SUMMARY
This study aimed to confirm that vertical transmission of hepatitis B virus (HBV) can occur via the infected ovum. Specimens studied were obtained from discarded test-tube embryos from mothers with chronic HBV infection who had received in vitro fertilization treatment. Single-cell reverse transcriptase–polymerase chain reaction was used to detect HBV mRNA in the embryos. HBV mRNA was detected in the cleavage embryos of patients with chronic HBV infection, with a detection rate of 13·2% (5/38). The level of serum HBV DNA was not related to the HBV mRNA positivity rates in embryos. In this study, HBV mRNA was detected in test-tube embryos from HBV-infected mothers who had received in vitro fertilization treatment. This confirms the theory of vertical transmission of HBV via the ovum, thereby providing an important theoretical basis for further study on the mechanism of HBV vertical transmission, influencing factors and blocking measures.
Key words: Embryo, hepatitis B, ovum, vertical transmission
INTRODUCTION
Hepatitis B virus (HBV) can infect and replicate within the ovum [1], but vertical transmission of HBV via the ovum is a theory that has not been fully confirmed. To fully confirm this means of transmission involves resolving a number of questions, e.g. whether an HBV-infected ovum can be fertilized, whether an HBV-infected ovum can transfer HBV to the embryo, and whether HBV can continue to replicate in the embryo thereby facilitating clinically significant vertical transmission of HBV. A number of difficult technical issues are involved, e.g. in vitro culture of the ovum, in vitro infection of the ovum, in vitro fertilization (IVF) of infected ova, etc., there are even some ethical issues involved. Therefore, it is difficult to confirm vertical transmission of HBV via the ovum with conventional research methods. In order to confirm this theory, we sought to detect HBV mRNA in a specimen of discarded test-tube embryos from HBV-infected mothers who had received IVF treatment. If we could detect HBV mRNA in a discarded embryo (the provider of sperm had no HBV infection, and the IVF and embryo culture process was conducted under strict conditions), the following points could be confirmed: (1) the ovum of a HBV-infected mother was infected with HBV and had replication activity; (2) this ovum can be fertilized successfully and transmit HBV to the embryo; (3) HBV in the embryo has replication activity. The above three points fully confirm that HBV is able to infect the ovum and that clinically significant vertical transmission of HBV has occurred.
SUBJECTS AND METHODS
Subjects
Thirty-eight discarded single-cell cleavage embryos were obtained from 14 couples who had received assisted reproductive technology treatment at the Reproductive Centre of Shaanxi Maternal and Child Care Service Centre from February 2007 to December 2008. Of these 14 couples, all the women had chronic HBV infection and the men had no infection. In the same period, 84 discarded single-cell cleavage embryos were obtained from 50 IVF couples with negative HBV markers for both men and women. These were taken as negative controls. Consent was obtained from the patients and the hospital ethics committee before the embryos were obtained, in accordance with the Declaration of Helsinki.
Ten millilitres of venous blood were collected from each patient before surgery and this was used for the quantitative detection of serum HBV DNA and the detection of hepatitis B serum markers. Meanwhile, HAV, HCV, HDV, and HIV antibodies were tested for in the serum of both husband and wife to rule out these viral infections.
In the ovum retrieval process, blood contamination was avoided as far as possible and a thorough cleaning was conducted; HBV serum markers and HBV DNA were detected as negative in the final wash fluid.
Methods
Single-cell reverse transcriptase–polymerase chain reaction (RT–PCR) was the major test technique in this experiment.
Cell lysis
The discarded single-cell cleavage embryos were washed eight times with PBS/DEPC and then placed in sub-packaged lysate for 2 h of lysis. The final wash fluid was retained for the detection of HBV DNA, and the specimens with negative HBV DNA in the final wash were used for subsequent experiments [2, 3].
Reverse transcription of RNA into cDNA
The reverse transcription reaction was conducted using a reverse transcription kit (Superscript III First-Strand Synthesis; Invitrogen, USA) according to the manufacturer's instructions, and the whole reaction was performed on ice. Denaturation: the reaction system is given in Table 1. The reaction mixture was mixed and centrifuged at 12 000 g for 3–5 s. Then after the mixture was incubated at 65 °C for 5 min, it was quickly placed on ice for at least 1 min and centrifuged at 12 000 g for 3–5 s. Extension: cDNA was synthesized. The reagents shown in Table 1 were added into the mixture one by one and gently shaken. When the total volume of the reaction system was 20 μl, it was centrifuged at 12 000 g for 3–5 s, followed by a water bath at 25 °C for 10 min, and a further water bath at 50 °C for 50 min. Termination: the mixture was incubated at 85 °C for 5 min, and cooled on ice to terminate the reaction. After instantaneous centrifugation, 1 μl RNase H was added to the mixture which was kept at 37 °C for 20 min to decompose untranscribed RNA and then stored at −20 °C or used immediately.
Table 1.
Reverse transcription reagents
| Reagents | Volume |
|---|---|
| 10× buffer | 2 μl |
| 25 mm MgCl2 | 4 μl |
| 0·1 m DTT | 2 μl |
| RNase out | 1 μl |
| Super III RT | 1 μl |
Amplification of housekeeping genes
Primer sequences and characteristics: the primers (Table 2) of housekeeping gene β-actin were designed with Oligo 6.0 biological software [4].
Table 2.
Primer sequences and characteristics
| β-actin | Primers' sequences (5′–3′) (bp) | Amplification length |
|---|---|---|
| P1 | 5′-ATCGTGCGTGACATTAAGGAGAAG-3′ | 179 bp |
| P2 | 5′-AGGAAGGAAGGCTGGAAGAGTG-3′ |
Amplification of HBV DNA by nested PCR
Primer sequences and characteristics: a pair of primers was designed according to mRNA gene sequences of the S area of HBV using Oligo 6·0 biological software (Table 3) [5]. The primers were P1, P3 (upstream) and P2, P4 (downstream).
Table 3.
Primer sequences and characteristics
| HBV S area | Nucleic acid position | Primer sequences (5′–3′) (bp) | Amplification length | |
|---|---|---|---|---|
| Outer primers | P1 | 300–321 | 5′-CATCTTCTTGTTGGTTCTTCTG-3′ | 417 bp |
| P2 | 715–695 | 5′-TTAGGGTTTAAATGTATACCC-3′ | ||
| Inner primers | P3 | 421–441 | 5′-TCTATGTTTCCCTCTTGTTGC-3′ | 206 bp |
| P4 | 626–605 | 5′-TACCACATCATCCATATAACTG-3′ |
Electrophoresis of PCR end products on 1·5% agarose gel
The prepared 1·5% agarose gel was heated and melted in a microwave oven and after cooling to 60 °C, it was poured into a gel mould with a preset electrophoresis comb. After 30–45 min at room temperature, the electrophoresis comb was removed and the gel was placed into an electrophoresis tank and 1× TBE electrophoresis buffer added. A 10-μl real-time quantitative PCR product of each gene was taken from each group, loading buffer was then added in order to display DNA and make the sample sink to the bottom. Voltage electrophoresis at 80 V was conducted for 35 min and electrophoresis fragments were observed under ultraviolet light. An imaging system (Bio-Rad, USA) was employed for scanning and the images were printed.
Controls
Negative controls: (a) the discarded embryos from the couples with negative HBV serum markers for both man and woman; (b) no template was added in the reverse transcription process; (c) no reverse transcriptase was added in the reverse transcription process; (d) no template was added in the PCR reaction system.
Positive control: HBV patients with positive serum DNA.
Follow-up visits
The infants with HBV-infected mothers were followed up every 3 months. The infants' peripheral blood was obtained for the detection of HBV serum markers. The follow-up visits lasted for 12 months.
RESULTS
RT–PCR results
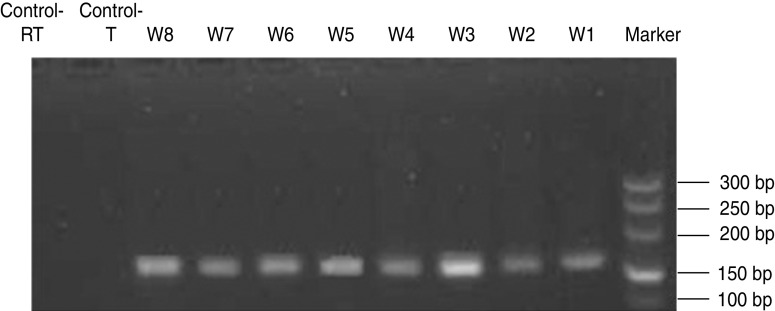
All single-cell cleavage embryos were placed in lysate and reverse transcription was performed to generate cDNA in the same Eppendorf tube. The housekeeping gene β-actin was amplified by PCR with an amplified fragment length of 179 bp. Except for the negative controls, the target fragments were amplified in every tube (Fig. 1).
Fig. 1.
Electrophoresis of β-actin. With the exception of negative control the target fragments were amplified in each tube. The length of the amplified fragment was 179 bp.
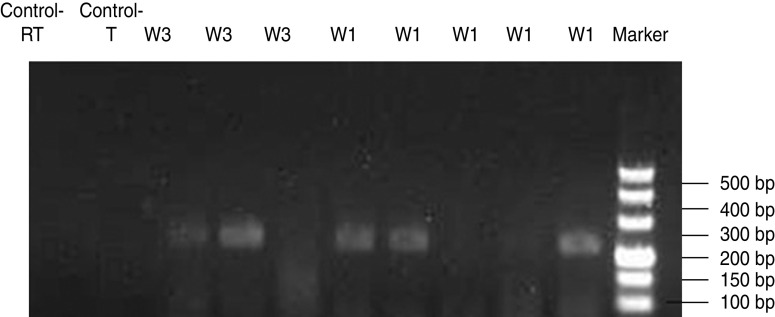
The target fragments were amplified by nested PCR and the products were 206 bp in the second round (Fig. 2). The positive rate of cleavage embryos was 13·2% (5/38). These five positive embryos were from two cases of the 14 couples; in one case, three positive signals were detected in five embryos with serum HBeAg positive and HBV DNA quantification at 4·7×105 IU/ml; in the other case, two positive signals were detected in the three cleavage embryos with serum HBeAg negative and HBV DNA quantification ⩽103 IU/ml. No specific HBV mRNA signal was detected in the embryos of the negative controls.
Fig. 2.
Electrophoresis of HBV mRNA PCR products in a single cleavage embryo. The target fragments were amplified by nested PCR and the product was 206 bp. No specific HBV mRNA signal was detected in the embryos of the negative control. These five positive embryos were from two cases of the 14 couples (W1 and W3).
The target fragments were amplified by nested PCR, and there was no amplified fragment in the first round, the product was 206 bp in the second round. In the HBV-positive mother group, for one case three positive signals were detected in five embryos and in the other case two positive signals were detected in three cleavage embryos.
Follow-up results
After follow-up visits, it was found that of the two pregnant women from the couples with the mother infected with hepatitis B and HBV RNA detected in cleavage embryos, one woman was successfully pregnant and hepatitis B was negative in this woman's infant at both birth and at age 6 months.
DISCUSSION
HBV is capable of infecting oocytes [1] and replicating therein. On this basis, the hypothesis that vertical transmission of HBV occurs via the ovum is put forward. Furthermore, some scholars have transfected the HBV gene into a mouse ovum which was then fertilized with normal sperm, or directly transfected the HBV gene into a fertilized ovum. In these mice not only were integrated HBV DNA fragments [6, 7] found, there was also evidence of HBV replication and expression [8], and even 3·5 kb mRNA and a small amount of double-stranded DNA packaged in Dane particles was found [9, 10]. These studies show that after transfection, HBV could enter the embryos via the animal's ovum and replicate in them, and could even mature in the mother's body and generate new individuals. However, HBV has strict species specificity, and a large gap exists between the transgene process and natural infection; therefore there is a great difference between the above research and human HBV vertical transmission via the ovum in the natural process. Human oocytes are not readily available, therefore research on confirmation of vertical transmission of HBV via the ovum is very slow.
In this study, HBV mRNA was detected for the first time in early human embryos, which suggests that HBV in these embryos has a certain replication activity and is capable of being transcribed and translated under certain conditions. All the patients in the study were receiving IVF treatment and blood contamination was avoided as far as possible in the ovum retrieval process and a thorough cleaning was conducted; both HBV serum markers and HBV DNA were detected as negative in the final wash fluid. The sperm providers were not HBV-infected individuals, and the IVF process was conducted under very strict conditions where no contamination factors existed. Therefore, the presence of HBV in the embryos could only have come from the ovum and the HBV that infected the ovum was able to enter the embryos during the process of ovum fertilization and then replicate actively within; vertical transmission of HBV then occurs via the ovum in the real sense.
The infant of the only successfully pregnant mother with HBV mRNA detected in the embryo was followed up, and no HBV evidence was detected in the infant's serum at birth or at age 6 months. This result is consistent with previous experimental results on HBV-infected ova, which suggests that not all ova of patients with chronic HBV infection can be infected with HBV. Moreover, it was also found in this study that only part of the cleavage embryo of the same patient with chronic HBV infection was infected with HBV, which further confirms the above result.
This study found that HBV patients' embryos infected with HBV was not correlated with viral load, which indicates that transmission of HBV via the ovum is not correlated with quantitative level of virus. At present, a blocking measure, i.e. lowering the serum HBV viral load of HBV patients, is not effective in this transmission via the ovum. The mechanism may be related to the person carrying virus, which needs further study.
In this study, HBV mRNA was detected in discarded test-tube embryos from HBV-infected mothers after IVF treatment, which confirms clearly for the first time the theory of vertical transmission of HBV via the ovum. This provides an important theoretical basis for further study on the mechanism of vertical transmission of HBV, influencing factors and blocking measures.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China, No. 30371277.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Zhou SL, Zhao LS, Li F. HBV DNA was detected in ovum of the patients with chronic hepatitis B virus infection. Journal of Heredity Disease 1989; 6: 46–48. [Google Scholar]
- 2.Kathryne MK, et al. Laser capture microdissection and single-cell RT-PCR without RNA purification. Journal of Immunological Methods 2005; 302: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu B, et al. Single cell RT-PCR demonstrates differential expression of GABAC receptor U subunits in rat hippocampal pyramidal and granule cells. Molecular Brain Research 2004; 123: 1–6. [DOI] [PubMed] [Google Scholar]
- 4.Barone M, et al. Gene expression analysis in HBV transgenic mouse liver: a model to study early events related to hepatocarcinogenesis. Molecular Medicine 2006; 12: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabrerizo M, et al. Molecular analysis of hepatitis B virus DNA in serum and peripheral blood mononuclear cells from hepatitis B surface antigen-negative cases. Hepatology 2000; 32: 116–123. [DOI] [PubMed] [Google Scholar]
- 6.Chan AW, et al. Transgenic cattle produced by reverse transcribed gene transfer in oocytes. Proceedings of the National Academy of Sciences USA 1998; 95: 14028–14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C. The construction of HBV DNA oocyte vector and the detection of HBV DNA in the hepatitis B patients' ovaries. Qing Dao University, 2006. [Google Scholar]
- 8.Anne SM. Improving gene transfer into live tock. Science 1998; 282: 1619–1620. [DOI] [PubMed] [Google Scholar]
- 9.Bagis H, Arat S, Merean HO. Stable transmission and expression of the hepatitis B virus total genome in hybrid transgenie mice until F10 generation. Journal of Experimental Zoology, Part A: Comparative Experimental Biology 2006; 305: 420–427. [DOI] [PubMed] [Google Scholar]
- 10.Chen GL, et al. Replication and expression of oocyte-mediated HBV DNA in early mouse embryo. Carcinogenesis, Teratogenesis & Mutagenesis 2005; 17: 179–182. [Google Scholar]