse temperatures. The platinum resistance thermometers (British Standard Grade II) were calibrated to an accuracy of ±0.1°C over the range of use. At the end of each experiment, the data were analyzed to determine the mean temperature and temperature variation.
Enumeration of survivors.
Enumeration of survivors was done as described previously (10) and conducted separately from the experiments with meat medium. Sterile lysozyme (hen egg white lysozyme), prepared as previously described, was added to the PYGS broth to give a final concentration of 480 U/ml (10 μg/ml). Lysozyme was added to represent a REPFED containing lysozyme. Five replicate tubes were used per dilution, and three dilution series were prepared for each sample. Growth was assessed by visible turbidity and production of gas. From the number of vials that showed growth, the most probable number (MPN) of viable spores in the original sample was calculated (15). Enumeration of viable spores in unheated controls was also carried out to establish the initial spore number per tube of meat slurry. The probability that a single spore would initiate growth and form toxin (P) was calculated as MPN of spores that resulted in growth/MPN of spores inoculated. The value log 1/P represents the logarithm (log10) of the number of spores required for one spore to result in growth (5).
Determination of growth and toxin production in meat medium.
Tubes were examined at least every 2 or 3 days for signs of visible growth. Growth of C. botulinum in meat medium was indicated by obvious formation of gas. In some circumstances a few gas bubbles or minor cracks in the meat medium occurred as a result of the manipulations; this was not taken to indicate growth. At the end of the experiment, samples of medium to be tested for toxin were centrifuged (15,000 × g, 10°C, 15 min), and the supernatant was stored at 1°C until it was tested. Tests for the presence of growth/toxin were done by using an enzyme-linked immunosorbent assay (ELISA) procedure (36) modified from a method originally described by Potter et al. (30). For each heat treatment, samples from the lowest incubation temperature that showed growth and the highest incubation temperature that did not show growth were tested with the ELISA. Under most of the sets of conditions in which some, but not all, of the five replicate vials showed visible signs of growth and gave positive results in the ELISA, samples from each of the five replicate vials were tested for toxin by intraperitoneal injection into mice, as described previously (27).
Modeling.
The incubation times required before the first observation of growth were modeled as a function of the heating temperature (H), heating time (t), and incubation temperature (I). The data from unheated tubes of meat were not included in the development of the model. A quadratic response surface was used, which was represented by a polynomial of the form ln (y) = c1 + (c2 × H) + (c3 × t) + (c4 × I) + (c5 × H × t) + (c6 × H × I) + (c7 × t × I) + (c8 × H2) + (c9 × t2) + (c10 × I2), where ln (y) is the natural logarithm of the dependent variable of the model, the time to the first tube showing growth, and c1 to c10 are the coefficients to be estimated. The response surface fitting was carried out by standard linear regression as performed with the commercial Microsoft Excel spreadsheet.
RESULTS
Heat treatments applied.
When the temperature approached the target values, there was a slow rate of increase in temperature. Therefore, for the heat treatments of short duration, the period during which the temperature was brought to the target value contributed significantly to the lethality of the heat treatment (Table 1). The cooling period was between 4 and 10 min in most of the treatments, and the fall in temperature was rapid (Table 1). The lethal effect of the total heat treatment is expressed as the equivalent time at the target temperature (Table 1).
TABLE 1.
Heat treatments applied to spores of nonproteolytic C. botulinum in a meat medium
| Expt no. | Heating temp (°C) | Time (min)
|
Heat lethality (equivalent time [min] at target temp)d | ||
|---|---|---|---|---|---|
| Come-upa | Target tempb | Coolingc | |||
| 1 | 70.0 | 8.2 | 100.6 | 7.8 | 104.9 |
| 2 | 70.0 | 7.0 | 527.0 | 7.5 | 529.1 |
| 1 | 70.0 | 12.7 | 989.6 | 5.8 | 998.9 |
| 1 | 70.0 | 12.8 | 1,587.9 | 6.2 | 1,596.3 |
| 2 | 70.0 | 14.5 | 2,056.0 | 12.5 | 2,065.9 |
| 2 | 70.0 | 14.0 | 2,537.5 | 7.5 | 2,544.5 |
| 2 | 75.0 | 9.5 | 280.0 | 8.0 | 284.6 |
| 1 | 75.0 | 11.8 | 457.4 | 7.1 | 463.1 |
| 2 | 75.0 | 9.0 | 730.0 | 7.0 | 734.2 |
| 2 | 75.0 | 12.5 | 1,065.5 | 5.0 | 1,071.5 |
| 1 | 75.0 | 15.8 | 1,373.7 | 5.7 | 1,376.5 |
| 2 | 75.0 | 11.0 | 1,785.0 | 12.0 | 1,793.0 |
| 1 | 80.0 | 10.0 | 7.8 | 4.7 | 11.4 |
| 2 | 80.0 | 13.5 | 64.5 | 5.0 | 69.7 |
| 1 | 80.0 | 15.3 | 93.7 | 6.8 | 98.0 |
| 1 | 80.0 | 15.3 | 123.7 | 5.3 | 127.9 |
| 2 | 80.0 | 14.5 | 178.0 | 10.0 | 183.8 |
| 1 | 80.0 | 10.1 | 226.1 | 5.7 | 229.6 |
| 2 | 80.0 | 12.5 | 289.0 | 8.5 | 294.9 |
| 2 | 80.0 | 15.0 | 355.0 | 8.0 | 362.7 |
| 2 | 85.0 | 9.6 | 18.7 | 6.9 | 23.3 |
| 1 | 85.0 | 14.8 | 28.0 | 9.1 | 35.7 |
| 2 | 85.0 | 11.0 | 46.6 | 6.3 | 52.0 |
| 1 | 85.0 | 11.5 | 53.5 | 5.1 | 57.8 |
| 2 | 85.0 | 10.8 | 78.4 | 5.5 | 83.8 |
| 1 | 90.0 | 11.6 | 5.8 | 4.5 | 10.3 |
| 2 | 90.0 | 9.3 | 6.4 | 5.7 | 10.9 |
| 2 | 90.0 | 11.9 | 10.1 | 5.4 | 15.3 |
| 2 | 90.0 | 12.7 | 17.3 | 4.3 | 23.5 |
| 2 | 90.0 | 11.9 | 27.8 | 5.9 | 33.5 |
Time required for the temperature at the center of the meat medium to reach within 0.1°C of the target maximum temperature.
Time that the temperature at the center of the meat medium was within 0.1°C of the target maximum temperature.
Time required for the temperature at the center of the meat medium to fall from within 0.1°C of the target maximum temperature to less than 10°C.
Determination of the number of spores that survived each heat treatment.
In general, the number of survivors decreased when the heating time increased (Table 2). The decreases that resulted from heat treatments at 70°C for 2,545 min, 75°C for 1,793 min, 80°C for 363 min, 85°C for 84 min, and 90°C for 34 min were all less than a factor of 103 when lysozyme was included in the enumeration medium. The number of spores required for one spore to grow and produce toxin at 30°C was substantially lower when lysozyme was included than when lysozyme was not included in the enumeration medium (Table 2).
TABLE 2.
Determination of the number of spores of nonproteolytic C. botulinum that survived each heat treatment and resulted in growth at 30°C in the presence of lysozyme and of the log10 number of spores required for one spore to grow at 30°C in the presence and absence of lysozyme
| Heating temp (°C) | Heating time (min) | Log MPN, viable spores/tubea | Log 1/Pb
|
|
|---|---|---|---|---|
| With lysozyme | No lysozymec | |||
| Unheated | 0 | 5.79 | ||
| 70 | 104.9 | 5.57 | 0.21 | 1.17 |
| 529.1 | 5.07 | 0.71 | 1.04 | |
| 998.9 | 4.47 | 1.30 | 2.95 | |
| 1,596.3 | 4.21 | 1.56 | 2.62 | |
| 2,065.9 | 3.51 | 2.27 | 1.97 | |
| 2,544.5 | 4.26 | 1.51 | 2.36 | |
| 75 | 284.6 | 4.52 | 1.26 | 3.47 |
| 463.1 | 4.12 | 1.66 | 3.87 | |
| 1,071.5 | 3.65 | 2.12 | 4.52 | |
| 1,376.5 | 2.89 | 2.88 | >4.8 | |
| 1,793.0 | 3.65 | 2.12 | >4.8 | |
| 80 | 11.4 | 5.47 | 0.31 | 2.66 |
| 69.7 | 4.79 | 0.98 | >4.8 | |
| 98.0 | 4.98 | 0.80 | >4.8 | |
| 127.9 | 4.54 | 1.23 | >4.8 | |
| 183.8 | 3.97 | 1.81 | >4.8 | |
| 229.6 | 4.16 | 1.61 | >4.8 | |
| 294.9 | 4.15 | 1.62 | >4.8 | |
| 362.7 | 3.90 | 1.88 | >4.8 | |
| 85 | 23.3 | 4.45 | 1.32 | 4.00 |
| 35.7 | 4.68 | 1.09 | >4.8 | |
| 52.0 | 4.12 | 1.66 | >4.8 | |
| 57.8 | 4.20 | 1.58 | >4.8 | |
| 83.8 | 3.51 | 2.27 | >4.8 | |
| 90 | 10.3 | 4.04 | 1.74 | >4.8 |
| 10.9 | 3.11 | 2.67 | >4.8 | |
| 15.3 | 3.27 | 2.51 | >4.8 | |
| 23.5 | 3.43 | 2.34 | >4.8 | |
| 33.5 | 3.52 | 2.26 | >4.8 | |
The number of surviving spores was determined by estimation of MPN in PYGS medium containing lysozyme (480 U/ml) at 30°C.
Log10 number of spores required for one spore to grow and produce toxin at 30°C.
Value when recovery was at 30°C in the absence of lysozyme (data are from reference 10).
Growth of nonproteolytic C. botulinum in meat medium after heat treatment and incubation at chill temperature.
The actual mean incubation temperatures were 4.9, 7.8, 11.5, 15.8, and 25.0°C for the heat treatments tested in experiment 1 and 4.7, 7.9, 11.8, 15.9, and 25.3°C for the heat treatments tested in experiment 2 (Table 1). At no time did the measured incubation temperature exceed the target incubation temperature by more than 1°C.
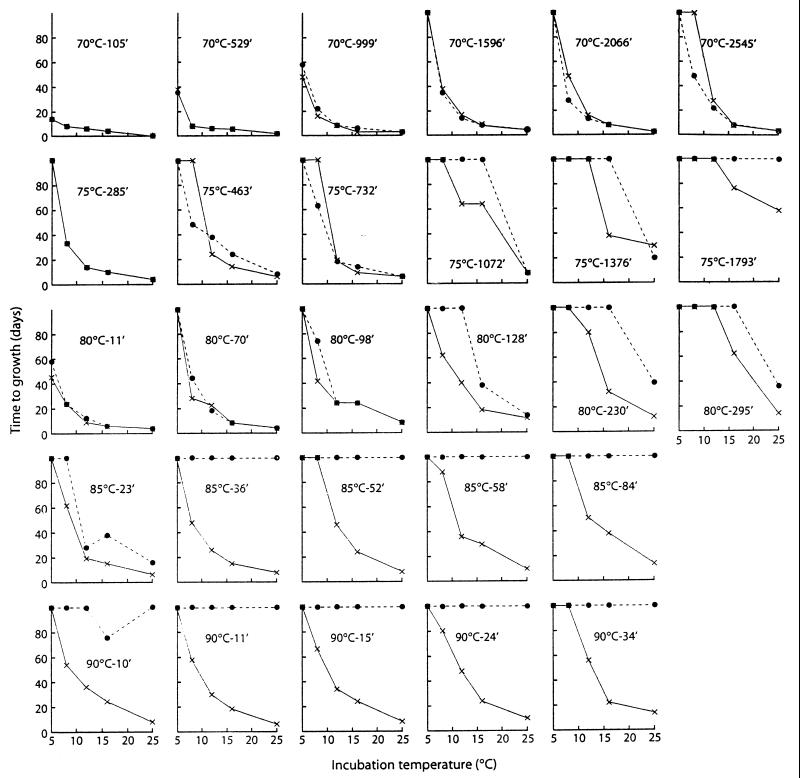
The effect of heat treatment and subsequent incubation temperature on the time to visible growth from an inoculum of 106 spores of nonproteolytic C. botulinum is shown in Table 3. A comparison of the results obtained in this study with those obtained in a previous parallel study in which lysozyme was not included (10) shows that the inclusion of lysozyme (2,400 U/ml) prior to heating reduced the time to growth when a relatively severe heat treatment was applied (i.e., higher heating temperature and/or longer heating times) or when the incubation temperature was low (Fig. 1). From previous studies (20, 22, 23, 26), it may be inferred that the presence of lysozyme resulted in germination of a fraction of the heat-damaged spores, giving a reduced time to growth or enabling growth under conditions where it was previously not observed in the absence of added lysozyme. This occurred at the longer heating times at 75 and 80°C and at most heating times at 85 and 90°C (Fig. 1). A sufficiently large number of spores survived the milder heat treatments (e.g., at 70°C [Table 2]) to ensure that lysozyme-induced recovery of small numbers of heat-damaged spores had little effect on time to growth (Fig. 1).
TABLE 3.
Effect of heat treatment and incubation temperature on time to growth from 106 spores of nonproteolytic C. botulinum (types B, E, and F) in five subsequent replicate tubes of meat medium containing lysozyme (2,400 U/ml)
| Heat treatment
|
Time (days) to growtha at the following incubation temp (°C)
|
|||||
|---|---|---|---|---|---|---|
| °C | min | 5 | 8 | 12 | 16 | 25 |
| Unheated | 0 | 14, 17, 17, 17, 17 | 7, 7, 7, 7, 7 | 5, 5, 5, 5, 5 | 2, 2, 2, 2, 2 | 1, 1, 1, 1, 1 |
| 70 | 104.9 | 14, 14, 16, 17, 17 | 9, 9, 9, 11, 11 | 6, 6, 6, 6, 6 | 2, 2, 2, 2, 3 | 1, 1, 1, 1, 1 |
| 529.1 | 39, 39, 43, 43, 43 | 9, 9, 10, 10, 12 | 7, 7, 7, 7, 7 | 4, 4, 4, 4, 5 | 1, 1, 1, 1, 1 | |
| 998.9 | 48, 55, 60, 70, 87 | 17, 21, 24, 26, 26 | 8, 8, 9, 10, 11 | 3, 5, 6, 6, 6 | 2, 2, 2, 2, 2 | |
| 1,596.3 | NGb | 38, 45, 52, 54, 82 | 16, 16, 17, 17, 19 | 9, 9, 11, 11, 12 | 3, 3, 3, 3, 5 | |
| 2,065.9 | NG | 46, 53, 58, 76 | 16, 17, 22, 24, 27 | 8, 8, 9, 9, 10 | 3, 3, 3, 4, 4 | |
| 2,544.5 | NG | NG | 27, 29, 36, 38, 47 | 9, 10, 11, 12, 12 | 4, 4, 5, 5, 5 | |
| 75 | 284.6 | NG | 33, 33, 33, 35, 35 | 13, 15, 18, 21, 21 | 11, 11, 11, 11, 11 | 4, 4, 4, 4, 5 |
| 463.1 | NG | NG | 23, 25, 40, 40, 80 | 15, 25, 30, 30, 38 | 6, 8, 9, 10, 11 | |
| 734.2 | NG | NG | 21, 35, 54, 59 | 11, 18, 21, 21, 28 | 7, 8, 8, 12, 15 | |
| 1,071.5 | NG | NG | 63 | 63 | 10, 20, 22, 22, 29 | |
| 1,376.5 | NG | NG | NG | 38, 82 | 30, 34 | |
| 1,793.0 | NG | NG | NG | 56 | 48, 60, 69 | |
| 80 | 11.4 | 46, 58, 65, 73, 73 | 24, 26, 26, 29, 29 | 10, 11, 14, 14, 15 | 8, 8, 8, 8, 8 | 4, 4, 4, 4, 5 |
| 69.7 | NG | 26, 35, 39, 44, 50 | 21, 21, 26, 28, 33 | 8, 13, 14, 14, 14 | 4, 4, 5, 6, 6 | |
| 98.0 | NG | 42, 59, 73, 80 | 24, 31, 35, 38, 40 | 24, 24, 26, 29, 29 | 8, 11, 11, 11, 15 | |
| 127.9 | NG | 63, 77, 77, 87 | 40, 47, 47, 49, 56 | 17, 31, 31, 35, 38 | 11, 11, 11, 11, 12 | |
| 183.8 | NG | 86 | 62, 69 | 19, 24, 39, 41, 48 | 11, 13, 16, 16, 19 | |
| 229.6 | NG | NG | 80, 84 | 31, 45, 45, 47, 49 | 16, 17, 17, 19, 19 | |
| 294.9 | NG | NG | NG | 62, 65, 65, 76, 76 | 16, 23, 26, 28, 30 | |
| 362.7 | NG | NG | NG | NG | 19, 28, 28, 31, 37 | |
| 85 | 23.3 | NG | 61, 64, 68, 68, 70 | 23, 30, 34, 34, 36 | 15, 18, 20, 20, 20 | 6, 6, 7, 7, 7 |
| 35.7 | NG | 48, 53, 56, 56, 77 | 26, 31, 33, 33, 49 | 15, 23, 23, 23, 28 | 7, 9, 9, 9, 9 | |
| 52.0 | NG | NG | 46, 51, 51, 51, 54 | 23, 25, 25, 30, 32 | 8, 11, 11, 11, 11 | |
| 57.8 | NG | 87, 87, 89, 89, 89 | 37, 37, 49, 52, 54 | 33, 33, 33, 37, 40 | 9, 10, 10, 10, 12 | |
| 83.8 | NG | NG | 51, 64, 64, 65, 72 | 36, 38, 38, 40, 47 | 13, 15, 15, 15, 18 | |
| 90 | 10.3 | NG | 54, 59, 70, 73, 75 | 38, 38, 38, 42, 42 | 24, 24, 26, 26, 26 | 8, 8, 8, 8, 8 |
| 10.9 | NG | 58, 61, 61, 68, 68 | 30, 30, 32, 32, 32 | 18, 18, 18, 20, 20 | 6, 7, 7, 7, 7 | |
| 15.3 | NG | 68, 72, 75, 75 | 34, 36, 38, 42, 47 | 23, 23, 23, 25, 25 | 8, 9, 9, 9, 11 | |
| 23.5 | NG | 79 | 47, 47, 47, 54, 56 | 23, 27, 27, 27, 30 | 9, 11, 11, 11, 11 | |
| 33.5 | NG | NG | 56, 56, 56, 61, 65 | 23, 30, 34, 38, 38 | 14, 14, 14, 14, 17 | |
As determined visually. When fewer than five values are included, the rest of the tubes did not show visible grow after 90 days.
NG, no growth.
FIG. 1.
Effect of inclusion of lysozyme, heat treatment, and incubation temperature on time to growth of nonproteolytic C. botulinum. ×, 2,400 U of lysozyme per ml added before heating; ●, no lysozyme added. Treatments showing no growth/toxin production in 90 days are plotted at the top of each y axis.
Toxin formation by nonproteolytic C. botulinum.
Tubes were tested for growth/toxin at the end of the experiment by using an ELISA. Growth/toxin were detected in at least one tube in each treatment in which visible growth was recorded, and growth/toxin were not detected in any tubes in which visible growth had not been detected by gas formation. In samples that were tested in the mouse test as well as by the ELISA, the results of the two tests were in agreement in all cases.
Modeling.
Coefficients obtained for the second-order polynomial were as follows: ln y = −39.14 + (1.0054 × H) − (0.01190 × t) − (0.2748 × I) + (0.00018818 × H × t) + (0.000488 × H × I) − (0.0000136 × t × I) − (0.005633 × H2) − (0.0000001535 × t2) + (0.003676 × I2), where ln y is the natural logarithm of the time (days) to the first observation of growth, H is the heating temperature, t is the heating time, and I is the incubation temperature.
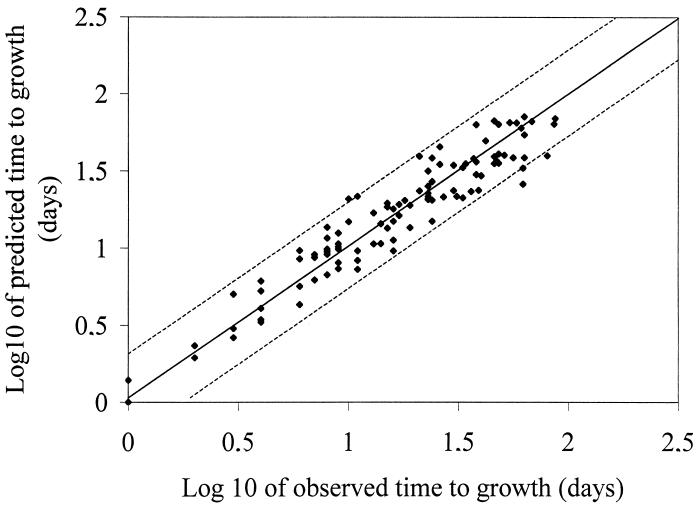
The percentage of variance accounted for was 89%, and the value of the residual mean square error for ln y was 0.33. The residual mean square error provides a measure of the goodness of fit of the model to the data (4). The predicted time to growth compares well with the observed time to growth; the 95% confidence interval of the prediction is shown in Fig. 2. Thus, the model provides a valid description of the data used to generate it.
FIG. 2.
Comparison of measured (observed) incubation time required before first observation of growth of nonproteolytic C. botulinum with fitted (predicted) time to first visualization of growth from the model. The 95% confidence interval of the prediction is shown as dashed lines.
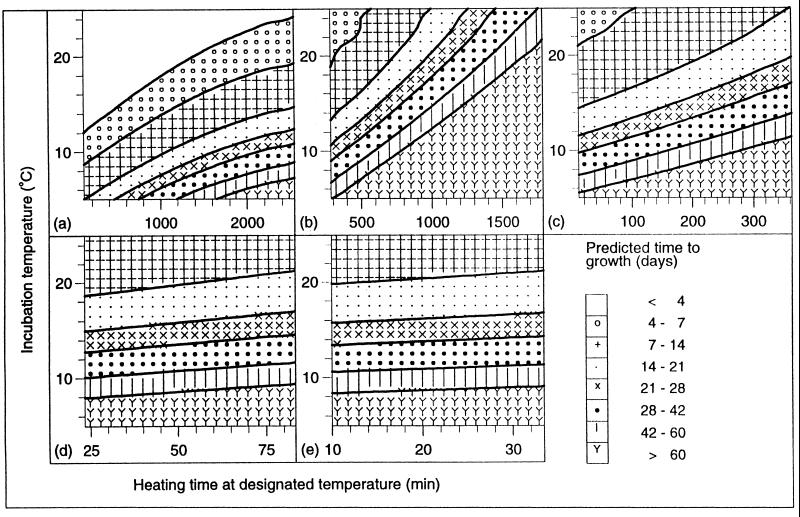
This equation should be used only to give predictions for a range of conditions within the limits of the model. The boundaries of the model are the shortest and longest heat treatments at each temperature and the incubation temperatures shown in Table 3. Contour plots produced from the model are shown in Fig. 3.
FIG. 3.
Effect of heat treatment at 70°C (a), 75°C (b), 80°C (c), 85°C (d), and 90°C (e), followed by incubation at temperatures of between 5 and 25°C, on the predicted time to growth from an inoculum of 106 spores of nonproteolytic C. botulinum types B, E, and F.
DISCUSSION
This study has evaluated, in a model food containing lysozyme, the effect of extended heat treatments in the range 70 to 90°C with subsequent incubation at refrigeration temperatures on growth from spores of nonproteolytic C. botulinum. A model that predicts the time to growth of this pathogen from an inoculum of 106 spores as a function of the heating temperature (70 to 90°C), period of heat treatment, and subsequent incubation temperature has been developed. This initial number of spores was chosen because the heat treatments recommended by the Advisory Committee on the Microbiological Safety of Food (ACMSF) (1) and the European Chilled Food Federation (ECFF) (9) were established with the aim of reducing the number of spores of nonproteolytic C. botulinum by a factor of 106 (6D process). The rationale for this criterion has been discussed previously (27).
The heat treatments applied in this study reduced the number of viable spores of nonproteolytic C. botulinum enumerated on medium containing lysozyme at 30°C by a factor of less than 103 (3D process) (Table 2). The heat treatments advocated by the ACMSF (1) and ECFF (9) as giving a 6D process gave only a 1D to 2D process. Spore thermal inactivation was, perhaps, marginally lower than that recorded previously when spores were heated in buffer and recovered in the presence of lysozyme (25), and this may be due to a protective effect of the meat slurry. It is well known that foods can have a protective effect on the spores subjected to heat treatment (see, e.g., references 16 and 19). When spores were enumerated on medium lacking lysozyme at 30°C, the measured reduction in the number of viable spores was considerably greater than it was when lysozyme was included, with a 6D process indicated for some, but not all, of the heat treatments advocated by the ACMSF and ECFF (1, 9). A recent article (29) indicates that with pasteurized crabmeat inoculated with 106 spores of nonproteolytic C. botulinum, heat treatments of 88.9°C for 65 min, 92.2°C for 45 min, or 94.4°C for 25 min were needed to prevent growth and toxin production during subsequent incubation at 27°C for 150 days. These data are more similar to those obtained in this study, with lysozyme added prior to heating, than to those obtained in our previous study without lysozyme (10). Enzymes with lysozyme activity have been reported in crustacea (20).
The combination (heating and incubation) treatments used in this and a previous parallel study (10) can be divided into two categories. These are the relatively mild combination treatments (mostly heat treatments at 70°C and short periods of heating at 75 or 80°C) and the more stringent combination treatments (mostly longer periods of heating at 75 and 80°C and heating at 85 and 90°C). For the relatively mild combination treatments, a large number of spores were able to germinate and give growth in the absence of lysozyme; hence, the small increase in the number of germinating spores brought about by the inclusion of lysozyme had little effect on the observed time to growth. Under these conditions, predictions from models derived in the absence and in the presence of lysozyme were also similar. For the more stringent combination treatments, few or no spores were able to germinate and give growth in the absence of lysozyme. Under these conditions, growth was either very slow or was not observed in the absence of lysozyme (10) but was frequently rapid when lysozyme was added prior to heating. Under those stringent conditions where predictions from the previous (no-lysozyme) model are possible, the new (plus-lysozyme) model predicts faster growth than does the previous (no-lysozyme) model. The model generated in the present study (plus-lysozyme) is more comprehensive than the (no-lysozyme) model generated previously (10) in that it includes many additional combination treatments not covered by the previous model (e.g., heating at 85 and 90°C). The model derived from results in the presence of lysozyme should be used to predict growth under these stringent conditions.
Predictions from the new (plus-lysozyme) model were compared with observations in independent studies where the time to growth from 106 spores of nonproteolytic C. botulinum was determined in the presence of lysozyme (Table 4). Predictions from the model were similar to observations of growth. This indicates that the model can be used to predict the shelf life that will provide a safety factor of 106 in relation to spores of nonproteolytic C. botulinum for a food containing lysozyme-like activity given a mild heat treatment (70 to 90°C) and stored at refrigeration temperature. While several predictive models for growth of nonproteolytic C. botulinum (see, e.g., references 3, 12, and 38) and for thermal inactivation (see, e.g., references 17, 25, and 28), currently exist, this is only the second to include thermal inactivation and subsequent growth together. The previous model does not include lysozyme (10). The new model described here is a useful tool for the food industry, as it is a rapid method to describe the effect of heat treatment and subsequent storage temperature on the ability of spores of nonproteolytic C. botulinum to survive and result in growth and toxin production in a food that may contain lysozyme or enzymes with lysozyme activity. Because it is difficult to ascertain and ensure that a food does not contain lysozyme activity, it would be prudent to assume the presence of this activity and to select for conditions for treatment on this basis. The model will help to define the safety of new products and formulations, encouraging the safe development of new minimally processed, ready-to-eat foods (mainly REPFEDs). It will still be judicious to do a small number of challenge tests to confirm the predictions for a new type of food and process, but they may now be carefully targeted with the help of the model. This is important, since challenge testing is expensive and time-consuming.
TABLE 4.
Comparison of the results of previous studies of the effect of heat treatment and subsequent incubation temperature on growth from spores of nonproteolytic C. botulinum, in the presence of lysozyme, with results predicted from the present model
| Heat treatment
|
Incubation temp (°C) | Lysozyme activity (U/ml)a | Time to growth (days)
|
Reference | ||
|---|---|---|---|---|---|---|
| °C | min | Observedb | Predicted | |||
| 80 | 20 | 5 | 1,200 | 53 | 68 | 21a |
| 8 | 1,200 | 24 | 38 | 21a | ||
| 12 | 1,200 | 16 | 20 | 21a | ||
| 16 | 1,200 | 6 | 12 | 21a | ||
| 80 | 23 | 6 | 625 | 40 | 56 | 27 |
| 8 | 625 | 23 | 39 | 27 | ||
| 10 | 625 | 19 | 28 | 27 | ||
| 12 | 625 | 12 | 20 | 27 | ||
| 25 | 625 | 3 | 5 | 27 | ||
| 90 | 19.8 | 8 | 2,400 | 68 | 67 | 26 |
| 12 | 2,400 | 31 | 36 | 26 | ||
| 16 | 2,400 | 24 | 21 | 26 | ||
| 25 | 2,400 | 9 | 10 | 26 | ||
| 90 | 20 | 8 | 1,200 | 87 | 67 | 13 |
| 12 | 1,200 | 49 | 36 | 13 | ||
| 16 | 1,200 | 29 | 21 | 13 | ||
Prior to heating.
When 106 spores of a mixture of strains of nonproteolytic C. botulinum were inoculated in 20 ml of meat medium containing lysozyme, given the indicated heat treatment, and subsequently incubated at the indicated temperature.
A number of guidelines and recommendations for the safe production of minimally processed foods have been made. The heat treatments included in guidelines produced by the ACMSF and the ECFF do not, on their own, give the recommended 6D reduction with respect to spores of nonproteolytic C. botulinum when lysozyme is present (Table 5). In the absence of lysozyme, some, but not all, of the heat treatments do give the recommended 6D reduction (Table 5). It has been proposed that the safety of these foods should rely on combinations of mild heat treatment and subsequent refrigerated storage that, when combined with a specified shelf life, provide a defined safety margin with respect to nonproteolytic C. botulinum (22). Such combinations can be identified when the results of this study are combined with those from a previous parallel study (10). For example, some, but not all, of the heat treatments recommended by the ACMSF and ECFF can be combined with storage at 8 or 12°C and a 42-day shelf life to give the required 6D process with respect to spores of nonproteolytic C. botulinum (Table 5). The models developed in our studies predict that the following heat treatments will prevent growth from 106 spores of nonproteolytic C. botulinum at 8°C within 42 days: 70°C for more than 2,500 min, 75°C for 520 min, 80°C for 75 min, 85°C for 25 min, and 90°C for 10 min. The heat treatment at 70°C is longer than those in current recommendations, while those at 80°C (9) and 85°C (1, 9) are shorter.
TABLE 5.
Assessment of the ability of recommended treatments for REPFEDs to give a 6D reduction in the probability of growth from spores of nonproteolytic C. botulinuma
| Organization (reference) | Recommended heat treatment
|
6D reduction by heat treatment alone with lysozyme:
|
6D process by heat treatment and subsequent storage at the following temp (°C) for 42 days:
|
|||||
|---|---|---|---|---|---|---|---|---|
| 8
|
12
|
|||||||
| °C | min | Absent | Presentb | Lysozyme absent | Lysozyme presentb | Lysozyme absent | Lysozyme presentb | |
| ACMSF (1) | 70 | 1,675 | No | No | No | No | No | No |
| 75 | 464 | No | No | Yes | Yes | No | No | |
| 80 | 129 | No | No | Yes | Yes | No | No | |
| 85 | 36 | Yes | No | Yes | Yes | Yes | No | |
| 90 | 10 | Yes | No | Yes | Yes | Yes | No | |
| ECFF (9) | 80 | 270 | No | No | Yes | Yes | Yes | Yes |
| 85 | 52 | Yes | No | Yes | Yes | Yes | No | |
| 90 | 10 | Yes | No | Yes | Yes | Yes | No | |
Results are from this study and reference 10.
Lysozyme was present at 2,400 U/ml prior to heating.
The results should be used in the context of a full HACCP assessment that minimizes factors such as microbial numbers in the raw material and contamination after processing. In particular, the results from this study are relevant to defining adequate heat treatment, storage conditions, and shelf life with respect to nonproteolytic C. botulinum for minimally processed foods that may contain lysozyme. This is an important step forward in the development of rational processes for the safe production of REPFEDs with respect to nonproteolytic C. botulinum. This study has not taken into account the hazard posed by proteolytic C. botulinum. If the storage temperature exceeds 10°C, then the hazard presented by growth and toxin production by proteolytic C. botulinum must also be considered.
ACKNOWLEDGMENTS
We are grateful to David Mason for his valuable help with the experimental work, to József Baranyi and Gary Barker for mathematical assistance, and to Barbara Lund for helpful comments on the manuscript.
P. S. Fernández acknowledges the Spanish Ministerio de Educacion y Ciencia for awarding him a fellowship. This work was partially funded by the Competitive Strategic Grant of the BBSRC.
REFERENCES
- 1.Advisory Committee on the Microbiological Safety of Food, United Kingdom. Report on vacuum packaging and associated processes. London, United Kingdom: Her Majesty’s Stationery Office; 1992. [Google Scholar]
- 2.Alderton G, Chen J K, Ito K A. Effect of lysozyme on the recovery of heated Clostridium botulinum spores. Appl Microbiol. 1974;27:613–615. doi: 10.1128/am.27.3.613-615.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker D A, Genigeorgis C. Predictive modeling. In: Hauschild A H W, Dodds K L, editors. Clostridium botulinum: ecology and control in foods. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 343–406. [Google Scholar]
- 4.Box G E P, Draper N R. Empirical model-building and response surfaces. New York, N.Y: John Wiley and Sons Inc.; 1987. pp. 34–103. [Google Scholar]
- 5.Dodds K L. An introduction to predictive microbiology and the development and use of probability models with Clostridium botulinum. J Ind Microbiol. 1993;12:139–143. [Google Scholar]
- 6.Egan H, Kirk R S, Sawyer R. Pearson’s chemical analysis of foods. 8th ed. London, United Kingdom: Churchill Livingstone; 1981. p. 404. [Google Scholar]
- 7.Eklund M W, Poysky F T, Wieler D I. Characteristics of Clostridium botulinum type F isolated from the Pacific coast of the United States. Appl Microbiol. 1967;15:1316–1323. doi: 10.1128/am.15.6.1316-1323.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eklund M W, Wieler D I, Poysky F T. Outgrowth and toxin production of nonproteolytic type B Clostridium botulinum at 3.3°C to 5.6°C. J Bacteriol. 1967;93:1461–1462. doi: 10.1128/jb.93.4.1461-1462.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Chilled Food Federation. Guidelines for the hygienic manufacture of chilled foods. London, United Kingdom: European Chilled Food Federation; 1996. [Google Scholar]
- 10.Fernández P S, Peck M W. Predictive model describing the effect of prolonged heating at 70 to 80°C and incubation at refrigeration temperatures on growth and toxigenesis by nonproteolytic Clostridium botulinum. J Food Prot. 1997;60:1064–1071. doi: 10.4315/0362-028X-60.9.1064. [DOI] [PubMed] [Google Scholar]
- 11.Gould G W. Heat-induced injury and inactivation. In: Gould G W, editor. Mechanisms of action of food preservation procedures. London, United Kingdom: Elsevier; 1989. pp. 11–42. [Google Scholar]
- 12.Graham A F, Mason D M, Peck M W. A kinetic model on the effect of temperature, pH and sodium chloride on growth from spores of nonproteolytic Clostridium botulinum. Int J Food Microbiol. 1996;31:69–85. doi: 10.1016/0168-1605(96)00965-8. [DOI] [PubMed] [Google Scholar]
- 13.Graham A F, Mason D M, Peck M W. Inhibitory effect of combinations of heat treatments, pH, and sodium chloride on growth from spores of nonproteolytic Clostridium botulinum at refrigeration temperatures. Appl Environ Microbiol. 1996;62:2664–2668. doi: 10.1128/aem.62.7.2664-2668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham A F, Mason D M, Maxwell F J, Peck M W. Effect of pH and NaCl on growth from spores of non-proteolytic Clostridium botulinum at chilled temperatures. Lett Appl Microbiol. 1997;24:95–100. doi: 10.1046/j.1472-765x.1997.00348.x. [DOI] [PubMed] [Google Scholar]
- 15.Hurley M A, Roscoe M E. Automated statistical analysis of microbial enumeration by dilution series. J Appl Bacteriol. 1983;55:159–164. [Google Scholar]
- 16.Juneja V K, Eblen B S. Influence of sodium chloride on thermal inactivation and recovery of nonproteolytic Clostridium botulinum type B strain KAP B5 spores. J Food Prot. 1995;58:813–816. doi: 10.4315/0362-028X-58.7.813. [DOI] [PubMed] [Google Scholar]
- 17.Juneja V K, Marmer B S, Phillips J G, Miller A J. Influence of the intrinsic properties of food on thermal inactivation of spoes of nonproteolytic Clostridium botulinum: development of a predictive model. J Food Safety. 1995;15:349–364. [Google Scholar]
- 18.Lund B M, Graham A F, George S M, Brown D. The combined effect of incubation temperature, pH and sorbic acid on the probability of growth of type B nonproteolytic Clostridium botulinum. J Appl Bacteriol. 1990;69:481–492. doi: 10.1111/j.1365-2672.1990.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 19.Lund B M, Notermans S H W. Potential hazards associated with REPFEDs. In: Hauschild A H W, Dodds K L, editors. Clostridium botulinum: ecology and control in foods. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 279–301. [Google Scholar]
- 20.Lund B M, Peck M W. Heat resistance and recovery of spores of nonproteolytic Clostridium botulinum in relation to refrigerated, processed foods with an extended shelf-life. J Appl Bacteriol Symp Suppl. 1994;76:115–128. doi: 10.1111/j.1365-2672.1994.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 21.Makki F, Durance T D. Thermal inactivation of lysozyme as influenced by pH, sucrose and sodium chloride and inactivation and preservative effect in beer. Food Res Int. 1996;29:635–645. [Google Scholar]
- 21a.Mason, D. R., G. A. Mitchell, and M. W. Peck. Unpublished data.
- 22.Peck M W. Clostridium botulinum and the safety of refrigerated processed foods of extended durability. Trends Food Sci Technol. 1997;8:186–192. [Google Scholar]
- 23.Peck M W, Fairbairn D A, Lund B M. Factors affecting growth from heat-treated spores of nonproteolytic Clostridium botulinum. Lett Appl Microbiol. 1992;15:152–155. doi: 10.1111/j.1472-765X.1992.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 24.Peck M W, Fairbairn D A, Lund B M. The effect of the recovery medium on the estimated heat-inactivation of spores of nonproteolytic Clostridium botulinum. Lett Appl Microbiol. 1992;15:146–151. doi: 10.1111/j.1472-765X.1992.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 25.Peck M W, Fairbairn D A, Lund B M. Heat-resistance of spores on nonproteolytic Clostridium botulinum estimated on medium containing lysozyme. Lett Appl Microbiol. 1993;16:126–131. [Google Scholar]
- 26.Peck M W, Fernandez P S. Effect of lysozyme concentration, heating at 90°C, and then incubation at chilled temperatures on growth from spores of nonproteolytic Clostridium botulinum. Lett Appl Microbiol. 1995;21:50–54. doi: 10.1111/j.1472-765x.1995.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 27.Peck M W, Lund B M, Fairbairn D A, Kaspersson A S, Undeland P C. Effect of heat treatment on survival of, and growth from, spores of nonproteolytic Clostridium botulinum at refrigeration temperatures. Appl Environ Microbiol. 1995;61:1780–1785. doi: 10.1128/aem.61.5.1780-1785.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peck M W, Stringer S C. Proceedings of Second European Symposium On Sous Vide. Leuven, Belgium: ALMA; 1996. Clostridium botulinum: mild preservation techniques; pp. 181–197. [Google Scholar]
- 29.Peterson M E, Pelroy G A, Poysky F T, Paranjpye R N, Dong F M, Pigott G M, Eklund M W. Heat pasteurization process for inactivation of nonproteolytic types of Clostridium botulinum in picked dungeness crabmeat. J Food Prot. 1997;60:928–934. doi: 10.4315/0362-028X-60.8.928. [DOI] [PubMed] [Google Scholar]
- 30.Potter M D, Meng J, Kimsey P. An ELISA for detection of botulinal toxin types A, B, and E in inoculated food samples. J Food Prot. 1993;56:856–861. doi: 10.4315/0362-028X-56.10.856. [DOI] [PubMed] [Google Scholar]
- 31.Proctor V A, Cunningham F E. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit Rev Food Sci Nutr. 1988;26:359–395. doi: 10.1080/10408398809527473. [DOI] [PubMed] [Google Scholar]
- 32.Rhodehamel E J. FDA’s concerns with sous vide processing. Food Technol. 1992;46(12):73–76. [Google Scholar]
- 33.Schmidt C F, Lechowich R V, Folinazzo J F. Growth and toxin production by type E Clostridium botulinum below 40°F. J Food Sci. 1961;26:626–630. [Google Scholar]
- 34.Scott V N, Bernard D T. The effect of lysozyme on the apparent thermal resistance of nonproteolytic type B Clostridium botulinum. J Food Safety. 1985;7:145–154. [Google Scholar]
- 35.Sebald M, Ionesco H. Germination 1zP-dépendante des spores de Clostridium botulinum type E. C R Acad S Sci Ser D. 1972;275:2175–2177. [PubMed] [Google Scholar]
- 36.Stringer S C, Haque N, Peck M W. Growth from spores of nonproteolytic Clostridium botulinum in heat-treated vegetable juice. Appl Environ Microbiol. 1999;65:2136–2142. doi: 10.1128/aem.65.5.2136-2142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stringer S C, Peck M W. Vegetable juice aids the recovery of heated spores of non-proteolytic Clostridium botulinum. Lett Appl Microbiol. 1996;23:407–411. [Google Scholar]
- 38.Whiting R C, Oriente J C. Time-to-turbidity model for non-proteolytic type B Clostridium botulinum. Int J Food Microbiol. 1997;35:49–60. doi: 10.1016/s0168-1605(96)01244-5. [DOI] [PubMed] [Google Scholar]