SUMMARY
In Vietnam, highly pathogenic avian influenza (HPAI) H5N1 infections in poultry often occur without concomitant clinical signs and outbreaks are not consistently reported. Live bird markets represent a convenient site for surveillance that does not rely on farmers' notifications. Two H5N1 surveys were conducted at live bird markets/slaughter points in 39 districts (five provinces) in the Red River, Mekong delta, and central Vietnam during January and May 2011. Oropharyngeal and rectal swab samples from 12 480 ducks were tested for H5N1 by reverse transcription–polymerase chain reaction in pools of five. Traders and stallholders were interviewed using standardized questionnaires; 3·3% of pools tested positive. The highest prevalence (6·6%) corresponded to the Mekong delta, and no H5N1 was detected in the two Red River provinces. The surveys identified key risk behaviours of traders and stallholders. It is recommended that market surveys are implemented over time as a tool to evaluate progress in HPAI control in Vietnam.
Key words: Avian influenza, epidemiology, public health emerging infections
INTRODUCTION
Highly pathogenic avian influenza (HPAI) caused by H5N1 has been of great concern in Vietnam since the winter of 2003/2004 when a high number of poultry outbreaks, followed shortly by a high number of human cases, were reported for the first time in the country [1]. In late 2005 the introduction of a mass vaccination programme in poultry flocks was associated with a considerable reduction in both poultry outbreaks and human cases in Vietnam [2]. However, H5N1 outbreaks in poultry continued to be reported in subsequent years, although in considerably lower numbers [2, 3]. Outbreaks of disease are more common before or around the Tet festival, coinciding with a build-up of poultry production [4], although seasonality has been less marked in recent years.
Detection of H5N1 in poultry in Vietnam is largely based on ‘passive’ reporting of clinical outbreaks by farmers to the Government's public veterinary system. Surveillance systems for avian influenza (as for many other livestock diseases) are recognized as having limited sensitivity, even in industrialized countries [5]. In Vietnam incentives for reporting poultry outbreaks often do not outweigh the potential negative consequences for the producer and other stakeholders that result from a confirmed H5N1 report, i.e. mass culling, trade restrictions, insufficient monetary compensation, etc. Furthermore, H5N1 infection in poultry does not always result in clinical disease: published studies have shown H5/H5N1 in unvaccinated healthy looking ducks sampled at farms [6, 7] and markets [8] in Vietnam. Results from annual Government-sponsored virus circulation surveys have consistently shown carriage of H5/H5N1 in ducks (and to a lesser extent in layer chickens). Experimental challenge studies have shown variability in clinical presentation and mortality due to H5N1 in ducks, with older ducks generally showing less obvious clinical and pathological signs than young ducks [9, 10]. This is likely to result in episodes of infection not leading to overt signs of disease. In Vietnam mass vaccination against H5N1 of poultry in general, and ducks in particular, has been used as an important measure to control infection. It is suspected that imperfect vaccination of ducks (e.g. application of only one dose early in life, absence of a booster dose after 6 months, etc.) may further compromise outbreak detection in this species [11]. Because of these two factors (subclinical infection and under-reporting of outbreaks), trends of H5N1 infection based on analysis of passive surveillance data only are not able to provide a reliable estimate of any progress in the H5N1 control programme in Vietnam. During 2009 and 2010 only 54 and 46 poultry outbreaks, respectively, were officially reported, but the number of infectious events is believed to be of a much greater order of magnitude. An alternative to passive surveillance would be to conduct ‘active’ surveillance by sampling live poultry from farms and markets/slaughter points. Market surveys are likely to be particularly appropriate in the context of Vietnam, where mass vaccination of ducks against H5N1 has been an important control measure. Sampling poultry from markets/slaughter points is thought to be more efficient, since these sites represent a convenient assembly point of birds from potentially many sources (farms). Live bird markets in Vietnam are ubiquitous and those catering for rural areas (i.e. the majority of the population) tend to be small scale, mostly trading in poultry from farms close to the areas where the market is located. This provides a potential opportunity for tracing back to the farm/areas where the infected poultry originate from.
This study reports the results of two surveys of HPAI H5N1 virus in ducks sampled from live bird markets at two time points (January and May 2011) in five provinces, including provinces in both in the Red River and Mekong River deltas, considered to be ‘high-risk’ zones. The specific aims of the surveys were to (1) detect H5N1 virus in ducks (Anas platyrhynchos domesticus) from live bird markets in order to identify districts where HPAI is circulating; (2) to investigate any variation in the presence of HPAI in the districts over the two sampling points at two different times of the year; and (3) to improve understanding of the poultry market chain and risk behaviours of both traders and stallholders.
MATERIALS AND METHODS
Study area and study period
The study was conducted in five provinces representative of the main geographical areas of Vietnam: the Red River delta (two provinces: Nam Dinh and Ninh Binh), Mekong delta (two provinces: Soc Trang and Hau Giang) and the central region (one province: Quang Binh) over two time periods (January and May 2011). The Red River and Mekong deltas are considered to be high-risk zones since they have the highest density of both poultry and humans, as well as a history of a large number of outbreaks reported. Consequently these areas have been subjected to intensive vaccination programmes in all districts, whereas vaccination in Quang Binh is only practised in four of its seven districts. All 42 districts in the five study provinces were eligible for the study. The sampling frame (i.e. list of markets per district) was obtained from a previous study conducted in July 2010 where all live bird markets in the five provinces were geo-referenced and their basic characteristics (daily number of chickens, ducks and Muscovy ducks traded) were gathered. From each district the two largest markets trading the largest number of live ducks for human consumption were selected.
Statistical considerations
Two identical surveys were performed, one in January representing the high-risk ‘pre-Tet’ season and the other in May, representing the low-risk ‘early summer’ season. For each survey the required sample size was calculated with the aim of having sufficient power to detect the presence of H5N1 virus in ducks from a given district. The target population were ‘all market ducks sold in large markets from the districts over one week’ in January and May. It was assumed that market ducks in districts were clustered in ‘market days’. A sample size to detect disease was estimated based on two levels of prevalence: ‘between-’ and ‘within-’ cluster prevalence [12]. Between-cluster prevalence was estimated as 0·33 based on a survey conducted in other Vietnamese provinces in 2009/2010, based on the finding that on average, H5N1 was detected in birds in 33% of market days) The within-cluster prevalence (prevalence of infected ducks in H5N1-positive markets) was estimated as 10% based on the analysis of positive pools from that study (Dr J. Weaver, unpublished data). Both sensitivity and specificity of the reverse transcription–polymerase chain reaction (RT–PCR) test were assumed to be 100%.
For each survey the number of ducks required from each district was 160 (80 from each of the two selected markets). This was achieved, where possible, by randomly sampling 20 ducks on each of four visits to each market. Visits were carried out on consecutive days where possible. In districts with only one eligible market, sampling from slaughter points identified by the District Veterinary Station was also allowed.
Live ducks were sampled as traders arrived at the market using systematic random sampling, following the sequence of their arrival into the market so that all traders could be interviewed. Because of this, the total number of ducks traded on a given day needed to be estimated a priori. This estimate was obtained after consultation with the market manager, and the given estimate was then slightly reduced to guarantee representative sampling from most of the ducks brought to the market on that day. If, for example, 50 ducks were expected to arrive at the market on a given day, an estimate of 75–80% of the figure (i.e. 40 ducks) was used and thus every other duck arriving at the market (40/20 = 2) was sampled, allowing us to obtain the 20 ducks required. When ducks arrived in groups, a system of sorting the birds was developed based on their position in the vehicle.
Sample collection and laboratory methods
From each duck a cloacal and an oropharyngeal swab were taken to collect faecal material and bronchial/oral secretions, respectively. The two swabs were placed into the same tube containing 1 ml of virus transport medium. Pooling of cloacal and oropharyngeal swabs was done with the aim of increasing sensitivity of detection, since shedding by the oral and faecal routes may differ in ducks [13]. Swab samples were transported to the laboratory under refrigerated conditions (4 °C). In the laboratory, each tube with the material collected from each individual duck was immediately vortexed and the eluates were kept refrigerated and were processed within 1 week of collection. Ducks were tested in pools of five, so swab eluates from five ducks were pooled. RNA was extracted using Qiagen RNeasy Mini kit (Qiagen, USA) according to the manufacturer's instructions, and tested for H5N1 using Taqman real-time RT–PCR as described previously for H4 [14] and N1 [15] with modifications for the H5 forward primer (5′-AAACAGAGAGGAAATAAGTGGAGTAAAATT-3′) and for the H5 probe (5′-HEX-TCAACAGTNGCGAGTTCYCTAGCA-BHQ1-3′).
Data collection and analysis
Swabs were individually identified so that test results could be linked to specific ducks and traders. All stallholders and poultry traders were interviewed using questionnaire forms. Estimation of individual duck prevalence data from pooled test results was performed using a frequentist approach [16]. Data collected using questionnaire forms (the questionnaire is available upon request) from traders and stallholders as well as laboratory results from the January survey were entered into a database built in MS Office Access 2003 (Microsoft, USA).
Questionnaires were collected from all poultry stallholders and from traders bringing ducks to the market; this information was linked to the laboratory testing results from swab pools collected/tested from the ducks. The data were analysed to investigate risk factors associated with the probability of a pool testing positive for H5N1 using multivariable logistic regression modelling. The variables investigated were: (1) the trader sells ducks directly to the public (i.e. the trader is also a stallholder) (yes/no); (2) species traded by the trader (ducks only/ducks and chickens); (3) number of farms from which the ducks brought by the trader on that day come from (1/ > 1); (4) age of the ducks (in months) (quintiles); (5) trader enters farms with vehicle/baskets/crates; (6) ducks originate from outside the district. The variable ‘number of ducks and chickens and ducks in the market on that day (log transformed)’ was forced into the model to investigate potential transmission within the market (because if ongoing transmission occurred in the markets, it would be expected that markets with the larger number of birds would pose the greater risk). The association between each of these variables and the outcome were first investigated in univariable analysis. Variables were candidates for multivariable analysis if the P value in the univariable analysis was <0·15, and were ranked by their degree of significance. Candidate variables were included one by one in the multivariable logistic regression, starting with the most significant variables using a step-wise forward approach. In the multivariable model, variables were kept if their P value was <0·1. The significance of each new variable included in the model was assessed using the Wald test [17]. All interactions between the variables in the final model were tested. Individual H5N1 prevalence was inferred from pooled test results based on Cowling and co-workers (method 2) [16] assuming 100% sensitivity and specificity (i.e. no loss of power of detection due to pooling). All statistical analyses were performed using R 2.13.1 (R Foundation for Statistical Computing, 2011) except the estimation of prevalence based on pooled data which were calculated using EpiTools [18].
RESULTS
Districts, markets and visits
A total of 39 districts were surveyed. Three districts (Tuyen Hoa and Minh Hoa, in Quang Binh) and Chau Thanh (Soc Trang) were excluded from the study because they either had no live duck markets or had very little duck trade during the period of the visits. Overall, ducks from a total of 78 sites (74 markets and four slaughter points) were sampled in January 2011, and from 76 sites (72 markets and four slaughter points) in May 2011. A total of 344 market visits were undertaken in the January survey and 327 in the May survey. The number of markets sampled by province was: 20 in Nam Dinh (10 districts), 16 in Ninh Binh (eight districts), 12 in Hau Giang (seven districts), 16/14 (January/May) in Soc Trang (nine districts), and 10 in Quang Binh (five districts). In addition three slaughter points were sampled in Hau Giang and one in Soc Trang.
Pool and market H5N1 RT-PCR prevalence
A total of 12 480 ducks were sampled and tested for H5N1 using RT–PCR in 2496 pools (i.e. 6240 ducks and 1248 pools per survey). The January survey resulted in a total of 56/1248 (4·5%) H5N1 RT–PCR positive pools, and the May survey in 26/1248 (2·1%) (χ2 = 10·6, d.f. = 1, P = 0·001). A total of 22/78 (28·2%) and 9/75 (12·0%) markets tested positive for H5N1 at least on one visit in the first and second surveys, respectively. All pools from ducks in Nam Dinh and Ninh Binh (Red River delta provinces) tested negative in both surveys. Results for the Mekong delta provinces were consistently higher than for the other provinces, and were lower in the second survey: the pool prevalence in Soc Trang was 23/288 (8·0%) and in Hau Giang 23/224 (10·3%) in the first survey, decreasing to 11/288 (3·8%) (P = 0·051) and 11/224 (4·9%) in the second survey, respectively (P = 0·051). In these two provinces a combined total of 19/32 (59%) markets tested positive in the first survey, and 8/29 (27%) in the second survey. In the central low-risk province of Quang Binh the pool prevalence during the first survey was 1·2% and decreased to 0·5% in the second survey (P = 0·120). Of the 10 markets investigated in this province, three (30%) tested positive in the first survey and one (10%) in the second survey (Table 1). In Quang Binh the ducks that tested positive in the first survey were detected in two districts (Bo Trach and Quang Trach), that had never previously reported a H5N1 outbreak.
Table 1.
H5N1 RT–PCR test results from two live market surveys in 39 districts of five provinces in Vietnam (January and May 2011)
| Province | District | No. pools (per survey) | No. ducks (per survey) | First survey (January 2011) | Second survey (May 2011) | ||
|---|---|---|---|---|---|---|---|
| No. pos. sampling sites/total | No. pos. pools | No. pos. sampling sites/total | No. pos. pools | ||||
| Nam Dinh | Giao Thuy | 32 | 160 | 0/2 | 0 | 0/2 | 0 |
| Hai Hau | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| My Loc | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Nam Dinh city | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Nam Truc | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Nghia Hung | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Truc Ninh | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Vu Ban | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Xuan Truong | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Ý Yên | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Total Nam Dinh | 320 | 1600 | 0/20 | 0 | 0/20 | 0 | |
| Ninh Binh | Gia Vien | 32 | 160 | 0/2 | 0 | 0/2 | 0 |
| Hoa Lu | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Kim Son | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Nho Quan | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Ninh Binh city | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Tam Diep | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Yen Khanh | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Yen Mo | 32 | 160 | 0/2 | 0 | 0/2 | 0 | |
| Total Ninh Binh | 256 | 1280 | 0/16 | 0 | 0/16 | 0 | |
| Quang Binh | Bố Trạch | 32 | 160 | 1/2 | 4 | 0/2 | 0 |
| ðồng Hới | 32 | 160 | 0/2 | 0 | 1/2 | 4 | |
Lệ Thu
|
32 | 160 | 0/2 | 0 | 0/2 | 0 | |
Qu ng Ninh ng Ninh |
32 | 160 | 0/2 | 0 | 0/2 | 0 | |
Qu ng Trạch ng Trạch |
32 | 160 | 2/2 | 6 | 0/2 | 0 | |
| Total Quang Binh | 160 | 800 | 3/10 | 10 | 1/10 | 4 | |
| Soc Trang | Cù Lao Dung | 32 | 160 | 0/3 | 0 | 0/1 | 0 |
| Kế Sách | 32 | 160 | 2/2 | 5 | 0/2 | 0 | |
| Long Phú | 32 | 160 | 1/1 | 2 | 0/1 | 0 | |
| Mỹ Tú | 32 | 160 | 2/2 | 4 | 0/2 | 0 | |
| Mỹ Xuyên | 32 | 160 | 1/2 | 1 | 0/2 | 0 | |
| Ngã Năm | 32 | 158 | 1/2 | 6 | 1/2 | 9 | |
| Thạnh Trị | 32 | 160 | 1/2 | 4 | 0/2 | 0 | |
| TP.Sóc Trăng | 32 | 160 | 1/1 | 1 | 1/1 | 1 | |
| Vĩnh Châu | 32 | 160 | 0/2 | 0 | 1/2 | 1 | |
| Total Soc Trang | 288 | 1438 | 9/17 | 23 | 3/15 | 11 | |
| Hau Giang | Châu Thành | 32 | 160 | 1/1 | 3 | 0/1 | 0 |
| Châu Thành A | 32 | 160 | 1/2 | 1 | 2/2 | 5 | |
| Long Mỹ | 32 | 160 | 2/4 | 5 | 0/4 | 0 | |
| Phụng Hiệp | 32 | 160 | 2/2 | 4 | 2/2 | 3 | |
| TP. Vị Thanh | 32 | 160 | 1/2 | 3 | 1/2 | 3 | |
TX. Ngã B y y |
32 | 160 | 2/2 | 4 | 0/2 | 0 | |
| Vị Thưy | 32 | 160 | 1/2 | 3 | 0/2 | 0 | |
| Total Hau Giang | 224 | 1120 | 10/15 | 23 | 5/15 | 11 | |
| All | 1248 | 6240 | 22/78 | 56 | 9/76 | 26 | |
Individual duck-level H5N1 RT–PCR prevalence
Overall, 82/2496 (3·3%) pools tested positive by RT–PCR, equivalent to an overall duck-level prevalence of 0·67% (with 0·53 as a 2·5% confidence limit, and 0·83 as a 97·5% confidence limit). The estimated duck-level prevalence in the first and second surveys was 0·91% [95% confidence interval (CI) 0·69–1·19] and 0·42% (95% CI 0·27–0·62), respectively. These differences were statistically significant (χ2 = 31·5, P < 0·001).
The combined estimated H5N1 duck-level prevalence (i.e. both surveys) for the Mekong delta provinces (Soc Trang, Hau Giang) was 1·36% and for Quang Binh was 0·89%. Results for the second survey were 0·88% (Mekong delta) and 0·51% (Quang Binh), compared to 1·87% and 1·28% in the first survey in the Mekong delta and Quang Binh, respectively. No ducks tested positive in the Red River delta provinces in either of the surveys (Table 2).
Table 2.
Estimations of duck-level H5N1 prevalence from pool test results for the Mekong delta provinces and Quang Binh, assuming 100% test sensitivity and specificity
| Mekong delta | Quang Binh | |
|---|---|---|
| January 2011 survey | ||
| No. positive pools/total (%) | 46/512 (9·0%) | 10/160 (6·2%) |
| Estimated duck-level prevalence, % (95% CI) | 1·87% (1·37–2·48) | 1·28% (0·20–2·35) |
| May 2011 survey | ||
| No. positive pools/total (%) | 22/512 (4·3%) | 4/160 (2·5%) |
| Estimated duck-level prevalence, % (95% CI) | 0·88% (0·55–1·32) | 0·51% (0·14–1·29) |
| Both surveys combined | ||
| No. positive pools/total (%) | 68/1024 (6·6%) | 14/320 (4·4%) |
| Estimated duck-level prevalence, % (95% CI) | 1·36% (1·06–1·73) | 0·89% (0·49–1·49) |
CI, Confidence interval.
Age and probability of H5N1 infection
The association between the age of a duck and the probability of a H5N1-positive result was investigated. Data on age of ducks collected in the questionnaires during the January 2011 survey and pools tested were assigned the average age of the ducks included in each pool. A total of 177 (14%) pools corresponded to ducks without age information. The median age of the ducks tested was 3 months [interquartile range (IQR) 3–4·5 months].
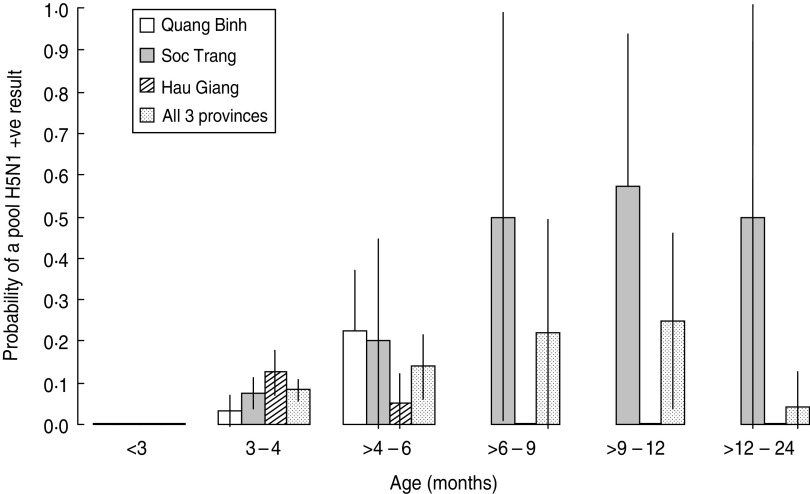
No pools from ducks aged <2 months (of 31 tested) resulted in a positive H5N1 result. Overall results indicated increasing prevalence with age, with the highest overall prevalence in pools from ducks aged between 6 and 12 months. In Soc Trang the pool H5N1 prevalence increased with age, whereas Hau Giang had a higher prevalence in pools from birds aged 3–4 months and all pools from ducks aged >6 months tested negative (Fig. 1).
Fig. 1.
Probability of a duck pool testing positive for H5N1 in the first market survey (January 2011) by province.
Poultry stallholders (sellers)
Overall, 1120 questionnaires were completed from a total of 635 poultry stallholders interviewed during the 344 market visits. In some cases the same stallholder was present at the market on different visits. The median number of different stallholders per market over the whole study period was seven (IQR 4·25–12·0), but on a given visit, the median number of stallholders was four (IQR 2–6). In some provinces there seemed to be a greater rotation of stallholders from one day to the next: whereas in Nam Dinh and Hau Giang typically the same stallholders were present at the markets on different days, in the other provinces there was a greater turnover of stallholders. On 515 (46%) occasions stallholders brought poultry to the market on that day (i.e. stallholders were also identified as traders). This figure was highest for Quang Binh (91%) and lowest for Ninh Binh (15%). The proportion of sellers selling both species (chickens and ducks) was highest for Quang Binh (83%) and lowest for Nam Dinh and Ninh Binh (21–23%). A total of 8% of stallholders reported accepting/selling sick birds. The highest proportion of stallholders accepting sick birds was in Nam Dinh (15%), Quang Binh (14%), and Ninh Binh (7·2%). A small percentage (∼1%) of stallholders accepting dead birds was found in Nam Dinh, Ninh Binh and Soc Trang, but not in the remaining two provinces (Table 3).
Table 3.
Summary of poultry stallholders (sellers) in markets visited in the five provinces in the January 2011 survey
| Nam Dinh | Ninh Binh | Quang Binh | Soc Trang | Hau Giang | All | |
|---|---|---|---|---|---|---|
| No. total of visits | 80 | 74 | 60 | 65 | 55 | 334 |
| Total no. stallholders | 133 | 182 | 57 | 158 | 105 | 635 |
| Total no. stallholder interviews | 169 | 354 | 157 | 315 | 125 | 1120 |
| Total no. stallholders per market (over all visits) | 4 (1–8) | 9 (7–13) | 6 (3·2–7·7) | 11 (5–15) | 5 (2–12) | 7 (4–12) |
| No. stallholders per visit | 4 (1–6) | 5 (4–7) | 3 (2–7) | 5 (4–7) | 4 (2–6) | 4 (2–6) |
| Stallholders bringing their own poultry to the market (%) | 57 | 15 | 91 | 47 | 57 | 46 |
| Stallholders trading ducks only/chickens only/both species (%) | 42/35/23 | 69/10/21 | 17/0/83 | 52/19/29 | 52/6/42 | 51/14/35 |
| No. ducks sold daily (per market) | 37 (30–55) | 63 (31–111) | 21 (15–33) | 64 (32–103) | 54 (33–101) | 39 (24–86) |
| No. chickens sold daily (per market) | 22 (9–35) | 8 (0–38) | 25 (11–45) | 17 (0–41) | 11 (0–27) | 20 (0–37) |
| Stallholders reporting accepting sick poultry (%) | 15 | 7 | 14 | 3 | 0 | 8 |
| Stallholders reporting accepting dead poultry (%) | 1·0 | 1·2 | 0 | 0·7 | 0 | 0·7 |
Values in parentheses are interquartile range.
Duck traders
Data from the 1054 trader interviews indicated that 588 (55%) brought ducks to the market by motorbike, 273 by bicycle (26%), 16 (1·5%) by car/van/truck and 187 (17%) by other means (including 23 by boat and four on foot). Only 32/1054 (3%) traders indicated that they sold to more than one market. Overall, poultry brought to the market on a given day originated from a median of one farm (IQR 1-2), and 31% of traders reported bringing poultry on the same day from more than one farm. The proportion of traders that brought poultry from two farms or more was highest in Quang Binh (45%) and lowest in Hau Giang (8%). The percentage of traders that sold ducks directly (i.e. were also stallholders) was also greatest in Quang Binh (67%) and lowest in Ninh Binh (15%). On average 60% of traders brought ducks to the markets and 40% brought both chickens and ducks (Table 4).
Table 4.
Summary of poultry traders in markets visited in the five provinces in the January 2011 survey
| Nam Dinh | Ninh Binh | Quang Binh | Soc Trang | Hau Giang | All provinces | |
|---|---|---|---|---|---|---|
| No. total of visits | 80 | 74 | 60 | 65 | 55 | 334 |
| Total no. trader interviews | 169 | 181 | 213 | 294 | 197 | 1054 |
| No. traders per market per visit | 5 (3–6) | 3 (2–4) | 4 (3–5) | 3 (2–5) | 3 (2–4) | 4 (2–7) |
| Traders directly selling poultry to the public in the market (%) | 42 | 15 | 67 | 50 | 36 | 49 |
| Traders trading ducks only (%) | 66 | 71 | 24 | 72 | 60 | 60 |
| Traders trading ducks and chickens (%) | 34 | 29 | 76 | 27 | 40 | 40 |
| Traders bringing their own poultry to the market (%) | 45 | 47 | 64 | 63 | 69 | 57 |
| Traders bringing vehicle/crates/baskets onto farms (%) | 68 | 88 | 49 | 69 | 51 | 75 |
| Traders bringing poultry sourced from more than one farm (%) | 32 | 38 | 45 | 15 | 8 | 31 |
Values in parentheses are interquartile range.
Model results
Significant independent factors for a pool testing positive for H5N1 were: (1) trader sells ducks directly to the public [odds ratio (OR) 1·88]; (2) ducks brought by the trader on the day come from more than one farm (OR 2·14); and (3) ducks are aged >6–12 months (OR 5·01). Pools of ducks from traders that also carried chickens had a lower probability of testing positive (OR 0·47). Duck pools from traders that entered the farms with their vehicle/baskets/crates were not associated with an increased risk (OR 0·95) and therefore this variable was not entered into the multivariable model (Table 5). Interaction terms between any of the four significant variables remaining in the final model were not significant.
Table 5.
Logistic regression models (univariable and multivariable analyses) to investigate risk factors for a pool testing positive (data from Quang Binh, Soc Trang and Hau Giang provinces, January 2011). Only factors that are candidates for multivariable modelling are shown
| Variable | Level | Univariable level | Multivariable level | |||
|---|---|---|---|---|---|---|
| OR | P value | OR | 95% CI | P value | ||
| Trader sells directly to the public (i.e. without stallholder intermediary) | No | Ref. | ||||
| Yes | 1·73 | 0·178 | 1·88 | 1·03–3·45 | 0·047 | |
| Trader trades chickens | No | Ref. | ||||
| Yes | 0·47 | 0·013 | 0·43 | 0·22–0·82 | 0·010 | |
| Ducks sold by trader originate from ⩾1 farms | 1 | Ref. | ||||
| >1 | 2·14 | 0·007 | 2·43 | 1·33–4·47 | 0·004 | |
| Ducks aged >6–12 months | Yes | 7·25 | <0·001 | 5·01 | 1·95–12·8 | <0·001 |
| Other age | Ref. | |||||
| Log (no. ducks traded + 1) | 1·05 | 0·606 | ||||
| No. ducks in market (log + 1) | 0·95 | 0·522 | 0·97 | 0·79–1·18 | 0·725 | |
| No. chickens in market (log + 1) | 0·87 | 0·094 | ||||
OR, Odds ratio; CI, confidence interval.
Model intercept: –2·48 (s.e. = 0·36).
DISCUSSION
The main findings from this study are: (1) a higher prevalence of H5N1 in the two Mekong delta provinces compared to the other three provinces surveyed; (2) a higher prevalence of H5N1 infection in January compared to mid-year (May); (3) a high proportion of traders reporting entering farms without adequate biosecurity measures, representing a risk of spread of H5N1 (and other diseases); (4) the identification of hazardous practices among a minority of stallholders such as accepting dead and sick poultry, representing a public health risk to slaughterers and consumers; (5) a higher probability of H5N1 infection in ducks aged 6–12 months. This study confirms the feasibility of live market surveys to investigate H5N1 in Vietnam. Live bird markets are a feature of many Asian countries since most Asian customers prefer freshly slaughtered poultry [19]. In Vietnam, there are a large number of small live bird markets catering mostly for the rural population.
The catchment area of the live duck markets investigated in this study was typically short-range and therefore ducks sampled in these markets are likely to represent prevalence of H5N1 in ducks at the end of their productive life in the districts where the surveys were conducted. However these markets may not be representative of those catering for the larger urban centres (i.e. Hanoi, Hue, Ho Chi Minh city, etc.). In our study only 13% of ducks were reported to originate from outside the district where the market was located (data not shown), and this was more common in markets located close to district/provincial boundaries and less common among those in the centre of the district.
Trader movements are likely to contribute to the focal spread of H5N1 (as well as other infectious diseases) and results from this study highlight a potential risk of spreading infection since most traders do enter farms with their vehicles/crates, and handle the birds themselves without any additional protection. The median number of farms visited per trader per day was one, but over a third of traders reported visiting more than one farm on a single day. These findings suggest that education campaigns should be targeted at this important group in order to improve biosecurity during access to farms and reduce the spread of infection posed by the trader. We found a higher H5N1 prevalence in ducks sold by farmer-traders, as opposed to through a stallholder ‘intermediary’ suggesting that some traders may be deliberately selling unhealthy looking ducks to reduce losses due to infectious disease.
A key feature of H5N1 infection in Vietnam is its geographical clustering [4], and it has been shown that poultry traders tend to operate in communes with similar HPAI status [4, 20], although this may have partly been a reflection of reporting bias and the typical short-range movements of most poultry trade in Vietnam.
Some studies have shown that poultry markets may sustain infection given the mixing of species and contacts with birds from a large number of sources [21] and it has been shown that closing the market for 1 day would eliminate transmission [22]. Most of the markets investigated in this study were small operations without poultry holding facilities, and most ducks traded originated from relatively nearby farms and were harvested and sold at the market on the same day. No markets investigated in this study had holding facilities and in most cases poultry were reported to be sold within the day of arrival.
A study in North Vietnam identified the presence of a trader in a village as a risk factor (OR 11·5) for outbreaks at the village level, and it was hypothesized that some traders may keep poultry at home over several days creating an active focus of infection [23]. However, this finding can also be a reflection of higher trade activity due to other factors (bigger farms, more contacts between farms, etc.) which is expected to be associated with an increased risk. The observed higher probability of H5N1 infection in older birds is not suggestive of acquisition of infection in transit, since in that case, a higher prevalence of infection in younger ducks would be expected, since younger ducks are more susceptible to H5N1 infection and tend to have lower vaccination coverage. Moreover, results from the model do not suggest that larger markets increase the probability of a sample testing positive.
A small but significant proportion of stallholders reported accepting dead and/or sick poultry. This is likely to pose a considerable risk since birds with clinical HPAI are likely to excrete high levels of virus, and handling poultry has been found to be a risk factor for H5N1 infection in humans [24]. We observed a greater tendency to accept sick/dead poultry by stallholders in the two Northern provinces. Although these two Northern provinces are not known for their high number of human cases, most H5N1 human cases in Vietnam occurred in the North and it is not known whether any cultural differences relating to handling sick poultry may partly explain these differences.
In the past both the Mekong and the Red River deltas have been considered to be high-risk (incidence) areas for HPAI, partly because of their higher poultry (and human) densities, rice and associated duck production [25]. Results from these surveys indicate absence of H5N1 in ducks sampled the two Red River provinces at the time of the study, but endemic presence in the Mekong provinces. It has been suggested that the epidemiology of H5N1 may differ considerably between those areas: in the North periodic introduction of new viruses from China are suspected, although these do not seem to persist over many years.
It is thought that conditions in the Mekong delta provinces are more conducive to avian influenza transmission (a higher density of waterways, higher density of waterfowl associated with higher rice yield, practice of mobile duck grazing, etc.). Mobile ducks are a feature typical of the Mekong provinces. These are flocks that travel beyond the commune boundaries to graze on harvested rice fields [26]. Mobile duck flocks tend to consist of layer ducks, which are typically older than ducks raised for meat. Older ducks are also more likely to be more efficient vectors of H5N1 infection since they show less clinical signs and may excrete H5N1 viruses for a longer period. Mobile ducks are not thought to be present in the Red River delta region.
Previous studies on asymptomatic poultry have demonstrated subclinical circulation of H5/H5N1 in the Mekong delta. The Government of Vietnam carried out bi-annual virus circulation surveys (one after each vaccination campaign) during 2007–2009. Over the five virus circulation surveys conducted, a total of 2571 swabs were collected from waterfowl, of which 151 (5·9%) tested positive for H5, compared to 68/1024 (6·6%) that tested positive for H5N1 in the Mekong delta provinces in this study. The proportion of these H5 strains identified in the Government surveys as H5N1 is not known. However, a previous longitudinal study conducted in 2007/2008 in poultry in four Mekong provinces (including Soc Trang) reported a very low H5 prevalence by testing combined oropharyngeal/cloacal duck pools (2/1086, 0·2%) [7]. It is not known to what extent these differences are due to: (a) real differences of prevalence over time; (b) a different age distribution of ducks sampled (i.e. older ducks in the market studies); or (c) an increase of shedding H5N1 viruses due to stress experienced by the ducks in the process of harvesting in the farm and transport to the market).
It is not known to what extent the pooling of samples may have reduced the sensitivity of the test, wrongly leading to the conclusion of absence of infection in some districts. To our knowledge no study investigating the detection threshold for pooled samples has yet been performed, so it is not possible to make any conclusions about a potential ‘dilution effect’. More generally, it would be desirable to develop and standardize more cost-effective (and ideally more sensitive) environmental sampling methods in markets adapted to the conditions of Vietnam, similar to those shown in other studies [27, 28]. It would theoretically be possible to include in this testing strategy swabs from crates/baskets where ducks are kept, as well as fluff/feather/faeces collected from key areas after poultry has been sold, as well as drain waste and other environmental samples. The systematic application of such sampling strategies would potentially be useful to monitor H5/H5N1 in markets located in districts that consistently test H5N1 negative. In this way the introduction of infection into such areas can be detected early allowing generic interventions (i.e. movement controls, enhanced public health awareness campaigns, etc.) to be put in place. However, if traceback to infected farms is desired then testing individually identified birds will be necessary, but that is of little practical use in endemically infected areas of Vietnam. Even with testing schemes based on individual-bird sampling it is theoretically possible to reduce the number of birds required in those markets/districts consistently showing a high level of infection (e.g. some Mekong districts) which would represent additional cost savings.
In summary, we conducted live market surveys for H5N1 in 39 districts of five provinces in Vietnam, and were able to consistently detect H5N1 in three of these provinces. These results provide important insights into risks associated with live market trading. Future public education campaigns need to address practices among traders, stallholders and slaughtering personnel. It is recommended that market survey methodology is introduced in active surveillance programmes in Vietnam.
ACKNOWLEDGEMENTS
We are grateful to all veterinary staff from the District Veterinary Stations and SDAH of each of the five participating provinces and the Department of Animal Health of the Government of Vietnam. We thank the directors and staff of NCVD, RAHO1, RAHO3, and RAHO7 for their support. We are indebted to VidaGIS Vietnam for carrying out the necessary training across the five provinces. We are grateful to Dr Santanu Bandyopadhyay (FAO) for reading the manuscript and making valuable comments. This work was funded by USAID (GETS Project, OSRO/801/US).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.World Health Organization. Evolution of H5N1 avian influenza viruses in Asia. Emerging Infectious Diseases 2005; 11: 1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eagles D, et al. H5N1 highly pathogenic avian influenza in Southeast Asia. Revue Scientifique et Technique 2009; 28: 341–348. [DOI] [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization. Approaches to controlling, preventing and eliminating H5N1 highly pathogenic avian influenza in endemic countries, 2011. (FAO Animal Health and Production, Paper No. 171).
- 4.Minh PQ, et al. Spatio-temporal epidemiology of highly pathogenic avian influenza outbreaks in the two deltas of Vietnam during 2003–2007. Preventive Veterinary Medicine 2009; 89: 16–24. [DOI] [PubMed] [Google Scholar]
- 5.Alba A, et al. Assessment of different surveillance systems for avian influenza in commercial poultry in Catalonia (North-Eastern Spain). Preventive Veterinary Medicine 2010; 97: 107–118. [DOI] [PubMed] [Google Scholar]
- 6.Henning J, et al. Scavenging ducks and transmission of highly pathogenic avian influenza, Java, Indonesia. Emerging Infectious Diseases 2010; 16: 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henning J, et al. Highly pathogenic avian influenza (H5N1) in ducks and in-contact chickens in backyard and smallholder commercial duck farms in Viet Nam. Preventive Veterinary Medicine 2010; 101: 229–240. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen DC, et al. Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001. Journal of Virology 2005; 79: 4201–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantin-Jackwood MJ, Swayne DE. Pathobiology of Asian highly pathogenic avian influenza H5N1 virus infections in ducks. Avian Diseases 2007; 51: 250–259. [DOI] [PubMed] [Google Scholar]
- 10.Pantin-Jackwood MJ, et al. Age at infection affects the pathogenicity of Asian highly pathogenic avian influenza H5N1 viruses in ducks. Virus Research 2007; 130: 151–161. [DOI] [PubMed] [Google Scholar]
- 11.Eggert D, Swayne DE. Single vaccination provides limited protection to ducks and geese against H5N1 high pathogenicity avian influenza virus. Avian Diseases 2010; 54: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 12.Dohoo I, Martyn W, Stryhn H. Veterinary Epidemiologic Research, 1st edn. Charlottetown, Canada: AVC Inc., 2003. [Google Scholar]
- 13.Londt BZ et al. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathology 2008; 37: 619–627. [DOI] [PubMed] [Google Scholar]
- 14.Spackman E, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology 2002; 40: 3256–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, et al. A multiplex real-time RT-PCR for detection and identification of influenza virus types A and B and subtypes H5 and N1. Journal of Virological Methods 2008; 148: 81–88. [DOI] [PubMed] [Google Scholar]
- 16.Cowling DW, Gardner IA, Johnson WO. Comparison of methods for estimation of individual-level prevalence based on pooled samples. Preventive Veterinary Medicine 1999; 39: 211–225. [DOI] [PubMed] [Google Scholar]
- 17.Thrusfield M. Veterinary Epidemiology, 3rd edn. Ames, Iowa: Blackwell Science, 2005. [Google Scholar]
- 18.Sergeant E.Epitools epidemiological calculators. AusVet Animal Health Services and Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease. (http://epitools.ausvet.com.au).
- 19.Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine 2007; 25: 5637–5644. [DOI] [PubMed] [Google Scholar]
- 20.Soares Magalhaes RJ, et al. Associations between attributes of live poultry trade and HPAI H5N1 outbreaks: a descriptive and network analysis study in northern Vietnam. BMC Veterinary Research 2010; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardona C, Yee K, Carpenter T. Are live bird markets reservoirs of avian influenza? Poultry Science 2009; 88: 856–859. [DOI] [PubMed] [Google Scholar]
- 22.Kung NY, et al. The impact of a monthly rest day on avian influenza virus isolation rates in retail live poultry markets in Hong Kong. Avian Diseases 2003; 47: 1037–1041. [DOI] [PubMed] [Google Scholar]
- 23.Desvaux S, et al. Risk factors of highly pathogenic avian influenza H5N1 occurrence at the village and farm levels in the Red River Delta Region in Vietnam. Transboundary and Emerging Diseases 2011; 58: 492–502. [DOI] [PubMed] [Google Scholar]
- 24.Dinh PN, et al. Risk factors for human infection with avian influenza A H5N1, Vietnam, 2004. Emerging Infectious Diseases 2006; 12: 1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeiffer DU, et al. An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. Veterinary Journal 2007; 174: 302–309. [DOI] [PubMed] [Google Scholar]
- 26.Minh PQ, et al. A description of the management of itinerant grazing ducks in the Mekong river delta of Vietnam. Preventive Veterinary Medicine 2010; 94: 101–107. [DOI] [PubMed] [Google Scholar]
- 27.Indriani R, et al. Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerging Infectious Diseases 2010; 16: 1889–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulaga LL, et al. Epidemiologic and surveillance studies on avian influenza in live-bird markets in New York and New Jersey, 2001. Avian Diseases 2003; 47: 996–1001. [DOI] [PubMed] [Google Scholar]