SUMMARY
The transmission of human metapneumovirus (hMPV) among family members is not well understood. We identified 15 families in which multiple members were diagnosed with hMPV infection by real-time PCR in 2008 and 2010. Index patients ranged in age from 2 years to 11 years (median 5 years), and all 15 index cases were children who attended primary school, kindergarten, or nursery school. Contact patients ranged in age from 2 months to 46 years (median 6 years). Excluding five adult cases, contact patients were significantly younger than index patients (P = 0·0389). Of the 12 contact children, seven (58%) were infants who were taken care of at home. The serial interval between the onset of symptoms in an index patient and the onset of symptoms in a contact patient was estimated to be 5 days. These results suggest that the control of school-based outbreaks is important for preventing hMPV infection in infants.
Key words: Infectious disease epidemiology, respiratory infections
INTRODUCTION
Human metapneumovirus (hMPV), first described in 2001, is a pathogen that is associated with respiratory infections. It has been classified as a member of the genus Metapneumovirus of the subfamily Pneumovirinae of the family Paramyxoviridae [1]. Based on genetic and phylogenetic analyses, hMPV can be separated into two subgroups, A and B, with each subgroup divided into two genotypes (i.e. A1 and A2, B1 and B2) [2]. This virus circulates predominately in late winter and spring, and several different hMPV genotypes co-circulate in the community [3–5]. For example, in the community of Yamagata, Japan, A2 and B2 are the major types in endemic circulation almost every year [5].
Human MPV is a common cause of respiratory infections worldwide, particularly in infants and young children, and it leads to conditions ranging from upper respiratory infections to severe lower respiratory infections, such as bronchitis, bronchiolitis, and pneumonia [1, 6, 7]. Serological studies have revealed that most individuals have been exposed to hMPV by the age of 5 years, and re-infections can occur in all age groups [1, 4, 8]. Outbreaks of this virus have been reported in long-term care facilities, hospitals, primary schools, and nurseries [9–11]. In infants, hMPV infection is most likely to be acquired from a family member. However, there have been no reports about the household transmission of hMPV. In this study, we identified 15 families in which multiple members had hMPV infection confirmed by molecular techniques in 2008 and 2010 and retrospectively investigated the hMPV infection in members of the same families.
METHODS
During the periods of January–April 2008 and January–May 2010, a total of 675 nasopharyngeal swab specimens were collected from patients with acute respiratory infection (ARI) at the Yamanobe Pediatric Clinic collaborating with the local health authority of the Yamagata Prefecture for the surveillance of viral diseases in Japan. Informed consent was obtained from the participating patients or their guardians. Specimens were transported to the Yamagata Prefectural Institute of Public Health and grown in a virus culture as previously described [5], and were further tested for hMPV by real-time PCR. HMPV-positive patients whose specimens were confirmed by real-time PCR were included in this study. Clinical information for patients who tested positive for hMPV was retrospectively obtained from their medical records. Statistical analysis was performed using StatView J-4.02 (Abacus Concepts, USA). The Mann–Whitney U test was used to compare median values. P values of <0·05 were regarded as statistically significant.
For real-time PCR, viral RNA was extracted from 200 μl specimens using the High Pure Viral RNA kit (Roche Diagnostics, Germany) according to the manufacturer's instructions and subsequently transcribed into cDNA using random primers. The resulting cDNA was used for real-time PCR with a TaqMan probe targeting the hMPV N gene, as previously described [12].
For sequencing analysis, the transcribed cDNA was subjected to PCR amplification of the fusion region, which was performed under the following conditions: 30 s at 94 °C; 35 cycles of 94 °C for 30 s, 53 °C for 30 s, and 72 °C for 2 min, with a final extension at 72 °C for 9 min. The primer pair hMPVM + 724 (5′-TGGAGYCAYCAAGGRACAAG-3′) and hMPVM2-92 (5′-GGCCAACTCCAGTAATTGTG-3′) was used to amplify the fusion region, which includes the full-length F gene (1620 bp). The PCR products were purified with a QIAquick PCR purification kit (Qiagen, Germany) and sequenced using a Big Dye Terminator v. 1.1 cycle sequencing kit (Applied Biosystems, USA) on an Applied Biosystems 3130 automatic sequencer. The nucleotide sequences of the primers used for sequencing were as follows: hMPVF + 75 (5′-AGARTCATGYAGYACYATAAC-3′), hMPVF + 574 (5′-AGCTTCAGTCAATTCAACAG-3′), hMPVF + 1081 (5′-TGCAAAGTYAGCACAGGAAG-3′), and hMPVF-302 (5′-GATTGTCTGGGATTYYCAATTTG-3′). Sequence data were analysed with CLUSTAL W version 1.83, and a phylogenetic tree was constructed by the neighbour-joining method using the same software. The nucleotide sequences determined in this study were deposited in GenBank under the accession numbers AB618745–AB618755, AB618758–AB618778 and AB693954–AB693960.
RESULTS
Real-time PCR for hMPV was positive in 141 of the 675 respiratory specimens. An analysis of these samples revealed 15 families in which two or more members had hMPV infection. The first hMPV-positive patient in a family was defined as the index case, and the second or third hMPV-positive patient in the same family was defined as a contact case. In 15 families, 15 index cases and 17 contact cases were identified. The demographic characteristics of the index and contact patients are shown in Table 1. Although one patient (F3, contact 1) was undergoing kidney dialysis due to chronic renal failure, no other subject had any immunosuppressive illness or other underlying disease.
Table 1.
Demographic characteristics of patients with human metapneumovirus infection
| Family | Subject | Age | Sex | Diagnosis | Situation | Strain name |
|---|---|---|---|---|---|---|
| F1 | Index | 6 yr | Male | URI | Primary school | 187-Yamagata-08 |
| Contact | 8 yr | Male | URI* | Primary school | 236-Yamagata-08 | |
| F2 | Index | 5 yr | Male | URI | Kindergarten | 234-Yamagata-08 |
| Contact | 34 yr | Female | URI* | Index's mother | 331-Yamagata-08 | |
| F3 | Index | 11 yr | Female | URI | Primary school | 428-Yamagata-08 |
| Contact 1 | 46 yr | Male | URI | Index's father | 495-Yamagata-08 | |
| Contact 2 | 41 yr | Female | URI | Index's mother | 484-Yamagata-08 | |
| F4 | Index | 2 yr | Male | Bronchitis | Nursery school | 489-Yamagata-08 |
| Contact | 31 yr | Female | URI | Index's mother | 560-Yamagata-08 | |
| F5 | Index | 5 yr | Female | URI | Kindergarten | 497-Yamagata-08 |
| Contact | 7 yr | Male | URI | Primary school | 562-Yamagata-08 | |
| F6 | Index | 3 yr | Male | URI | Nursery school | 535-Yamagata-10 |
| Contact | 2 mo. | Male | URI* | Taken care of at home | 618-Yamagata-10 | |
| F7 | Index | 10 yr | Female | URI | Primary school | 543-Yamagata-10 |
| Contact | 7 yr | Male | URI* | Primary school | 636-Yamagata-10 | |
| F8 | Index | 5 yr | Male | URI | Kindergarten | 553-Yamagata-10 |
| Contact | 1 yr | Female | Bronchiolitis | Taken care of at home | 626-Yamagata-10 | |
| F9 | Index | 4 yr | Male | Bronchitis | Kindergarten | 640-Yamagata-10 |
| Contact | 39 yr | Female | URI* | Index's mother | 748-Yamagata-10 | |
| F10 | Index | 6 yr | Female | URI | Kindergarten | 629-Yamagata-10 |
| Contact | 2 mo. | Female | URI* | Taken care of at home | 783-Yamagata-10 | |
| F11 | Index | 10 yr | Male | URI | Primary school | 630-Yamagata-10 |
| Contact | 2 yr | Female | URI* | Taken care of at home | 764-Yamagata-10 | |
| F12 | Index | 8 yr | Female | URI | Primary school | 651-Yamagata-10 |
| Contact 1 | 5 yr | Female | URI | Kindergarten | 737-Yamagata-10 | |
| Contact 2 | 1 yr | Female | URI | Taken care of at home | 738-Yamagata-10 | |
| F13 | Index | 5 yr | Male | URI | Kindergarten | 776-Yamagata-10 |
| Contact | 2 yr | Male | URI | Taken care of at home | 858-Yamagata-10 | |
| F14 | Index | 5 yr | Male | Bronchitis | Kindergarten | 869-Yamagata-10 |
| Contact | 6 mo. | Female | Bronchitis | Taken care of at home | 923-Yamagata-10 | |
| F15 | Index | 7 yr | Male | URI | Primary school | 1119-Yamagata-10 |
| Contact 1 | 6 yr | Male | URI | Primary school | 1158-Yamagata-10 |
URI, Upper respiratory infection.
Indicates that the onset symptom is cough.
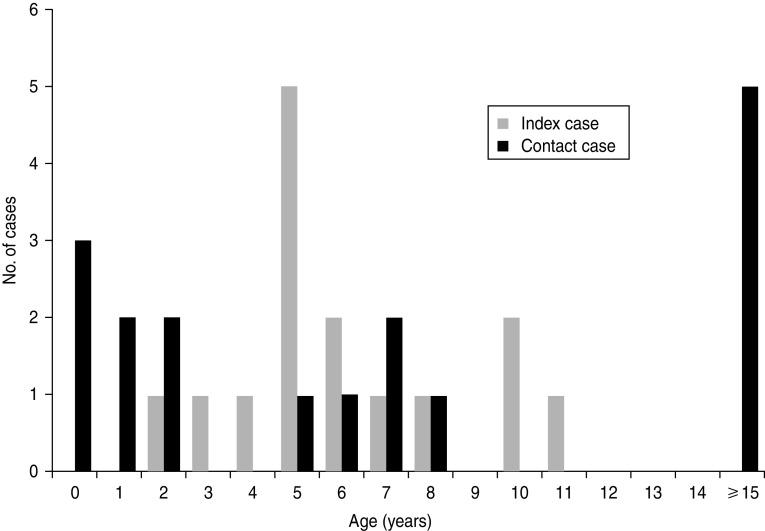
The index cases ranged in age from 2 years to 11 years (median 5 years) (Fig. 1); 10 (67%) of the index patients were male. All 15 index patients were children who attended primary school, kindergarten, or nursery school. The contact cases ranged in age from 2 months to 46 years (median 6 years); seven (41%) of the contact patients were male. Of the 17 contact patients, five were parents (aged 31–46 years), two were older siblings and 10 were younger siblings. Excluding the adult cases, seven (58%) of 12 contact cases were infants who were taken care of at home. The median age of the paediatric contact patients was 2 years (range 2 months to 8 years). Statistical analysis revealed that the contact patients, excluding the adult cases, were significantly younger than the index patients (P = 0·0389).
Fig. 1.
Age distribution of human metapneumovirus-positive index cases and contact cases.
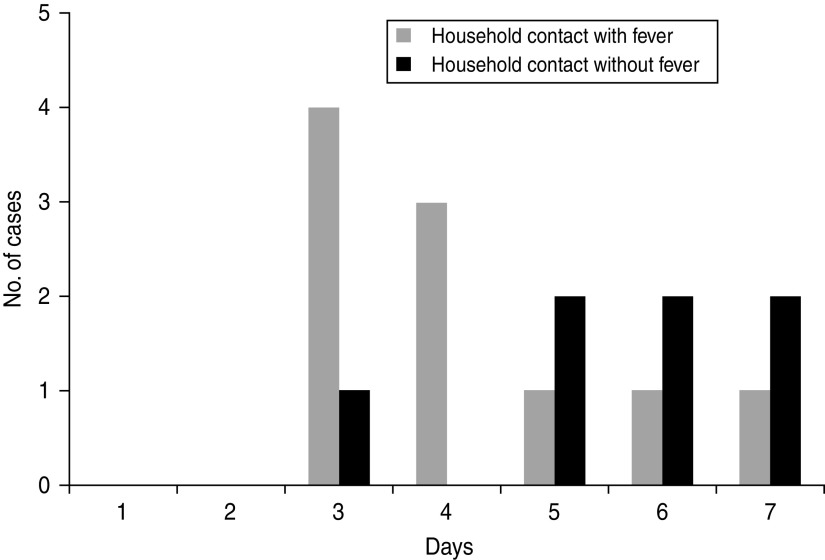
Of the 32 subjects, 27 (84%) were diagnosed with upper respiratory infection (URI). Bronchiolitis and bronchitis were diagnosed in one and four children, respectively. Fever (body temperature >38 °C) was noted in all of the index patients and in 10 of the 17 contact patients. Seven of the contact patients had no fever during the period of illness, and their onset symptom was cough. Of these patients, two were 2-month-old infants (contact patients of F6 and F10), and two were adults (contact patients of F2 and F9). The symptom onset interval was calculated as the number of days from the onset of fever in the index patient to the onset of fever (or cough, if fever was not present) in the household contact. This interval ranged from 3 days to 7 days (median 5 days) (Fig. 2). When the seven cases for which the onset symptom was cough were excluded from the analysis, the median interval was 4 days (range 3–7 days).
Fig. 2.
Interval from symptom onset in index cases and household contacts with or without fever.
As shown in Figure 3, phylogenetic analysis revealed that hMPV recovered from members of the same family in 2008 belonged to A2 (F1, F2, F4, F5) or B2 (F3) lineage and all isolates recovered in 2010 belonged into B2 (F6–F15) lineage. The nucleotide sequences of the hMPV isolates recovered within each family were completely identical to the 1620 bp of the F gene. A comparison of the interfamily sequences showed that the F gene sequences of hMPV from F2, F3, F6 and F9 differed from those of the viruses recovered from other families by at least one nucleotide. Furthermore, compared to the F gene of hMPV circulating within this community, they differed from those of viruses recovered from families identified in the same season by 0–21 bp.
Fig. 3.
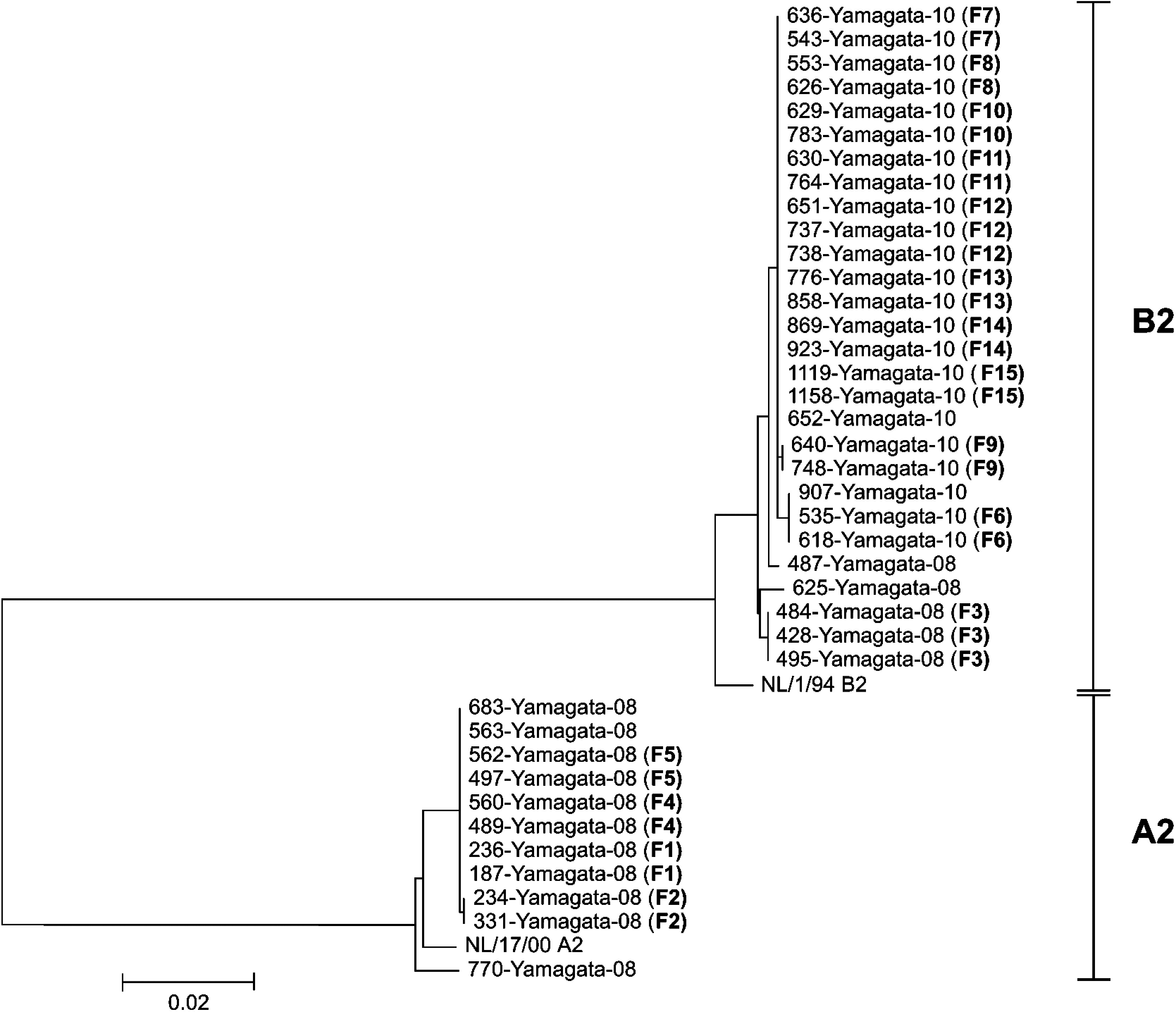
Comparison of the nucleotide sequences of the full-length F gene (1620 bp) of human metapneumovirus recovered from the members of the 15 studied families and other residents of Yamagata, Japan. Family identification numbers are shown in parentheses.
DISCUSSION
The present study shows that the nucleotide sequences of hMPV isolates recovered within each family were identical. Because viruses with identical nucleotide sequences were circulating elsewhere in this community, it is possible that in some families, the virus was acquired from a community contact rather than a family contact. However, the short intervals between symptom onset in index patients and contact patients suggest that secondary transmission most likely occurred within families.
Several studies have revealed that children aged <5 years are most susceptible to hMPV infection [3, 4, 6]. All of the index patients were children who attended primary school, kindergarten, or nursery school, and 58% of the contact children were infants who were taken care of at home. Thus, preschool- or school-aged children appeared most likely to introduce hMPV into the family, and infants are likely to acquire hMPV infection from their older siblings.
Knowledge of the incubation period, which is the time between infection and symptom onset, is useful for infectious disease control [13]. The incubation periods of several infectious diseases, such as influenza virus and respiratory syncytial virus, have been estimated by studying the experimental infection of volunteers or based on the observational study of secondary infections in families or facilities [13–15]. However, there have been only a few reports about the incubation period of hMPV infection. Based on a single case of nosocomial infection, two reports have suggested incubation periods of 5–6 days and 4–6 days [16, 17]. In another case of a paediatric haemato-oncology patient, the incubation period was estimated to be 7–9 days [10]. In this study, the serial interval for household transmission of hMPV was calculated to be 4–5 days (range 3–7 days). Our result is comparable to the incubation period of previous reports. However, in cases of transmission within families, the serial interval could not be considered to be the exact incubation period for a secondary case because it is possible that the contact patient was infected before or after the onset of symptoms in the associated index patient. In a previous study [18], we suggested that virus shedding in children with hMPV decreases by 4 days after the onset of fever. Therefore, it is likely that household contact between family members and the infected individual within 1–3 days after the onset of fever in the index patient is responsible for virus transmission.
One limitation of this study is the study design. The contact cases were retrospectively identified in patients diagnosed with hMPV infection by a molecular method in our surveillance work. Therefore, the total frequency of hMPV infection in household contacts of hMPV-positive index patients could not be estimated. There were five cases of transmission from the index child to the parent. Of these adult contacts, two had no fever during the period of illness. Because hMPV infection is associated with very mild symptoms of ARI in adults, they may not visit the clinic. If the index patient is confirmed as hMPV positive in a clinical setting, all household contacts should be prospectively investigated further. Infections in adults and the elderly are probably underreported [7]; in this group there may be more cases of hMPV infection acquired from a family member.
In conclusion, infants are likely to acquire hMPV infections from their older siblings. The control of hMPV outbreaks in nursery schools, kindergartens, and primary schools may be an important strategy for preventing hMPV infection in infants.
ACKNOWLEDGMENTS
This work was supported in part by a grant-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology. We thank Yoko Kadowaki for her assistance in the sequence analysis.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.van den Hoogen BG, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nature Medicine 2001; 7: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Hoogen BG, et al. Antigenic and genetic variability of human metapneumoviruses. Emerging Infectious Diseases 2004; 10: 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JV, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. Journal of Infectious Diseases 2006; 193: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuzaki Y, et al. Clinical impact of human metapneumovirus genotypes and genotype-specific seroprevalence in Yamagata, Japan. Journal of Medical Virology 2008; 80: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 5.Mizuta K, et al. Endemicity of human metapneumovirus subgenogroups A2 and B2 in Yamagata, Japan, between 2004 and 2009. Microbiology and Immunology 2010; 54: 634–638. [DOI] [PubMed] [Google Scholar]
- 6.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatric Infectious Disease Journal 2004; 23 (Suppl. 1): S25–32. [DOI] [PubMed] [Google Scholar]
- 7.Schildgen V, et al. Human Metapneumovirus: lessons learned over the first decade. Clinical Microbiology Reviews 2011; 24: 734–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto M, et al. Longitudinal course of human metapneumovirus antibody titers and reinfection in healthy adults. Journal of Medical Virology 2010; 82: 2092–2096. [DOI] [PubMed] [Google Scholar]
- 9.Louie JK, et al. A summer outbreak of human metapneumovirus infection in a long-term-care facility. Journal of Infectious Diseases 2007; 196: 705–708. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, et al. Molecular epidemiological investigation of a nosocomial outbreak of human metapneumovirus infection in a pediatric hemato-oncology patient population. Journal of Clinical Microbiology 2009; 47: 1221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abiko C, et al. Outbreak of human metapneumovirus detected by use of the Vero E6 cell line in isolates collected in Yamagata, Japan, in 2004 and 2005. Journal of Clinical Microbiology 2007; 45: 1912–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki Y, et al. Evaluation of a new rapid antigen test using immunochromatography for detection of human metapneumovirus in comparison with real-time PCR assay. Journal of Clinical Microbiology 2009; 47: 2981–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lessler J, et al. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infectious Diseases 2009; 9: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foy HM, et al. Influenza B in households: virus shedding without symptoms or antibody response. American Journal of Epidemiology 1987; 126: 506–515. [DOI] [PubMed] [Google Scholar]
- 15.Hall CB, Douglas RG Jr, Geiman JM. Quantitative shedding patterns of respiratory syncytial virus in infants. Journal of Infectious Diseases 1975; 132: 151–156. [DOI] [PubMed] [Google Scholar]
- 16.Peiris JS, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerging Infectious Diseases 2003; 9: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebihara T, et al. Human metapneumovirus infection in Japanese children. Journal of Clinical Microbiology 2004; 42: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzaki Y, et al. Comparison of virus isolation using the Vero E6 cell line with real-time RT-PCR assay for the detection of human metapneumovirus. BMC Infectious Diseases 2010; 10: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]