SUMMARY
The aim of this study was to evaluate potential sampling strategies for detection of infected flocks that could be applied during an outbreak of low pathogenicity notifiable avian influenza (LPNAI) initiated in duck holdings, following initial detection. A simulation model of avian influenza virus transmission and spread within and between holdings, respectively, was used to predict the impact on the size and duration of an outbreak of (i) changing the tracing window within which premises that might be the source of infection or that may have been infected by the index premises were sampled and (ii) changing the number of birds sampled in the flock being tested. It has shown that there is potential benefit in increasing the tracing window in terms of reducing the likelihood of a large outbreak. It has also shown that there is comparatively little benefit from increasing the number of birds sampled per flock.
Key words: Avian influenza, mathematical modelling, outbreaks, surveillance
INTRODUCTION
Avian influenza (AI) is a highly infectious disease of poultry and other birds caused by influenza A viruses. The level of risk to animal and public health posed by different influenza A viruses is both variable and unpredictable due to a range of factors including: their diversity and wide host range; the possibility that certain AI subtypes may mutate in poultry from low pathogenicity to a fully virulent phenotype and genotype [so-called highly pathogenic avian influenza (HPAI) viruses]; and their propensity for inter-species transmission and genetic reassortment. Infection of susceptible poultry with HPAI viruses can be devastating, resulting in high mortality in affected flocks and the implementation of statutory disease control measures. In combination this can lead to considerable economic losses. Consequently, modelling studies have been performed to explore the potential transmission of, and evaluate possible control measures for, HPAI in the poultry industry in Great Britain (GB) [1–4].
Within the European Union (EU) a legislative framework for the control of AI, based on a ‘stamping out’ policy, is laid down in Council Directive 2005/94/EC [5]. The AI virus subtypes and pathotypes that are subject to the implementation of sanitary and other disease control measures are also defined. These definitions describe the so-called notifiable avian influenza (NAI) viruses. Briefly, NAI viruses are defined as any HPAI isolates and all AI viruses of H5 and H7 subtypes, irrespective of pathotype. Therefore, all H5 and H7 low pathogenicity avian influenza (LPAI) viruses are also defined as being NAI viruses (LPNAI). This is due to the risk of mutation of H5 and H7 LPNAI viruses to virulence following infection and circulation in poultry hosts [6].
Statutory measures that are also described in Council Directive 2005/94/EC include compulsory notification and official investigation of suspected disease in poultry and minimum requirements for laboratory diagnosis, slaughter of infected birds, movement restrictions and surveillance programmes. The Directive also allows member states to take a flexible, proportionate and risk-based approach to the control of NAI outbreaks. In addition, the control measures implemented following the detection of LPNAI may differ from those in a highly pathogenic notifiable avian influenza (HPNAI) outbreak. Current contingency plans in GB in the event of an H5 or H7 LPNAI outbreak involve the establishment of a restricted zone with a radius of at least 1 km around the infected premises (IP), followed by culling of the IP and tracings to identify holdings that are either the potential source of infection or holdings that may have been infected by the IP. In contrast, confirmation of a HPNAI outbreak results in two concentric restriction zones of at least 3 km and 10 km radius around the IP as well as the implementation of sanitary measures as described above.
However, in contrast to HPNAI, no studies have been performed to model or determine the optimal surveillance strategy in response to an H5 or H7 LPNAI outbreak. Clinically, LPAI virus infections are often more problematical to detect in susceptible galliforme poultry (chickens and turkeys) than HPAI due to the less severe disease presentation including much lower mortality. Consequently scanning surveillance may not readily identify LPAI. This situation is compounded in anseriformes (ducks and geese), which may not show clinical signs. Therefore, key questions regarding the optimal approach to sampling strategy both prior to and during an LPNAI outbreak remain unanswered.
The aim of this study was to evaluate potential sampling strategies for detection of infected flocks during an LPNAI outbreak, following initial detection. The study focused on simulated outbreaks initiated in commercial duck holdings (where a holding represents at least one flock on the same premises) due to the ecology and epidemiology of AI viruses in anseriformes, facilitating subclinical or cryptic circulation of infection and absence of mutation to a fully virulent HPAI virus in these hosts.
MATERIALS AND METHODS
Model development
We developed a stochastic simulation model based on individual poultry premises. Each premises was classed as susceptible, infected and infectious, infected but not infectious, or detected. In order to assess potential transmission between premises, a network approach was adopted, where the poultry premises were nodes in the networks and contacts between premises were the links in the network. This approach has been adopted for various livestock diseases [7–9], and has previously been applied to the poultry industry in GB [1, 2]. Furthermore, this approach is especially useful where there is limited previous outbreak data with which to parameterize spatial transmission models, as is the case for LPNAI in GB. Premises in the network may be linked by the following:
-
•
spread via slaughterhouses;
-
•
spread between premises belonging to the same integrated company;
-
•
local spread.
The nodes of the network consisted of all commercial poultry premises (all species) as although the simulated outbreaks would be initiated in commercial duck holdings, there would be potential transmission to non-duck premises and such transmission, especially to galliformes where clinical signs of LPNAI are more apparent, could be an important means of detection.
Demographic poultry data and frequency of between-premises contacts
To ascertain the frequency of contacts between commercial duck holdings, a duck-producing company was approached and provided data on actual between-holding movements over a 2-month period, on slaughterhouse movements and those activities that were considered most likely to result in spread between premises belonging to the duck-producing company, i.e. the movement of live birds between premises, the movement of feed vehicles and between-premises contacts due to egg collection. This data was used to calculate the frequency of each type of movement.
For the non-duck holdings, the potential contacts between poultry premises were informed by a previous data collection exercise, described by Dent et al. [1], which provided information on the company affiliation of 1922 holdings, with chicken, turkey, goose and duck premises included in the survey, including both single and multispecies holdings. In this study, slaughterhouses and catching companies were approached to provide a list of the premises from which they collect birds and the species involved. A sample of single and multi-site companies were also sent a questionnaire on which they were asked for details about the frequency and type of movements from their premises.
Data on the location and size of poultry holdings was obtained from (i) the duck-producing company for its own premises and (ii) the GB Poultry Register (GBPR) for all other poultry premises, which contains the location and the number of birds of each species on all holdings with more than 50 birds, and for holdings with less than 50 birds that have registered voluntarily.
Rates of transmission
The relative risk of each type of between-farm contact was obtained from expert opinion, by asking two poultry veterinarians who work with the duck company to give a rating of the risk for each movement type, on a score from 1 to 10, along with their reasons for the rating. Slaughterhouse movements were considered the contact with the greatest risk of transmission, due to the difficulties associated with the cleaning of the vehicles and slaughterhouse crates and the equipment and clothing of the catchers, so was given a relative risk score of 8. Egg crates and feed delivery vehicles were considered as less of a risk, since it was assumed that there should be no source of virus production at the hatchery or feed mill, but there is a risk of fomite spread via the egg crates or delivery vehicles, so these contacts were given a risk score of 5. The ducklings were considered a relatively low risk since there is no evidence of virus infection at the hatchery. As LPAI virus infections are not systemic, vertical (or in ovo) transmission is considered unlikely [10] so the stock should be free of infection, and the vehicles are cleaned and disinfected between farms. Therefore, the movement of 1-day-old ducklings and hatching eggs was given a relative risk score of 2. The actual risk for each movement type was calculated by multiplying the relative risk score by a scaling factor, to give the following daily probabilities of 1-day-old ducklings and hatching eggs (1%), egg crates and delivery vehicles (2·5%), slaughterhouse/catchers (4%). These transmission rates were then scaled up and down for the sensitivity analysis (described below).
For premises not belonging to the duck-producing company, it was assumed that there was potential transmission between any two holdings in the same integrated company each day, according to the user-specified probability, which was set at a probability of a 1% risk of spread each day between premises with the same owner (owner details were obtained from a previous study [1]).
Links between pairs of premises belonging to a large duck-producing company were more explicitly specified; historical records were used to generate a matrix containing the frequency of slaughterhouse movements, which was then randomly sampled each day.
Local spread
We assumed that spatial transmission could occur up to 3 km between the same or different species located on the same premises or on different premises that are geographically close, due to either very short-range airborne transmission, shared staff between adjacent holdings or short distance fomite transmission by wildlife. The following spatial kernel, adapted from Boender et al. [11] for local transmission of AI in The Netherlands, was used to determine the daily probability of transmission at a distance d between identical species, with a maximum probability of infection of 0·001 at distance zero.
| (1) |
Transmission between different species at a given distance used the same formula, but was scaled to half the probability, to reflect the lower likelihood of between-farm contacts between different species compared to farms of the same species.
Since there was no direct connection via slaughterhouse, personnel movements or feed vehicles between the integrated duck company and non-duck holdings, local spread was the only route by which AI could spread to non-duck species in the model.
Within-premises transmission
The number of flocks in each premises was not available for the majority of holdings, and so all the birds of a given species on each holding were assumed to mix homogenously, i.e. each holding was divided into species rather than flocks. A deterministic Susceptible-Exposed-Infected-Recovered (SEIR) model was used to describe the expected number of birds in the susceptible, exposed, infectious or recovered states. Transmission rates and incubation periods were taken from the literature (Table 1).
Table 1.
List of model parameters used for a between-flock simulation model of low pathogenic avian influenza, and their source
| Parameter | Value | Source |
|---|---|---|
| Chickens | ||
| Transmission rate | 0·22 | [14] |
| Latent period | 1·00 | [14] |
| Infectious period | 4·17 | [14] |
| Turkeys | ||
| Transmission rate | 0·66 | To give R0 of 5·5 [15] |
| Latent period | 2·90 | |
| Infectious period | 8·2 | [16] |
| Ducks and geese | ||
| Transmission rate | 2·44 | [16] |
| Latent period | 1·75 | [16] |
| Infectious period | 10·42 | [16] |
| Virological test sensitivity | 72% in infectious period | Bayesian methods applied to data from [13] |
| Virological test specificity | 100% in infectious period | AHVLA data |
| Serological test sensitivity (HI) | 98% | Dr M. Arnold, AHVLA (unpublished results) |
| Serological test specificity (HI) | 99% | Dr M. Arnold, AHVLA (unpublished results) |
AHVLA, Animal Health and Veterinary Laboratories Agency; HI, haemagglutination inhibition.
Transmission between species in the same holding was determined by the rate of local transmission at distance = 0.
Detection of LPAI within premises
Detection of LPAI within a holding could occur via three routes: passive surveillance (according to clinical signs), random sampling of premises as part of the annual AI poultry serological survey or via tracing from an LPAI IP.
Mean time from infection to detection by passive surveillance was determined by expert opinion and then converted to daily odds of detection, broken down by species. On each day, the proportion of infectious birds on the premises is calculated (0 = 0%, 1 = 100%) and then multiplied by the odds of detection. The scaled odds of detection are evaluated every day, so that a premises which is 50% infected is half as likely to be detected as a premises that is 100% infected. The baseline time between infection and detection was set at 5 days for turkeys, 10 days for chickens, informed by experience of LPAI outbreaks in GB and 40 days for ducks (in line with Sharkey et al. [3]).
Serological sampling of premises for the annual AI poultry survey, stratified by species, is undertaken annually by all EU member states, including the UK. This survey was performed each year in the model, starting after the model had run for 1 year. The target number of holdings (N = 402) of each poultry species for the 2009 poultry survey was sampled in England, Scotland, and Wales.
Control measures
Silent (undetected) spread continued in the simulation until sufficient clinical signs were observed on a holding or the flock was sampled as part of the AI poultry survey, at which point the following measures would be triggered:
-
(1)
Tracing and sampling of all potential sources of infection of each newly detected IP using a 14-, 21- or 28-day window.
-
(2)
Tracing and sampling all holdings potentially infected by the newly detected IP, using a 14-, 21- or 28-day window.
-
(3)
Testing of all poultry holdings within a 1 km restricted zone by serology and virological testing.
We use the term ‘back-tracing window’ to describe the length of the time period in which potential sources of infection or potentially infected holdings are investigated (i.e. tracings) in measures (1) and (2) above.
The number of birds in each state (S, E, I or R according to the deterministic model) was used to determine the expected flock-level sensitivity of sampling; it was assumed that virological sampling would only detect infectious birds, and serological sampling would only detect recovered birds. Three different testing scenarios were considered: testing 20, 40 or 60 birds, where each bird was sampled using both oropharyngeal and cloacal swabs for virological sampling by real-time polymerase chain reaction (PCR), and also with serology, using the haemagglutination inhibition (HI) test. Test sensitivities and specificities were determined by Bayesian methods in the absence of a gold standard, assuming two conditionally independent tests [12] using data from (i) parallel testing of 70 flocks of ducks and geese (2129 sera) with HI and ELISA (Animal Health and Veterinary Laboratories Agency, unpublished observations) and (ii) data from the parallel testing of birds from live bird markets in the USA with PCR and virus isolation [13]. Estimates are given in Table 1.
Tracings were simulated using the contact information held in the model. However, to account for the likely resource/personnel constraints in carrying out tracings, a mean delay of 4 days was allowed to complete the tracings, drawn from a Poisson distribution. It was assumed that 98% of contacts between farms would be identified, to allow the possibility of incomplete farm records.
Model outputs
Infection was seeded in a random holding within an integrated duck company and 140 000 runs were performed. For each run of the simulation, the model recorded the total number of IPs. The duration of the outbreak was also recorded.
Sensitivity analyses
The rates of transmission of each route were highly uncertain. The relative risk of each route was obtained from expert opinion, but there was no information on the actual risk of each route. Therefore, the transmission rates were all modulated up and down fivefold to explore the impact on the conclusions of the model on the rate of transmission. They were also varied on an individual basis up and down twofold.
While there were published estimates available for the transmission rates and incubation periods of AI in ducks, it is possible that different strains of AI virus would have different rates of transmission and incubation period. We therefore ran the model considering two different scenarios for LPNAI; first, using parameter estimates from the literature from a single LPNAI strain (Table 1), and second, assuming a doubled transmission rate and halved infectious period, to determine whether changes in the transmission parameters would influence the conclusions.
RESULTS
Simulated LPNAI outbreak size and duration
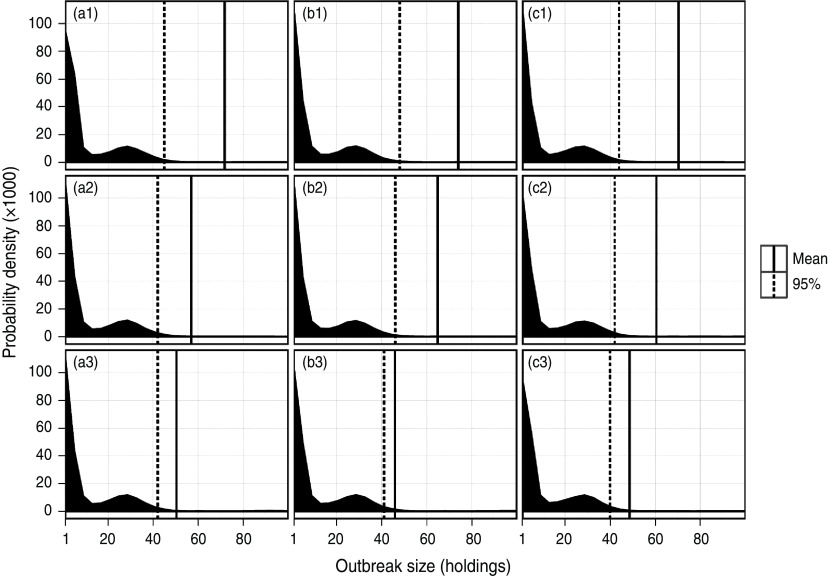
The total simulated LPNAI outbreak size under each surveillance scenario, i.e. under each variation of the back-tracing window and number of birds sampled, is given in Figure 1. For each surveillance strategy, a large proportion of outbreaks resulted in little onward spread (<10 premises) from the index premises, while a small proportion involved >20 premises. There is the potential for many holdings to be infected on rare occasions, which results in a highly skewed mean number of IPs (>95% percentile). An increase in the back-tracing window tended to reduce the likelihood of a very large outbreak, showed by a reduction in the mean outbreak size as the back-tracing window was increased (Fig. 1). However, the increase in the number of birds sampled per infected flock tended to result in very little reduction in the mean outbreak size (Fig. 1).
Fig. 1.
Distribution of the total number of infected premises resulting from a simulated incursion of LPNAI in a commercial duck premises in GB, according to the surveillance scenario adopted. Sample sizes of 20, 40 and 60 are represented in columns (a), (b) and (c), and back-tracing windows of 2, 3 and 4 weeks are represented in rows (1), (2) and (3), respectively.
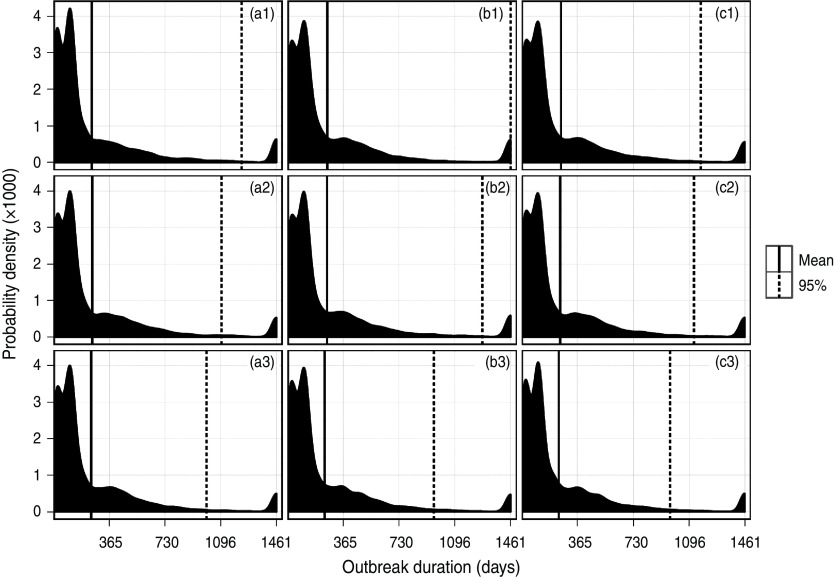
The mean simulated outbreak duration for each surveillance strategy is shown in Figure 2, which shows that for all strategies the majority of outbreaks last <1 year, with a small probability of persistence beyond 3 years. The most likely duration of an outbreak was either <1 month, or around 3–4 months. Beyond 6 months, there was a general a trend of a reducing likelihood of occurrence, the longer the outbreak duration. Increasing the back-tracing window had little impact on the mean duration of the outbreak, although it had an impact on the upper 95% percentile for most strategies, reducing the likelihood of a very long outbreak.
Fig. 2.
Distribution of the outbreak duration resulting from an incursion of LPNAI in a commercial duck premises in GB, according to the surveillance scenario adopted. Sample sizes of 20, 40 and 60 are represented in columns (a), (b) and (c), and back-tracing windows of 2, 3 and 4 weeks are represented in rows (1), (2) and (3), respectively.
Sensitivity analysis
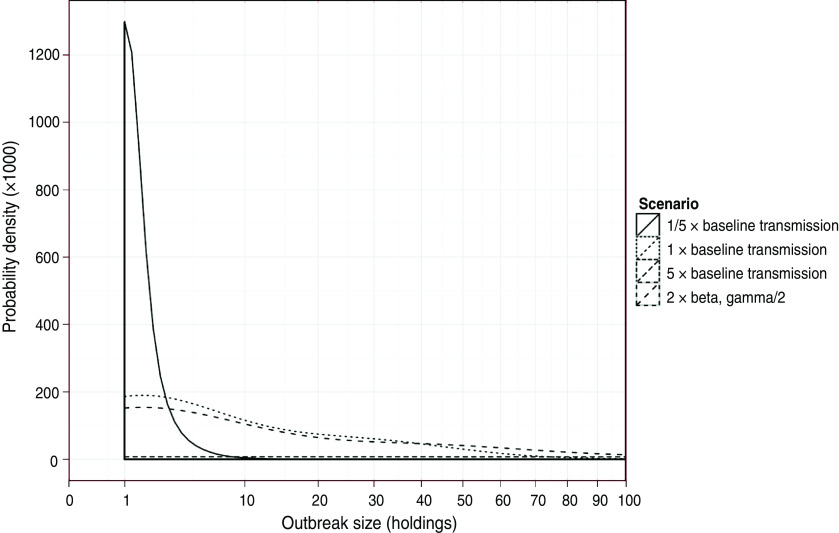
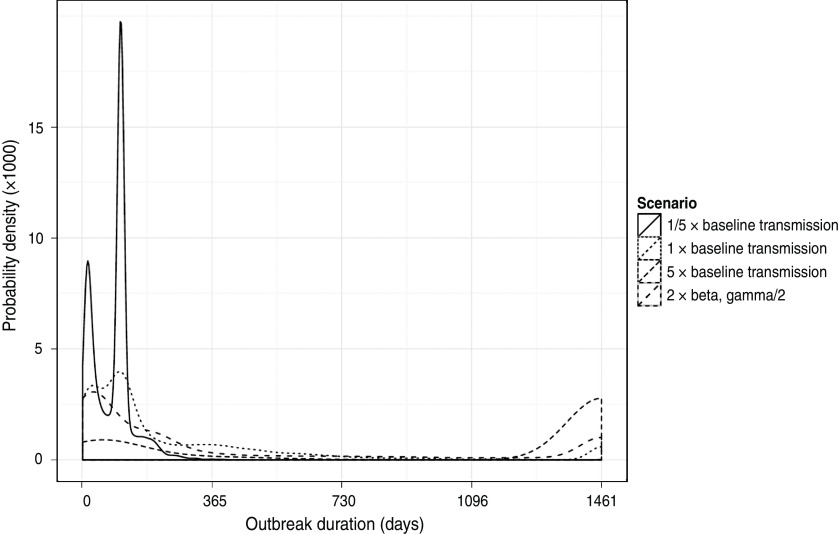
The impact on the simulated LPNAI outbreak size and duration of reducing and increasing the baseline rate of transmission by a factor of 5 is shown in Figures 3 and 4. The distribution of outbreak size was highly sensitive to the rate of between-holding transmission (Fig. 3). For fivefold the baseline rate of between-holding transmission, there was a substantial increase in the likelihood of a very large outbreak, with the majority of outbreaks having more than 100 IPs (72·3%), compared to 4·3% and 0·04% likelihood of an outbreak with more than 100 IPs for the baseline rate and one-fifth of the baseline rate, respectively. In particular, the outbreak size was highly sensitive to the rate of local transmission and owner transmission, where a doubling of the rate produced a fourfold increase in mean outbreak size (Table 2). Outbreak size was not sensitive to changes in the rate of slaughterhouse transmission, with a 20–30% change in outbreak size if the rate was halved or doubled (Table 2). The main impact of changes to the transmission rate on the outbreak duration was a larger proportion of outbreaks lasting >4 years (Fig. 4).
Fig. 3.
Distribution of simulated outbreak size up to 100 holdings of a simulated LPNAI outbreak seeded in a commercial duck holding in GB, and how it varies when the relative rate of transmission is increased and decreased by fivefold from a baseline level (the proportion of outbreaks of size >100 infected premises is provided in the text).
Fig. 4.
Distribution of simulated outbreak duration of a LPNAI outbreak seeded in a commercial duck holding in GB, and how it varies when the relative rate of transmission is increased and decreased by fivefold from a baseline level.
Table 2.
The impact of changes to the rate of transmission by contact type in a simulation model of low pathogenic notifiable avian influenza transmission in GB on the mean number of infected flocks (all species)
| Scenario | Number of infected premises | ||
|---|---|---|---|
| Mean | 90%* | Percentage of runs with >100 cases | |
| Baseline | 71 | 45 | 4 |
| Double local | 297 | 2523 | 12 |
| Half local | 21 | 35 | 2 |
| Double owner | 227 | 1712 | 14 |
| Half owner | 20 | 15 | 1 |
| Double slaughter | 92 | 993 | 6 |
| Half slaughter | 59 | 43 | 4 |
The 90th percentile of the number of infected premises.
In terms of the comparison of surveillance strategies, the sensitivity analyses indicated that an increase in the transmission rate had no impact on the conclusions (Figs S1, S2, supplementary online material), and neither did changes to the strain properties (Fig. S5, supplementary online material), i.e. there were reductions to outbreak size and duration in increasing the back-tracing window but little effect of increasing the number of samples. However, with a fivefold reduction in the transmission rate, there was comparatively little benefit in increasing the back-tracing window (Figs S3, S4, supplementary online material), with outbreaks effectively controlled with a 14-day back-tracing window and 20 birds sampled per flock (a1 in Fig. S3).
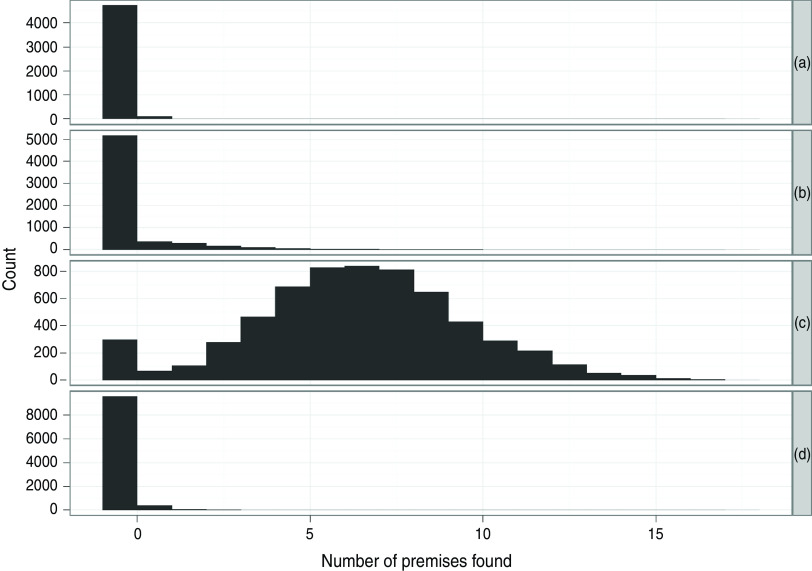
The distribution of the number of IPs identified by simulation of the annual AI poultry serosurvey in GB is shown in Figure 5 for each rate of between-holding transmission. For the majority of simulated LPNAI outbreaks seeded in GB duck flocks, the baseline rate and the one-tenth baseline rate of transmission had no holdings detected in the AI poultry serosurvey; this was because many of the outbreaks did not last sufficiently long for there to be IPs at the time the survey was carried out (every 365 days, counting from the first day of the simulation). In a few cases, the annual poultry survey did identify several IPs in the baseline transmission rate, and in these cases the poultry survey was predicted to assist in reducing the duration and size of the outbreak. For the tenfold increase in transmission rate, outbreaks were often of long duration and thus the annual poultry survey identified many IPs.
Fig. 5.
Distribution of the number of positive holdings identified in the simulated annual avian influenza poultry serosurvey during model simulations of LPNAI in the GB poultry flock for (a) one-fifth of baseline rate of transmission, (b) baseline rate of transmission, (c) fivefold baseline rate of transmission, (d) baseline rate of transmission but doubled rate of within-flock transmission.
DISCUSSION
This study has examined the potential outbreak size and duration of a simulated LPNAI outbreak seeded in a commercial duck holding in GB. It has shown that there is potential benefit in increasing the tracing window within which premises that might be the source of infection or that may have been infected by the index premises, in terms of reducing the likelihood of an outbreak of large size (in terms of IPs) and long duration. It has also shown there is comparatively little benefit of increasing the number of birds sampled per flock beyond that recommended in the AI Diagnostic Manual (20 per epidemiological group [6]).
Previous modelling studies on between-holding transmission of AI in GB have concentrated on HPNAI, estimating the likely outbreak size and evaluating control measures [1, 3, 4]. This study has instead focused on LPAI and the surveillance that is carried out once LPNAI has been detected, and assumed that confirmed IPs will be culled. Results indicate that increasing the back-tracing window could have an important impact on the mean outbreak size. This is probably due to the relatively long infectious period of ducks and geese, which results in premises being potentially infectious for up to 15 weeks, according to the output of the SEIR model using the assumed parameters for ducks (Table 1). The impact of increasing the number of birds sampled from 20 to 40 or 60 was much less marked, since the prevalence of infection was generally sufficiently high on sampled premises for detection; the virological sampling would be effective early after infection and the serological sampling likely to capture IPs in the tail of the within-flock outbreak or after birds have recovered.
There is considerable uncertainty in the rate of between-holding transmission, due to a lack of data with which to parameterize and validate the transmission models. The largest source of information regarding the exposure of poultry to LPAI virus infection arises from the annual AI poultry serosurvey. Even this is problematic to compare with the model estimates, since the number of poultry flocks identified in this survey with positive serology results indicative of prior exposure to infection could occur from several strains rather than being from exposure to one strain. It should be noted that since the first annual AI poultry survey was performed in 2002, no NAI outbreaks have been confirmed in the UK as a result of survey activities. Positive serological results have been identified from a small number of sampled poultry flocks each year. However, no evidence of circulating NAI virus has been detected on completion of follow-up laboratory investigations. Therefore, as circulating virus has not been detected, no tracings have been performed from the flocks identified during the course of the survey. Furthermore, as there is little indication of the timing of the infection from serological results, the incidence of seropositive farms cannot be determined. However, one would expect that if the order of magnitude of the rate of between-holding transmission in the model is the same as the true rate, then the distribution of the number of serologically positive farms detected by the poultry survey in the model should not generally exceed that observed in the poultry survey, except for a few outlying cases. This would suggest that the fivefold increase in the baseline rate was much higher than the true rate of between-holding transmission, and true rate of between-holding transmission could feasibly lie between the one-fifth baseline and the baseline rate of transmission.
Another area of uncertainty is the relative risk of each transmission route. These were obtained by expert opinion, and there is some basis to the relative ranking of these routes. However, the actual relative risk of each route is uncertain, and there could be, for example, a much greater difference in the relative risk of a slaughterhouse movement than the movement of ducklings than is currently assumed by the model. Results indicate that changes to individual rates of transmission could have a large impact on the model estimates of the outbreak size (Table 2), although not the conclusions of the model. Furthermore, there could be differences in the susceptibility of different types of premises. For example, levels of biosecurity might be correlated with the position of farms within the production chain, or correlated with farm size and whether they are part of a large integrated company. This could influence the type of farms which succumb to infection, and knowledge of this could lead to efficient targeted surveillance strategies. Without the occurrence of a large NAI outbreak in GB and details of the source of infection of each IP, it is very difficult to parameterize these models robustly.
The within-flock transmission element of the model assumed homogenous mixing between all birds of a given species within a holding. This is a simplification, since birds will be divided into flocks or houses, which will generally operate as separate epidemiological units within a holding. While it would be more realistic to include the information on the number of flocks of each species in a holding, there are two difficulties. First, data on the number of flocks of each species is not available. The GBPR does hold data on the number of flocks on each holding, but it is unclear how to divide flocks where there is more than one poultry species or production type on the holding, and the data could also be inaccurate or out of date. Second, there is no reported estimate on the rate of transmission of AI between flocks on a holding. The assumption of homogenous mixing means that the model cannot currently determine the actual number of samples required for each strategy. It is also likely to overestimate the rate at which all of the birds become infected in a holding, and underestimate the proportion of birds infected in the tail-end of an outbreak, since there is likely to be some delay between birds in different flocks from the index flock on a holding becoming infected, which is not currently included in the model. However, this will equally impact each strategy considered in the modelling, and thus is not likely to impact on the main results and conclusions of the model.
Little work has been done on modelling the time between detection and infection of LPNAI in poultry flocks, and previous studies have focused on detection triggers in commercial egg-laying chicken flocks: HPNAI [17] and H6N2 LPAI [18]. The H6N2 study [18] demonstrated that their early warning system, using mortality rates and loss of egg production relative to a baseline, would have detected H6N2 LPAI between 6–9 days post-infection, depending on the thresholds used, where the thresholds were based on deviation from a pre-set baseline, according to the expected mortality according to the age of the birds. However, this time to detection would vary between poultry production system, species and strain of AI virus. In the present study, we attempted to take into account likely species differences in the time of detection via passive surveillance, based on experience of LPAI outbreaks in GB, but this was based on a small number of outbreaks and it would be a useful area of further study.
There was a lack of published estimates of sensitivity and specificity of the virological and serological methods for detection of LPNAI, and the estimates of these used in the model remain uncertain. It is also possible that there is variability in the sensitivity of the PCR according to the nature of the virus and the epidemiological context in which the test is applied. For example, the data used to estimate the sensitivity of PCR in the present study was from a situation where PCR was applied to birds from a live bird market [13], which may have a different profile of virus excretion levels to an actively infected flock, and so it is possible that higher sensitivity of PCR may be found in many outbreak flocks. Interestingly, in contrast to Spackman et al. [13], a recent study [19] found a higher sensitivity of the M gene PCR than virus isolation, suggesting that it was able to detect low titres of virus and thus have relatively high sensitivity when applied in an outbreak situation. In contrast, when the M gene was applied in Vietnam to a virus with a different genetic profile to that usually found in Europe, the M gene performed relatively poorly, with only 58% sensitivity for ducks and 54% for chickens [20]. A recent study when applied to HPAI in the UK has found PCR to have a sensitivity of 82% (Dr M. Slomka, AHVLA, unpublished observations). However, although there is this potential variability and uncertainty in the sensitivity of PCR, the overall findings of the model were not sensitive to the assumption of test sensitivity, so it has a limited impact on the model.
We did not allow for the possibility of mutation of the LPNAI strain to HPNAI. This is problematic to model due to the unknown likelihood of mutation. Furthermore, LPNAI is not thought to mutate to HPNAI in anseriformes [21], which was the species predominantly infected in the simulation model. Mutation to HPNAI in a susceptible, non-anseriforme flock would result in earlier detection by passive surveillance due to higher rates of transmission and a clearer clinical presentation, including higher mortality rates. There would also be more action to control disease due to the larger restriction zones, and would therefore be likely to result in shorter outbreaks than those predicted in Figures 2 and 4.
Supplementary Material
Supplementary information supplied by authors.
ACKNOWLEDGEMENTS
This study was funded by the Department for the Environment, Farming and Rural Affairs, project SE0533.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812001483.
click here to view supplementary material
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Dent JE, et al. Contact structures in the poultry industry in Great Britain: Exploring transmission routes for a potential avian influenza virus epidemic. BioMed Central Veterinary Research 2008; 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dent JE, et al. The potential spread of highly pathogenic avian influenza virus via dynamic contacts between poultry premises in Great Britain. BioMed Central Veterinary Research 2011; 7: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharkey KJ, et al. Epidemiological consequences of an incursion of highly pathogenic avian influenza into the British poultry flock. Proceedings of the Royal Society of London, Series B: Biological Sciences 2007; 275: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truscott J, et al. Control of a highly pathogenic H5N1 avian influenza outbreak in the GB poultry flock. Proceedings of the Royal Society of London, Series B: Biological Sciences 2007; 274: 2287–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Council of the European Communities (CEC). Council directive 2005/94/EC of 20 December 2005 on Community measures on control of avian influenza and repealing 94/20/EEC. Official Journal of the European Commission 2006; L10: 16–65. [Google Scholar]
- 6.EU (European Union). Diagnostic manual for avian influenza. Official Journal of the European Union 2006; L237/1, 31 August 2006 (http://eur-lex.europa.eu/LexUriServ/site/en/oj/2006/l_237/l_23720060831en00010027.pdf). Accessed 23 November 2009. [Google Scholar]
- 7.Green DM, Kiss IZ, Kao RR. Modelling the initial spread of foot and mouth disease through livestock movements. Proceedings of the Royal Society of London, Series B: Biological Sciences 2006; 273: 2729–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao RR, et al. Disease dynamics over very different time-scales: foot-and-mouth disease and scrapie on the network of livestock movements in the UK. Journal of the Royal Society Interface 2007; 4:907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiss IZ, Green DM, Kao RR. The network of sheep movements within Great Britain: Network properties and their implications for infectious disease spread. Journal of the Royal Society Interface 2006; 3: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swayne DE, Halvorson DA. Influenza. In: Saif YM, ed. Diseases of Poultry, 12th edn. Ames, Iowa: Wiley-Blackwell, 2008, pp. 153–184. [Google Scholar]
- 11.Boender GJ, et al. The local threshold for geographical spread of infectious diseases between farms. Preventive Veterinary Medicine 2007; 82: 90–101. [DOI] [PubMed] [Google Scholar]
- 12.Branscum AJ, Gardner IA, Johnson WO. Estimation of diagnostic-test sensitivity and specificity through Bayesian modeling. Preventive Veterinary Medicine 2005; 68: 145–163. [DOI] [PubMed] [Google Scholar]
- 13.Spackman E, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology 2002; 40: 3256–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Goot JA, et al. Comparison of the transmission characteristics of low and high pathogenicity avian influenza (H5N2). Epidemiology and Infection 2003; 131: 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comin A, et al. Transmission dynamics of low pathogenic avian influenza in turkey flocks. PLoS ONE 2011; 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hénaux V, et al. Model-based evaluation of highly and low pathogenic avian influenza dynamics in wild birds. PLoS ONE 2010; 5: e10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savill NJ, St Rose SG, Woolhouse MEJ. Detection of mortality clusters associated with highly pathogenic avian influenza in poultry: a theoretical analysis. Journal of the Royal Society Interface 2008; 5: 1049–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltran-Alcrudo D, et al. A flock-tailored early warning system for low pathogenic avian influenza in commercial egg-laying flocks. Preventive Veterinary Medicine 2009; 92: 324–332. [DOI] [PubMed] [Google Scholar]
- 19.Slomka MJ, et al. Role of real time platform technology in the diagnosis and management of notifiable avian influenza outbreaks: experiences in Great Britain. Avian Diseases 2010; 54: 591–596. [DOI] [PubMed] [Google Scholar]
- 20.Slomka MJ, et al. Challenges for accurate and prompt molecular diagnosis of clades of highly pathogenic avian influenza H5N1 viruses emerging in Vietnam. Avian Pathology 2012; 41: 177–193. [DOI] [PubMed] [Google Scholar]
- 21.Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine 2007; 25: 5637–5644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information supplied by authors.
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268812001483.
click here to view supplementary material