SUMMARY
Faecal specimens collected from 2101 patients with acute gastroenteritis from three cities (Pune, Alappuzha, Belgaum) in India during 1994–1995 and 2004–2010 were tested for group B rotavirus (RVB) by amplification of the NSP2 gene using RT–PCR. Seventy-five (3·6%) specimens were shown to contain RVB RNA. The positivity rate in Pune, Alappuzha and Belgaum was 4·1%, 7·3% and 4·1%, respectively, in the 2000s which was not significantly different from the detection rate in the 1990s in Pune (2·5%, P>0·05). RVB infections prevailed in adolescents and adults (62/1082, 5·7%) compared to children (13/1019, 1·3%, P<0·001) and were detected throughout the year. Phylogenetically, all strains clustered in an NSP2 lineage together with Indian-Bangladeshi RVB strains belonging to VP7 genotype G2. The study confirmed the occurrence of RVB infections in western India and reported for the first time circulation of RVB strains in southern India, suggesting that an increased awareness and monitoring for RVB infections is necessary in India.
Key words: Human group B rotavirus, Indian-Bangladeshi lineage, NSP2 gene, RT–PCR
INTRODUCTION
Viral gastroenteritis continues to be one of the most frequently encountered health problems in both developed and developing countries. Rotaviruses have been recognized as the major viral agent of acute gastroenteritis known to occur in individuals of all age groups [1, 2]. These viruses belong to the family Reoviridae and appear as icosahedral particles consisting of 11 segments of double-stranded RNA encoding six structural proteins (VP1–VP4, VP6, VP7) and 5–6 non-structural proteins (NSP1–NSP5/6) [3]. Rotaviruses are classified into eight groups, A–G and a putative novel group H on the basis of antigenicity of the inner capsid protein, VP6 and genomic characteristics [3–6]. Rotavirus strains from groups A–C and H have been detected in human infections.
Group B rotaviruses (RVBs) are known to infect humans and porcine, bovine, caprine and murine animal species [7]. Association of RVBs with human diarrhoeal disease was identified in a large epidemic of adult diarrhoea affecting nearly a million people in China during 1982–1983 [8]. In subsequent years, several smaller outbreaks and also sporadic infections of RVB have been reported from China [9, 10]. Outside China, RVB was first detected in five sporadic cases of severe diarrhoea occurring in Kolkata, India during 1997–1998 [11]. Since 2000, detection of human RVB has been reported in sporadic cases of acute gastroenteritis from Bangladesh [12–14]. Furthermore, a human RVB was also detected in Myanmar in 2007 [15].
RVB infections were detected at a significant level (18·5%) in children from Kolkata, eastern India during 2002–2004 [16]. In western India, RVB has been reported from Pune city in sporadic diarrhoeal infections and from Daman, Surat, Sangli and Mumbai cities in diarrhoeal outbreaks [17–20]. However, a long-term surveillance of RVB infections has not been documented in India. The present study was conducted to examine the incidence of RVB infections in the 2000s in children, adolescents and adults with acute gastroenteritis from Pune, western India and Belgaum and Alappuzha, southern India. The data obtained in the study were compared to those of the 1990s and analysed in order to determine the temporal variations in RVB infections.
MATERIALS AND METHODS
Specimens
The study included patients with acute gastroenteritis from Indian cities Pune (Maharashtra, western India), Alappuzha (Kerala, southern India) and Belgaum (Karnataka, southern India). A total of 2101 stool specimens comprising 1794 from Pune, 110 from Alappuzha and 197 from Belgaum were collected from children (0–10 years, n=1019), adolescents (11–18 years, n=135) and adults (>18 years, n=947) at the two time points, 1994–1995 (n=924) and 2004–2010 (n=1177). The specimens collected during 1994–1995 were available only from Pune. A case of acute gastroenteritis in the present study was defined as the passage of ⩾3 loose or watery stools a day, with or without associated symptoms such as vomiting, fever and abdominal pain. One specimen per patient was collected for the study within 24 h of hospitalization or immediately after the visit of the patient to the outpatient department with prior informed consent from the parents/guardians (in the case of children and adolescents) or adult patients. Epidemiological data including age, sex, date of diarrhoea onset and date of specimen collection were available for the majority of the patients. Thirty percent diluted faecal suspensions [liquid faeces (v/v), semi-solid faeces (w/v)] were prepared in 0·01 m phosphate-buffered saline (PBS) (pH 7·4) containing calcium chloride (0·01 mm). The suspensions were stored at −20 °C until tested by enzyme-linked immunosorbant assay (ELISA) and reverse transcription–polymerase chain reaction (RT–PCR).
ELISA for detection of group A rotavirus (RVA) antigen
All faecal specimens were subjected to RVA antigen capture ELISA according to a protocol described previously [21]. Specimens having optical density (OD) values above the cut-off value (2·0×OD of negative control wells) were considered positive for RVA antigen.
RNA extraction and RT–PCR for detection of RVB RNA
Viral RNA was extracted from faecal specimens using TRIzol® LS reagent (Invitrogen, USA) according to the manufacturer's instructions. RT–PCR based on NSP2 gene-specific primers was performed using the one-step RT–PCR kit (Qiagen, Germany) according to the manufacturer's instructions. Briefly, PCR was performed at an initial denaturation at 94 °C for 5 min followed by 35 cycles of amplification (94 °C for 1 min, 55 °C for 30 s, 72 °C for 1 min) and a final extension at 72 °C for 10 min. In the first step, a 321-bp amplicon was generated using primers NSP2-AF (GCCATCAGACAGAGAATGTGTTGCA, primer positions 112–136) and NSP2-CR (TTGTCTGCCGAAGCTAAAACATCC, primer positions 432–409). This product was further used as a template for semi-nested PCR (229 bp) using primers NSP2-AF and NSP2-BR (CCAATCAGTCACAAGAGTCCATAGT, primer positions 340–316). All final PCR products were analysed on ethidium bromide-stained agarose gels (2%), visualized under UV light, excised from the gel and purified using the QIAquick gel extraction kit (Qiagen).
Nucleotide sequencing and phylogenetic analysis
All PCR products, 229 bp in size were sequenced using the ABI PRISM Big Dye Terminator cycle sequencing ready reaction kit (Applied Biosystems, USA) on an automated DNA sequencer (ABI PRISM 3100 Genetic analyser, Applied Biosystems). Nucleotide sequences of partial NSP2 gene were aligned with the sequences of reference strains available in GenBank using CLUSTAL W [22]. The phylogenetic analyses were conducted in the MEGA version 5 software package using p-distance and the neighbour-joining algorithm [23]. The reliability of different phylogenetic groupings was confirmed using the bootstrap test (1000 bootstrap replications) available in MEGA 5.
Statistical analysis
The proportions across two different periods as well as two different age groups were compared using the χ2 test with Yates's correction and P values <0·05 were considered statistically significant.
Accession numbers
Sixty-five of 75 NSP2 gene sequences of RVB strains derived in this study have been deposited in GenBank under the accession numbers JQ686121–JQ686185. Sequences of the remaining 10 strains containing <200 bp could not be submitted to GenBank.
RESULTS
RVB positivity rates
From a total of 2101 faecal specimens collected from patients with acute gastroenteritis, 75 (3·6%) were shown to contain RVB RNA.
The rate of positivity in Pune (2004–2010), Alappuzha (2009) and Belgaum (2008–2009) cities was found to be 4·1% (36/870), 7·3% (8/110) and 4·1% (8/197), respectively. Although the positivity rate appeared lower (2·5%, 23/924) during 1994–1995 compared to the rate in 2004–2010 in Pune, the difference was not significant (P>0·05).
Age distribution of RVB-infected patients
RVB positivity was significantly higher in adolescents/adults (20/538, 3·7% in the 1990s; 36/413, 8·7% in the 2000s) compared to that of children (3/386, 0·8% in the 1990s; 0/457, 0·0% in the 2000s) (P<0·005 for each comparison) in specimens analysed from Pune city. However, it was not significantly different in children and adolescents/adults from Alappuzha (3/32, 9·4% vs. 5/78, 6·4%) and Belgaum (7/144, 4·9% vs. 1/53, 1·9%). Overall, RVB infections were detected at a higher prevalence in adolescents and adults (62/1082, 5·7%) compared to those from children (13/1019, 1·3%) (P<0·001). In the group of children aged between 0 and 10 years the prevalence of RVB infection was highest in children aged ⩽2 years (8/13, 61·5%). However, the rate of RVB positivity in adolescents and in different age groups (>18–29, 30–39, 40–49, 50–59, ⩾60 years) of adults was not different (P>0·05).
Seasonality
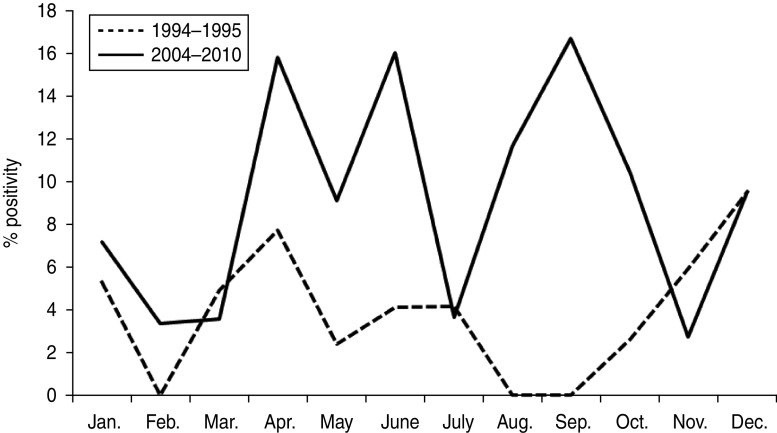
Monthly distribution of RVB infections identified in Pune in 1994–1995 and 2004–2010 is depicted in Figure 1. RVB-positive cases were detected throughout the year and no seasonal pattern of infection was observed as is known for RVA. However, peak activity was found in April and December during 1994–1995 and in April, June and September during 2004–2010.
Fig. 1.
Monthly distribution of group B rotavirus positivity in adolescent/adult patients with acute gastroenteritis from Pune, western India.
Mixed infection
Mixed infections of RVA and RVB were identified in 15/59 (25·4%), 1/8 (12·5%) and 2/8 (25%) specimens from Pune, Alappuzha and Belgaum, respectively. Adults from different age groups (n=4 for >18–29 years, and n=3 for each of the 30–39, 40–49 and ⩾60 years age groups) showed the highest number (13/18, 72·2%) of mixed infections. Adolescents and children also showed presence of mixed infections, but at lower levels (2/18, 11·1%; 3/18, 16·7%).
Sequencing of NSP2 genes and phylogenetic analysis of RVB strains
The presence of RVB RNA in 75 faecal specimens was confirmed by sequencing of the partial NSP2 gene (229 bp). Nucleotide sequence identity in the 75 Indian RVB strains was 93·9–100%. Phylogenetically, all strains clustered with other RVB strains in an NSP2 lineage containing Indian-Bangladeshi RVB strains belonging to VP7 genotype G2 (Fig. 2). The RVB sequences obtained from the different cities in India were indistinguishable and showed 93·1–100% and 92·6–95·1% nucleotide identity with their counterparts in other strains of Indian-Bangladeshi and Chinese lineages, respectively.
Fig. 2.
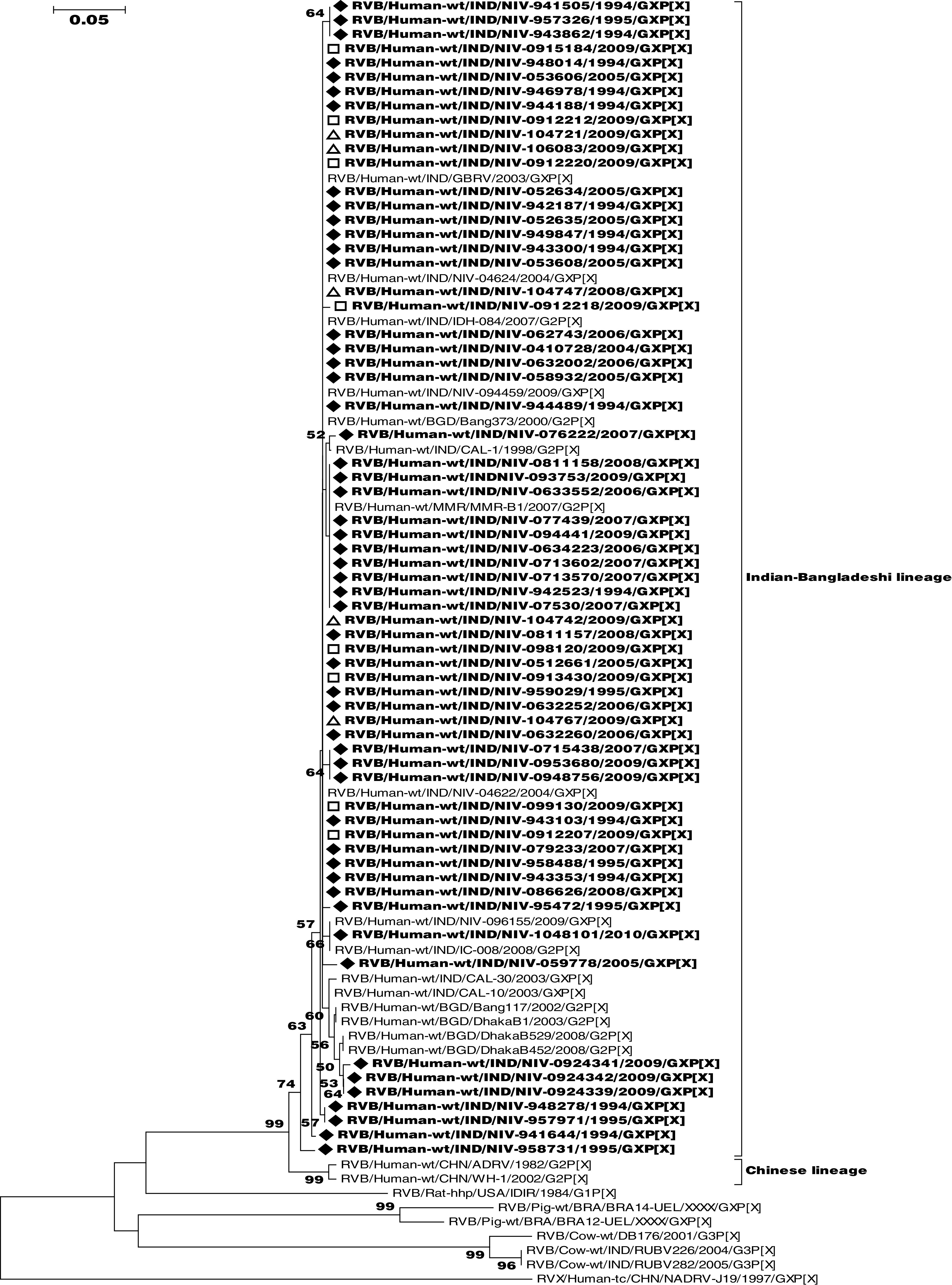
Phylogenetic dendrogram of partial group B rotavirus (RVB) NSP2 gene (137–340 bp). The strains of the present study (n=65) are indicated by the symbols: ◆ (strains from Pune city), □ (strains from Alappuzha city) and ▵ (strains from Belgaum city). The scale represents genetic distance. RVB strain names are according to the guidelines of the Rotavirus Classification Working Group [34].
DISCUSSION
Identification of non-RVA strains is known to be based on the characteristic electrophoretic migration patterns of their genomes or by electron microscopy of rotaviruses not reactive in common enzyme immunoassays for RVAs [24]. Surveillance conducted to identify non-RVA strains in the 1980s made use of electropherotyping, electron microscopy, immune electron microscopy and ELISA [8, 9, 25, 26]. In the 1990s, highly sensitive RT–PCR assays were utilized for detection of RVB and group C rotavirus (RVC) strains in faecal specimens from human and animal species [24, 27]. Using electropherotyping aided by RT–PCR, limited studies conducted in Pune, western India and Kolkata, eastern India reported variable (0·8–18·5%) frequencies of RVB infection in patients with acute gastroenteritis [16, 17]. Recently, different rates (0·5–26·2%) of RVB and RVC infections have been reported for humans or pigs from Ireland, South Korea, Myanmar and Bangladesh [14, 15, 28–30]. The present study documents similar findings (2·5–7·3%) on RVB infections in three different cities located in three different states of India.
RVB infections were detected in both genders and in all age groups. However, the rate of infection was higher in children aged ⩽2 years, compared to older children (aged between 2 and 10 years), similar to that reported earlier for RVA and RVB infections [16, 31]. It is interesting to note that RVB infection in Pune, western India was confined only to adolescent and adult cases of acute gastroenteritis during the seven consecutive years, 2004–2010 although a small proportion of the paediatric population (0·9%) was found affected by this virus in the 1990s. This may indicate a low exposure of children to RVB in the recent past or low shedding of RVB in faecal specimens, below the detection limit of the assay employed in the study [32]. Although our study has limitations because the analysis was restricted to a small number of specimens and/or a short period of collection, especially from Alappuzha (2009) and Belgaum (2008–2009), it revealed similar rates of RVB infections (P>0·05) in adolescents and different age groups of adults.
Mixed infections of RVA and RVB strains have been reported in humans and animals [16, 33]. In the present study, a significant proportion (24%) of RVB-infected patients was found to be co-infected with RVA as well. Interestingly, patients infected with RVB or with both, RVB and RVA strains only experienced mild to moderate gastroenteritis.
With respect to seasonality, the results of this study are in agreement with previous findings showing the absence of a seasonal pattern for RVB infections [16]. In Bangladesh, incidence of RVB was found highest between August and October during 2003 [13] and in March and May during 2008 [14]. In the present study, more RVB infections were noted in April and December in 1994–1995 and April, June and September during 2004–2010 in Pune compared to other months (Fig. 1).
The partial nucleotide sequences of the NSP2 gene of all 75 RVB strains of the present study clustered together (Fig. 2) and displayed a high nucleotide sequence similarity not only among themselves but also with other strains from eastern India, Bangladesh and Myanmar, thus revealing a single lineage of RVB circulating in the human population.
To summarize, this study has confirmed that human RVB strains are consistently causing infections in western India at low to moderate levels, and also documented for the first time the circulation of RVB strains in southern India. To our knowledge, this is the first description of a long-term surveillance of RVB infections from India. Similar studies would be useful for a better understanding of the disease burden caused by RVB infections.
ACKNOWLEDGEMENTS
The authors thank the Indian Council of Medical Research (ICMR), New Delhi for supporting the first author (A.L.) with a Junior Research Fellowship and Dr A.C. Mishra, Director, National Institute of Virology, Pune for his constant support during this study.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Parashar UD, et al. Rotavirus and severe childhood diarrhea. Emerging Infectious Diseases 2006; 12: 304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tatte VS, Gentsch JR, Chitambar SD. Characterization of group A rotavirus infections in adolescents and adults from Pune, India: 1993–1996 and 2004–2007. Journal of Medical Virology 2010; 82: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapikian AZ, Hoshino Y, Chanock RM. Rotaviruses. In: Knipe DM, et al. , eds. Fields Virology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, PA, 2001, pp. 1787–1834. [Google Scholar]
- 4.Jiang S, et al. Molecular characterization of a novel adult diarrhoea rotavirus strain J19 isolated in China and its significance for the evolution and origin of group B rotaviruses. Journal of General Virology 2008; 89: 2622–2629. [DOI] [PubMed] [Google Scholar]
- 5.Nagashima S, et al. Whole genomic characterization of a human rotavirus strain B219 belonging to a novel group of the genus Rotavirus. Journal of Medical Virology 2008; 80: 2023–2033. [DOI] [PubMed] [Google Scholar]
- 6.Matthijnssens J, et al. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Archives of Virology 2012; 157: 1177–1182. [DOI] [PubMed] [Google Scholar]
- 7.Estes MK, Kapikian AZ. Rotavirus. In: Knipe DM, et al. , eds. Fields Virology, 5th edn, vol. 2. Philadelphia, PA: Lippincott-Raven, 2007, pp. 1917–1974. [Google Scholar]
- 8.Hung T, et al. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by novel rotavirus. Lancet 1984; 1: 1139–1142. [PubMed] [Google Scholar]
- 9.Fang Z-Y, et al. Investigation of an outbreak of adult diarrhea rotavirus in China. Journal of Infectious Diseases 1989; 160: 948–953. [DOI] [PubMed] [Google Scholar]
- 10.Yang JH, et al. Phylogenetic analysis of a human group B rotavirus WH-1 detected in China in 2002. Journal of Medical Virology 2004; 74: 662–667. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan T, et al. Emergence of adult diarrhoea rotavirus in Calcutta, India. Lancet 1999; 353: 380–381. [DOI] [PubMed] [Google Scholar]
- 12.Sanekata T, et al. Human group B rotavirus infections cause severe diarrhea in children and adults in Bangladesh. Journal of Clinical Microbiology 2003; 41: 2187–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman M, et al. Sequence analysis and evolution of group B rotaviruses. Virus Research 2007; 125: 219–225. [DOI] [PubMed] [Google Scholar]
- 14.Saiada F, et al. Clinical presentation and molecular characterization of group B rotaviruses in diarrhea patients in Bangladesh. Journal of Medical Microbiology 2011; 60: 529–536. [DOI] [PubMed] [Google Scholar]
- 15.Aung TS, et al. Detecton of group B rotavirus in an adult with acute gastroenteritis in Yangon, Myanmar. Journal of Medical Virology 2009; 81: 1968–1974. [DOI] [PubMed] [Google Scholar]
- 16.Barman P, et al. RT-PCR based diagnosis revealed importance of human group B rotavirus infection in childhood diarrhoea. Journal of Clinical Microbiology 2006; 36: 222–227. [DOI] [PubMed] [Google Scholar]
- 17.Kelkar SD, Zade JK. Group B rotaviruses similar to strain CAL-1 have been circulating in Western India since 1993. Epidemiology and Infection 2004; 132: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelkar SD, et al. Outbreak of diarrhoea in Daman and detection of group B rotavirus from three adult cases. National Medical Journal of India 2007; 20: 41. [PubMed] [Google Scholar]
- 19.Chitambar SD, et al. Occurrence of group B rotavirus infections in the outbreaks of acute gastroenteritis from western India. Indian Journal of Medical Research 2011; 134: 399–400. [PMC free article] [PubMed] [Google Scholar]
- 20.Chitambar SD, et al. Diversity in the enteric viruses detected in outbreaks of gastroenteritis from Mumbai, western India. International Journal of Environmental Research and Public Health 2012; 9: 895–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelkar SD, et al. Rapid ELISA for the diagnosis of rotavirus. Indian Journal of Medical Research 2004; 119: 60–65. [PubMed] [Google Scholar]
- 22.Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequencing weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Research 1994; 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 2011; 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouvea V, et al. Detection of group B and C rotaviruses by polymerase chain reaction. Journal of Clinical Microbiology 1991; 29: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodger SM, Bishop RF, Holmes IH. Detection of a rotavirus-like agent associated with diarrhea in an infant. Journal of Clinical Microbiology 1982; 16: 724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai G-Z, et al. First report of an epidemic of diarrhoea in human neonates involving the new rotavirus and biological characteristics of the epidemic virus strain (KMB/R85). Journal of Medical Virology 1987; 22: 365–373. [DOI] [PubMed] [Google Scholar]
- 27.Chang KO, et al. Detection of group B rotaviruses in fecal samples from diarrheic calves and adult cows and characterization of their VP7 genes. Journal of Clinical Microbiology 1997; 35: 2107–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M, et al. Detection and characterization of human group C rotaviruses in Bangladesh. Journal of Clinical Microbiology 2005; 43: 4460–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins PJ, Martella V, O'Shea H. Detection and characterization of group C rotaviruses in asymptomatic piglets in Ireland. Journal of Clinical Microbiology 2008; 46: 2973–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong YJ, et al. Detection and molecular characterization of porcine group C rotaviruses in South Korea. Veterinary Microbiology 2009; 138: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahl R, et al. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. Journal of Infectious Diseases 2005; 192: S114–S118. [DOI] [PubMed] [Google Scholar]
- 32.Saif LJ, Jiang B. Non group A rotaviruses of humans and animals. In: Ramig RF, ed. Rotaviruses. New York: Springer-Verlag, 1994, pp. 339–371. [DOI] [PubMed] [Google Scholar]
- 33.Medici KC, et al. Porcine rotavirus groups A, B, and C identified by polymerase chain reaction in a faecal sample collection with inconclusive results by polyacrylamide gel electrophoresis. Journal of Swine Health and Production 2011; 19: 146–150. [Google Scholar]
- 34.Matthijnssens J, et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Archives of Virology 2011; 156: 1397–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]