SUMMARY
Completion of treatment is key to tuberculosis control. Using national surveillance data we assessed factors associated with tuberculosis patients being lost to follow-up before completing treatment (‘lost’). Patients reported in England, Wales and Northern Ireland between 2001 and 2007 who were lost 12 months after beginning treatment were compared to those who completed, or were still on treatment, using univariable and multivariable logistic regression. Of 41 120 patients, men [adjusted odds ratio (aOR) 1·29; 95% confidence interval (CI) 1·23–1·35], 15- to 44-year-olds (P<0·001), and patients with pulmonary sputum smear-positive disease (aOR 1·25, 95% CI 1·12–1·45) were at higher risk of being lost. Those recently arrived in the UK were also at increased risk, particularly those of the White ethnic group (aOR 6·39, 95% CI 4·46–9·14). Finally, lost patients had a higher risk of drug resistance (aOR 1·41, 95% CI 1·17–1·69). Patients at risk of being lost require enhanced case management and novel case retention methods are needed to prevent this group contributing towards onward transmission.
Key words: Epidemiology, treatment outcome, tuberculosis, UK
INTRODUCTION
Ensuring patients adhere to and complete treatment is key to the control of tuberculosis (TB) [1, 2]. Failure to ensure treatment completion can lead to increased levels of drug resistance, relapse of disease and further transmission.
In 1998 the WHO established a recommended classification of TB treatment outcomes, updated in 2001 by a European Working Group [3, 4]. This consists of a minimum of six treatment outcomes, with the option of adding more detailed subsets. One such subset is ‘lost to follow-up’ which refers to patients who start treatment, but for whom there is a failure to obtain contact before the end of their planned treatment. While in most countries this is captured under ‘default’ some, such as the UK, capture this separately, providing an opportunity to identify and compare risk groups as well as to consider potential interventions to prevent cases becoming lost [5, 6].
‘Lost to follow-up’ is an unsatisfactory outcome for TB control. Patients may be non-compliant and are not in contact with health services for contact tracing and monitoring, which is of concern for public health. These patients are a group that can have distinct problems and challenges, and interventions to improve treatment adherence need to be targeted to their characteristics. A previous study in London found that patients in hard to reach groups such as the homeless, prisoners, and those who misuse drugs and alcohol had higher levels of sputum smear-positive and drug-resistant disease and more often became lost to follow-up [7].
This study aimed to identify the characteristics of TB patients being lost to follow-up compared to those who were not, in England, Wales and Northern Ireland between 2001 and 2007, thereby identifying groups who may be more likely to become lost before completion of treatment. We also investigated the presence of drug resistant disease in cases lost to follow-up.
METHODS
Data sources and record linkage
In England, Wales and Northern Ireland cases of TB are reported to the Enhanced Tuberculosis Surveillance (ETS) system. The system entails the collection of demographic and clinical information on TB cases by the clinician using a standardized form. Since 2001, the treatment outcome of pulmonary and extra-pulmonary cases at 12 months after the start of treatment is also reported, using a second standardized form as described previously [5, 6]. Table 1 shows the treatment outcome options available. Where possible, information on drug susceptibility was obtained through linkage with information on isolates reported through the Mycobacterial Surveillance Network, as described previously [8].
Table 1.
Definitions of tuberculosis (TB) treatment outcomes used in England, Wales and Northern Ireland
| Treatment outcome | Definition |
|---|---|
| Completed | Reported case who successfully completed full course of treatment within 1 year of starting therapy, and was discharged by physician |
| Died | Patient who died from any cause while on TB treatment, or patients found to have TB post mortem |
| Lost to follow-up | Patient lost to follow-up before end of treatment. This includes patients lost to follow-up because they moved overseas, as well as all other patients lost to follow-up |
| Still on treatment | Patient still on treatment (either planned treatment lasts longer than 12 months, treatment was interrupted and resumed, or treatment was changed) |
| Treatment stopped | Treatment not completed for reason other than death or still being on treatment |
| Transferred out | Responsibility for patient's care transferred to another clinical team |
| Unknown | No details on treatment outcome available, e.g. due to lost patient notes |
| No outcome report received | No outcome indicated (all recoded to unknown) |
Definitions
TB cases were either culture confirmed as caused by Mycobacterium tuberculosis complex, or patients with clinical and/or radiological signs compatible with TB and a clinician's decision to treat with full course anti-TB therapy.
Drug resistance was defined as resistance to one or more of the first-line drugs: isoniazid, rifampicin, pyrazinamide, and/or ethambutol.
Inclusion and exclusion criteria
Cases of TB reported between 1 January 2001 and 31 December 2007 with a treatment outcome of ‘lost to follow-up’ were compared with those who ‘completed treatment’ or were ‘still on treatment’ at 12 months (Table 1). The ‘still on treatment’ cases were included in the comparison group as some forms of the disease can take more than 12 months to treat. Cases reported to have died, stopped treatment (e.g. those who had an adverse reaction to drugs), or who were without an outcome report (e.g. those with lost notes) were excluded from the analysis as their continuing contact with the TB service could either not be assessed or was not continued for reasons other than loss to follow-up.
Analysis of data
We assessed the association between loss to follow-up and age, sex, country of birth, year of entry into the UK, ethnicity, site of disease, sputum smear, and previous diagnosis. In order to investigate the effect of migration, country of birth was grouped into UK born and non-UK born with varying lengths of residence in the UK. Ethnic groups were based on Office for National Statistics (ONS) definitions. Within the variables length of residence in the UK and previous history of TB a value of ‘unknown’ was included, as it was hypothesized that those lost to follow-up may be less likely to have complete data in these variables.
Site of disease (pulmonary and extra-pulmonary TB) was combined with information on sputum smear status. Differences in presenting symptoms of pulmonary and extra pulmonary TB may result in different health-seeking behaviour and treatment adherence, while patients with infectious disease may be monitored more strictly by clinic staff and therefore have a different risk of loss to follow-up. Previous diagnosis was assessed as a predictor of loss to follow-up as this may indicate previous non-adherence.
Descriptive analysis was undertaken and proportions of TB cases lost were calculated for all variables. Univariable analysis was used to calculate odds ratios for each variable and lost to follow-up, and a likelihood ratio test (LRT) was used to assess the strength of evidence of an association. A multivariable model was built containing the a priori variables sex and age, followed by variables found to be associated with being lost to follow-up on univariable analysis (LRT, P value <0·20). These were added to the multivariable model in order of strength of evidence of association, and kept in if they improved the model significantly (LRT, P<0·05). The next variable was then added and assessed. Finally, interactions were investigated and retained if they improved the model (LRT, P<0·10).
Patients' initial drug resistance was also available for analysis. However, we hypothesized that drug resistance is an unlikely predictor of loss to follow-up, and more likely a consequence of non-adherence to treatment of cases that are lost to follow-up. Drug resistance was therefore not analysed as a potential predictor. To investigate the potential public health impact of loss to follow-up, we compared the proportion of drug resistance using χ2 test.
Data were analysed using Stata v. 11.1 (Stata Corporation, USA).
Ethical approval
Ethical approval was not required as the HPA has National Information Governance Board (NIGB) approval to hold and analyse national surveillance data for public health purposes under Section 251 of the NHS Act 2006.
RESULTS
Study population
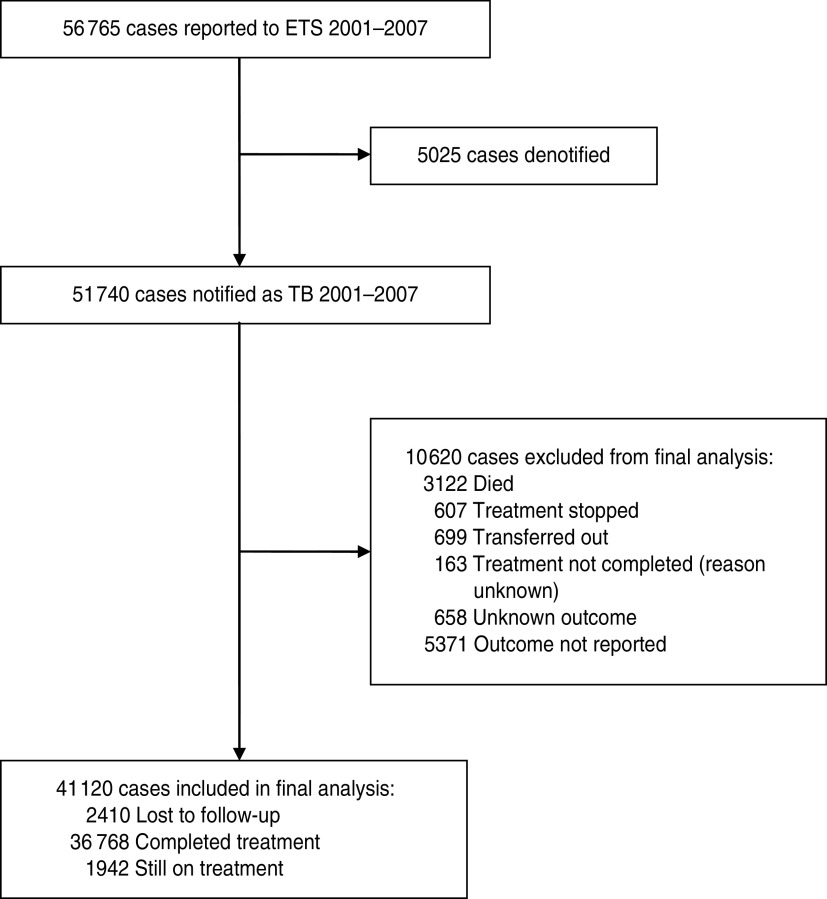
The inclusion process for the cohort is shown in Figure 1. Of the 41 120 cases included, 2410 (5·9%) patients were ‘lost to follow-up’ at 12 months, 36 768 (89·4%) had completed treatment and 1942 (4·7%) were still on treatment.
Fig. 1.
Inclusion process for cases of tuberculosis reported to the Enhanced Tuberculosis Surveillance (ETS) system.
The median age of the study population was 33 years [interquartile range (IQR) 25–49 years] and 54·4% were male. Black Africans made up 24·6% of the cases and those of Indian and White ethnicity accounted for 20·5% and 20·4% respectively (Table 2). Three-quarters (75·2%) of cases were non-UK born, with a median time since entry of 4 years (IQR 1–12 years). Nearly half (47·7%) of all cases were reported from London, 45·0% of cases presented with extra-pulmonary disease only and 7·9% had a previous diagnosis of TB (Table 2).
Table 2.
Univariable analysis of factors associated with becoming lost to follow-up in tuberculosis cases, England, Wales and Northern Ireland 2001–2007 (N=41 120)
| Characteristic | Total | Lost to follow-up (%) | OR (95% CI) | P value* |
|---|---|---|---|---|
| Gender (n=41 071†) | ||||
| Female | 18 703 | 801 (4·3) | 1 | <0·001 |
| Male | 22 368 | 1601 (7·2) | 1·31 (1·26–1·37) | |
| Age group (years) (n=41 115†) | ||||
| 0–14 | 2464 | 63 (2·6) | 0·34 (0·27–0·44) | |
| 15–44 | 26 313 | 1871 (7·1) | 1 | <0·001 |
| 45–64 | 7614 | 323 (4·2) | 0·58 (0·51–0·65) | |
| ⩾65 | 4724 | 153 (3·2) | 0·44 (0·37–0·52) | |
| Region of reporting (n=41 120) | ||||
| London | 19 627 | 1086 (5·5) | 1 | 0·007 |
| Outside London | 21 493 | 1324 6·2) | 1·12 (1·03–1·22) | |
| Year of notification (n=41 120) | ||||
| 2001 | 4480 | 214 (4·8) | 0·93 (0·78–1·11) | |
| 2002 | 5262 | 322 (6·1) | 1·21 (1·03–1·41) | |
| 2003 | 5463 | 329 (6·0) | 1·19 (1·02–1·39) | |
| 2004 | 5793 | 383 (6·6) | 1·31 (1·13–1·52) | |
| 2005 | 6438 | 416 (6·5) | 1·28 (1·11–1·48) | |
| 2006 | 6807 | 394 (5·8) | 1·14 (0·98–1·32) | |
| 2007 | 6877 | 352 (5·1) | 1 | 0·001 |
| Site of disease (n=41 064†) | ||||
| Extra-pulmonary | 18 461 | 983 (5·3) | 1 | <0·001 |
| Pulmonary sputum smear-positive | 8693 | 578 (6·0) | 1·27 (1·14–1·41) | |
| Pulmonary other | 13 910 | 844 (7·6) | 1·15 (1·04–1·26) | |
| Previous diagnosis (n=41 120) | ||||
| No | 30 531 | 1652 (5·4) | 1 | <0·001 |
| Yes | 2634 | 157 (6·0) | 1·11 (0·94–1·31) | |
| Not known | 7955 | 601 (7·6) | 1·43 (1·30–1·57) | |
| Ethnic group (n=38 068†) | ||||
| White | 8194 | 331 (4·0) | 1 | <0·001 |
| Black-African | 9864 | 663 (6·7) | 1·71 (1·50–1·96) | |
| Indian | 8237 | 525 (6·4) | 1·62 (1·40–1·86) | |
| Pakistani | 6224 | 303 (4·9) | 1·22 (1·04–1·43) | |
| Bangladeshi | 1475 | 46 (3·1) | 0·76 (0·56–1·05) | |
| Other‡ | 4074 | 306 (7·5) | 1·89 (1·63–2·19) | |
| Lived in UK (n=37 965†) | ||||
| UK born | 10 207 | 354 (3·5) | 1 | <0·001 |
| ⩾5 years | 10 783 | 373 (3·5) | 1·00 (0·86–1·16) | |
| 2–4 years | 6265 | 360 (5·7) | 1·70 (1·46–1·97) | |
| <2 years | 6954 | 793 (11·4) | 3·58 (3·15–4·08) | |
| Time unknown | 3756 | 325 (8·7) | 2·64 (2·26–3·08) | |
OR, Odds ratio; CI, confidence interval.
Likelihood ratio test.
Missing data.
Including Black Caribbean, Black Other, Chinese and Mixed/Other.
Characteristics associated with loss to follow-up
On univariable analysis, the risk of being lost to follow-up was highest in males [odds ratio (OR) 1·31, 95% confidence interval (CI) 1·26–1·37], cases with no information regarding a previous diagnosis (OR 1·43, 95% CI 1·30–1·57), cases residing outside London (OR 1·12, 95% CI 1·03–1·22), those with pulmonary disease (smear positive: OR 1·27, 95% CI 1·14–1·41, and negative/not tested: OR 1·15, 95% CI 1·04–1·26) and those aged 15–44 years (reference group, see Table 2). Compared to the White ethnic group, all groups other than Bangladeshi had an increased risk of being lost to follow-up at 12 months (P<0·001) and cases that had lived in the UK for <5 years were at increased risk compared to UK-born cases.
There was strong evidence of an interaction between length of residence in the UK and ethnic group (LRT for interaction P<0·001). Therefore in multivariable analysis this interaction was kept in the model.
On multivariable analysis after adjusting for the other factors, being male (aOR 1·29, 95% CI 1·23–1·35), aged 15–44 years (reference group, see Table 3), residing outside London (aOR 1·39, 95% CI 1·27–1·53), having pulmonary disease (smear positive: aOR 1·25, 95% CI 1·12–1·41, and negative/not tested: OR 1·16, 95% CI 1·04–1·28) and having no information on previous diagnosis (aOR 1·26, 95% CI 1·13–1·41) still had higher odds ratios of being lost to follow-up at 12 months (Table 3). Within each ethnic group, patients that had lived in the UK for <2 years prior to diagnosis or for an unknown period of time had higher odds for loss to follow-up than UK-born patients of the same ethnic group and those who had lived in the UK for a longer period of time (Fig. 2). The odds were highest in recent entrants (<2 years in the UK) of the White ethnic group (aOR 6·39, 95% CI 4·46–9·14 compared to White UK-born patients) followed by those of the Indian (aOR 5·03, 95% CI 4·04–6·25) and other (aOR 4·42, 95% CI 3·52–5·55) ethnic groups. Compared to UK-born patients of White ethnicity, no increased risk was found in Indian, Pakistani, or Bangladeshi ethnic groups who were born in the UK or had lived in the UK for a longer period of time (Indian group >5 years; Pakistani and Bangladeshi groups >2 years). There was also evidence that TB patients in the Bangladeshi group residing in the UK for >5 years may have a decreased risk of loss to follow-up (aOR 0·50, 95% CI 0·26–0·99). Patients from White, Black and other ethnic groups that had lived in the UK for a longer period of time remained at a slightly increased risk compared to the White UK-born group.
Table 3.
Multivariable analysis of possible risk factors for loss to follow-up of tuberculosis cases in England, Wales and Northern Ireland 2001–2007 (N=37537*)
| Characteristic | aOR† (95% CI) | P value‡ |
|---|---|---|
| Gender | ||
| Female | 1 | <0·001 |
| Male | 1·29 (1·23–1·35) | |
| Age group (years) | ||
| 0–14 | 0·42 (0·32–0·56) | |
| 15–44 | 1 | <0·001 |
| 45–64 | 0·86 (0·75–0·99) | |
| ⩾65 | 0·70 (0·58–0·85) | |
| Region of reporting | ||
| London | 1 | <0·001 |
| Outside London | 1·39 (1·27–1·53) | |
| Year of notification | ||
| 2001 | 1·03 (0·85–1·24) | |
| 2002 | 1·26 (1·06–1·49) | |
| 2003 | 1·23 (1·04–1·46) | |
| 2004 | 1·32 (1·12–1·55) | |
| 2005 | 1·28 (1·10–1·50) | |
| 2006 | 1·17 (0·99–1·37) | |
| 2007 | 1 | 0·004 |
| Site of disease | ||
| Extra-pulmonary | 1 | 0·004 |
| Pulmonary sputum smear-positive | 1·25 (1·12–1·41) | |
| Pulmonary other | 1·16 (1·04–1·28) | |
| Previous diagnosis | ||
| No | 1 | 0·001 |
| Yes | 1·18 (0·99–1·41) | |
| Not known | 1·26 (1·13–1·41) | |
| Ethnic group, length of residence in the UK and the interaction between these | See Figure 2 | |
aOR, Adjusted odds ratio; CI, confidence interval.
Excluding cases with data missing for sex, age, ethnic group, place of birth or site of disease.
Adjusted for all variables in the table and the interaction between ethnic group and length of residence in the UK.
Likelihood ratio test for adding the variable to the model.
Fig. 2.
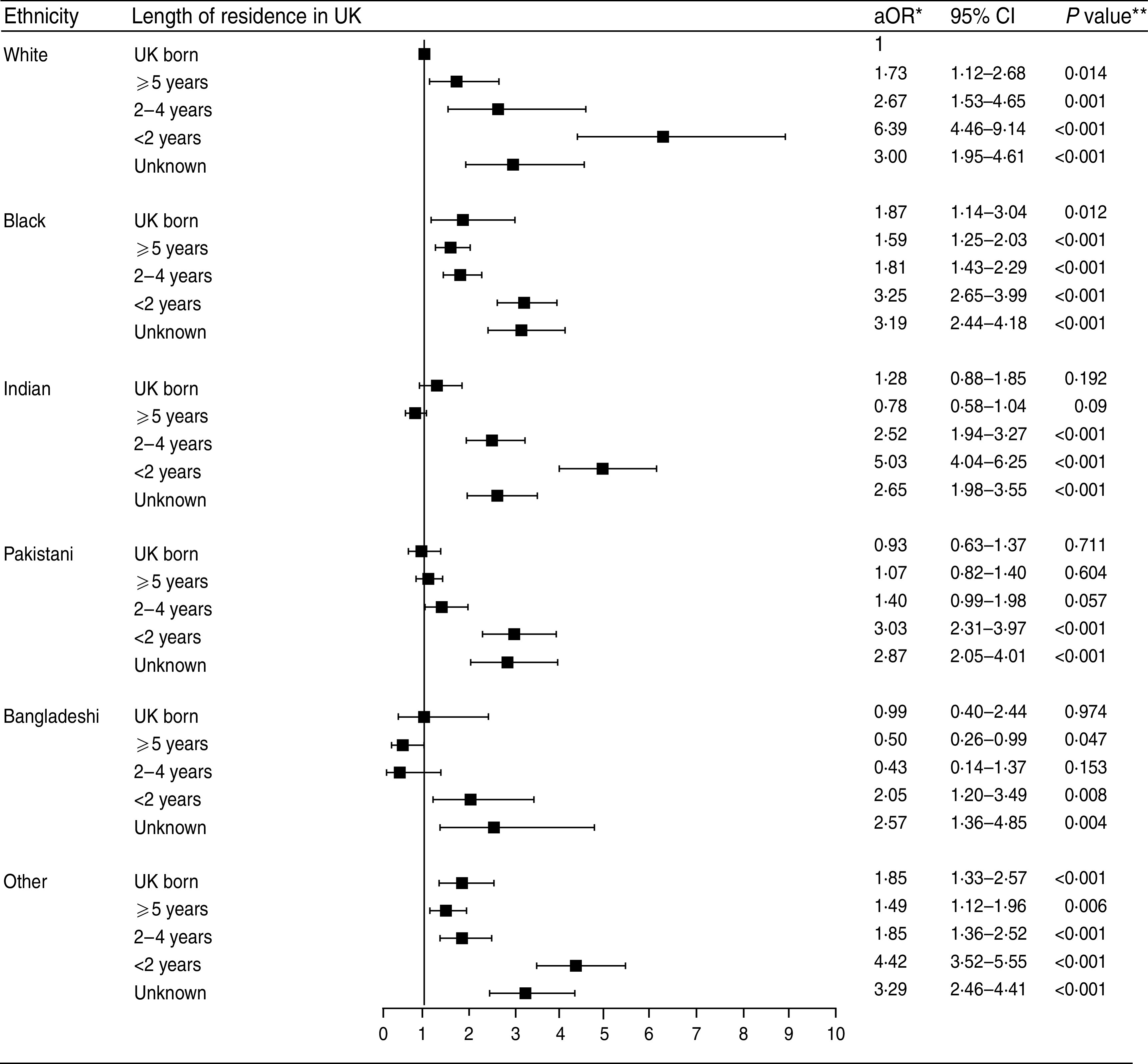
Adjusted odds ratios (aOR) and 95% confidence intervals (CI) for the interaction of length of residence and ethnic group on loss to follow-up of tuberculosis cases in England, Wales and Northern Ireland 2001–2007, N=37 537 (excluding cases with data missing for sex, age, ethnic group, place of birth or site of disease). * Adjusted for age, sex, region of reporting, year of notification, site of disease and previous diagnosis; ** Likelihood ratio test.
Drug resistance
Among patients lost to follow-up a significantly higher proportion was identified at diagnosis as being resistant to any first-line drug compared to patients who completed or were still on treatment [11% (168/1518) vs. 7·4% (1641/22177), P<0·001].
DISCUSSION
Loss to follow-up in TB cases in England, Wales and Northern Ireland appears to be occurring primarily in young male adults and those born outside the UK, particularly those who migrated within the 2 years prior to diagnosis.
The higher risk of being lost to follow-up in pulmonary sputum smear-positive cases, and to a lesser extent other pulmonary cases, emphasizes the potential of this group to contribute to ongoing transmission. The higher proportion of drug resistance in cases lost to follow-up further accentuates the public health implications of this group. Few studies have investigated the contribution of loss to follow-up to transmission of drug-resistant strains. Dahle et al. describe a case of isoniazid-resistant TB imported from Somalia that acquired multidrug-resistant TB (MDR-TB) after the patient failed to attend check-up appointments, with subsequent transmission within Norway [9]. It has previously been estimated that 14% of isoniazid resistance and 27% of MDR-TB in the UK is due to treatment failure [10]. Reducing the number of patients lost to follow-up would help to decrease the transmission and amount of home-grown drug-resistant disease.
There are a number of reasons that could explain the association between loss to follow-up and pulmonary and drug-resistant disease. Patients with severe forms of extra-pulmonary disease may be less likely to become lost because they are likely to be in closer contact with their healthcare provider and may even be hospitalized due poor health. Extra-pulmonary disease has also been shown to be related with longer-term migration, which we show in this study to have a lower risk of loss to follow-up [11]. Finally, it has been shown that a high proportion of patients in hard to reach groups is lost to follow-up and that these patients account for a high proportion of smear-positive and drug-resistant disease [7].
The increased odds of loss to follow-up in recent migrants could be due to these individuals returning home more often than those who have lived for longer in the UK, and could relate to the extent of family ties and the purpose of their visit. Although treatment can be continued outside the UK, it is not always possible for the clinician to properly transfer a case. The use of over-the-counter drugs and private hospitals in some countries may result in further drug resistance and transmission.
The risk of loss in recent entrants was particularly high in the White and Indian ethnic groups. Since these are also the groups with the highest numbers of TB cases in the UK [12], enhanced retention efforts and novel methods to improve treatment completion are most required in these groups. In the UK, TB patients of White ethnicity are mostly UK born or born in another European country. Logistics may thus explain the particularly high risk in recent entrants of this ethnic group: it is both quicker and cheaper to return to your country of origin if from a European nation rather than from more distant locations, such as Asia or Africa. In addition, returning home may be the only option for work-migrants when they cannot work due to illness; ONS migration statistics show a greater proportion of EU citizens immigrated for work-related reasons to the UK compared to migrants from other areas, some of which will have been for short-term work [13].
Those born in the UK and of White ethnicity are a diverse group, including patients in hard to reach groups with high levels of loss to follow-up [7].
All ethnic groups that had resided in the UK for an unknown period of time were more likely to be lost to follow-up than the UK-born White group, as were cases with an unknown previous TB history (compared to those known to have had or not had TB). Migrants in Sweden have expressed concern over reporting complete information to health professionals due to perceived fear of deportation [14] – this may explain some of the missing information in our study. Confidentiality of medical information needs to be reiterated particularly for vulnerable patients. Data completeness in the ETS system is continuously monitored and efforts are made to improve this.
Strengths and limitations
This study used seven consecutive years of national surveillance data making this a large and representative dataset; however, use of surveillance data for research means that we could not control nor account for all possible variables that might influence loss to follow-up. For example, information for other risk factors, such as substance misuse (including alcohol), HIV status or other underlying health conditions, homelessness or incarceration, and the use of directly observed therapy (DOT) may be important but are not currently available. Some of this information has recently been included in the enhanced TB surveillance system.
The definitions of treatment outcome are subject to a level of interpretation, and it should be noted that investigation of the comments field within the ETS indicated a low level of outcome misclassification across the dataset. For instance, patients who started treatment in the UK but then moved abroad while still on treatment were occasionally classified as completed or transferred and this may have led to an underestimation of the number of cases lost to follow-up. Similar problems in interpretation of outcome definitions were noted by Faustini et al. and further clarification of the UK and European guidelines may be required [15]. The recent addition of ‘moved abroad’ as a subset of those with a treatment outcome at 12 months of lost to follow-up in the UK will allow differentiation between those who are not being treated and those who are expected to be seeking treatment abroad. Treatment outcome was not reported or unknown for almost 12% of all reported TB cases. While some of these cases may have been lost to follow-up, many are likely to be due to loss of medical records as clinicians completing the 12-month follow-up forms are directed to use this outcome classification if correct. We also judge that given the large number of cases included in our study, any missing data on losses to follow-up will be unlikely to have a large effect on our results.
CONCLUSIONS
We have identified a demographic group at higher risk of loss to follow-up after a diagnosis of TB. It may be sensible to target resources on such patients to improve treatment completion levels and prevent onwards transmission. Consideration should be given for enhanced case management of young men who have recently arrived in the UK, and particularly those with pulmonary disease. This may include the use of DOT which is not very widespread and is often sub-optimal in the UK. Within hard to reach groups, it may be valuable to extend the work of ‘Find and Treat’, a service that helps re-engage lost patients with health services, to other cities in addition to London [16].
Some patients may be lost as a result of moving abroad. Strengthening of international collaboration and support for clinicians to ensure such patients are properly transferred, on the correct treatment regimen, and non-infectious when travelling should be actively considered to prevent transmission of TB across borders. Additionally, the feasibility of allowing patients to stay in the UK to complete treatment should be assessed as it could reduce this risk and contribute to the global control of TB especially multidrug-resistant disease.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Department of Health. Stopping tuberculosis in England: an action plan from the Chief Medical Officer. London, UK: Department of Health, 2004; CWP 264538. [Google Scholar]
- 2.World Health Organization. Treatment of Tuberculosis: Guidelines for National Programmes, 4th edn. Geneva, Switzerland: World Health Organization, 2009. WHO/HTM/TB/2009.420. [Google Scholar]
- 3.Veen J, et al. Standardized tuberculosis treatment outcome monitoring in Europe. Recommendations of a Working Group of the World Health Organization (WHO) and the European Region of the International Union Against Tuberculosis and Lung Disease (IUATLD) for uniform reporting by cohort analysis of treatment outcome in tuberculosis patients. European Respiratory Journal 1998; 12: 505–510. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization et al. Revised international definitions in tuberculosis control. International Journal of Tuberculosis and Lung Disease 2001; 5: 213–215. [PubMed] [Google Scholar]
- 5.Antoine D, et al. Tuberculosis treatment outcome monitoring in England, Wales and Northern Ireland for cases reported in 2001. Journal of Epidemiololgy & Community Health 2007; 61: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Health Protection Agency. First annual report on tuberculosis treatment outcome surveillance in England, Wales and Northern Ireland: outcome results on tuberculosis cases reported in 2001. London, UK: Health Protection Agency, 2004. [Google Scholar]
- 7.Story A, et al. Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax 2007; 62: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ditah IC, et al. Monitoring tuberculosis treatment outcome: analysis of national surveillance data from a clinical perspective. Thorax 2008; 63: 440–446. [DOI] [PubMed] [Google Scholar]
- 9.Dahle UR, et al. Deciphering an outbreak of drug-resistant Mycobacterium tuberculosis. Journal of Clinical Microbiology 2003; 41: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayward AC, Herbert J, Watson JM. Tuberculosis drug resistance in England and Wales. How much is home-grown? Epidemiology and Infection 2000; 125: 463–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax 2009; 64: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 12.Pedrazzoli D, et al. Tuberculosis in the UK: annual report on tuberculosis surveillance in the UK, 2011. London: Health Protection Agency, 201. [Google Scholar]
- 13.Office for National Statistics. Migration Statistics 2008 Annual Report. London, UK: Office for National Statistics, 2008. [Google Scholar]
- 14.Kulane A, Ahlberg BM, Berggren I. ‘It is more than the issue of taking tablets’: the interplay between migration policies and TB control in Sweden. Health Policy 2010; 97: 26–31. [DOI] [PubMed] [Google Scholar]
- 15.Faustini A, Hall AJ, Perucci CA. Tuberculosis treatment outcomes in Europe: a systematic review. European Respiratory Journal 2005; 26: 503–510. [DOI] [PubMed] [Google Scholar]
- 16.Jit M, et al. Dedicated outreach service for hard to reach patients with tuberculosis in London: observational study and economic evaluation. British Medical Journal 2011; 343: d5376. [DOI] [PMC free article] [PubMed] [Google Scholar]