SUMMARY
A population-based anti-hepatitis C virus (HCV) prevalence is important for surveillance purposes and it provides insight into the burden of disease. The outcomes of recent studies in the general Dutch population as well as recent HCV data from specific risk groups including migrants, men who have sex with men (MSM) and injecting drug users (IDUs), were implemented in a modified version of the Workbook Method (a spreadsheet originally designed for HIV estimations), to estimate Dutch HCV seroprevalence. The estimated national seroprevalence of HCV was 0·22% (min 0·07%, max 0·37%), corresponding to 28 100 (min n = 9600, max n = 48 000) HCV-infected individuals in The Netherlands. Of these, first-generation migrants from HCV-endemic countries (HCV prevalence ⩾2%) accounted for the largest HCV-infected group, followed by IDUs and HIV-positive MSM.
Key words: HCV seroprevalence, hepatitis C, national estimation
INTRODUCTION
The World Health Organization (WHO) estimated that 3% of the world's population has been infected with the hepatitis C virus (HCV). Globally an estimated 130–170 million people are chronically infected, including about nine million in the WHO European region [1]. The estimated HCV prevalence in Europe ranges from 0·1% to 6·0%, with the highest prevalence rates (>1·2%) in Southern and Eastern European countries [2, 3]. Up-to-date knowledge on the general population prevalence of HCV in The Netherlands is limited. A Dutch cross-sectional serosurvey in 1995–1996 (PIENTER-1 study) estimated a national prevalence of 0·1% [4]. However, groups with higher risk profiles like individuals of non-Dutch nationality were underrepresented in this study, resulting in a possible underestimation of HCV prevalence. In a second serosurvey in 2006–2007 (PIENTER-2 study) more individuals of non-Dutch nationality were included leading to an estimated prevalence of 0·3% [5]. Two recent population-based studies in the two largest cities, Amsterdam and Rotterdam, where a substantial proportion of the migrant population resides, found prevalences of 0·6 and 1·1%, respectively [6, 7]. As expected, the prevalence found in specific risk groups, e.g. injecting drug users (IDUs), (HIV-positive) men who have sex with men (MSM), and haemophilia and dialysis patients, is much higher (2·6–82·2%) [8–14].
In the literature, mathematical modelling has been used to estimate national HCV prevalence rates. An overall HCV prevalence of 0·4% was modelled in The Netherlands in 2004 using suboptimal data of HCV risk groups [15]. In the UK, a Bayesian approach was used which was based on three main groups: current IDUs, ex-IDUs and non-IDUs, resulting in an overall prevalence of 0·6% (0·4–1·0%) [16]. Australia included IDUs, migrants and other risk groups (including transmission by transfusion of blood or blood products) and estimated 264 000 HCV-infected persons at the end of 2005 [17]. Considering a population size of 20 409 100 (Year Book Australia, 2008) this would correspond to a prevalence of about 1·3%. Canada, distinguishing five different risk groups: migrants, IDUs, blood transfusion patients, haemophilia patients and others, estimated the HCV prevalence at 0·8% in 2007 [18].
Since the HCV epidemiology in The Netherlands has changed with (HIV-positive) MSM now accounting for most new infections, and lower numbers of HCV infections are observed in former high-risk groups like haemophilia patients and IDUs, there is a need for a new HCV estimation [8, 14, 19, 20]. Recent studies in the general population and specific (risk) groups, gave us the unique opportunity to estimate the national HCV seroprevalence.
MATERIALS AND METHODS
Workbook method
The Workbook Method was used to estimate the national HCV seroprevalence. The Workbook Method is an Excel spreadsheet designed to estimate adult HIV prevalence, it was also recently used to estimate HIV prevalence in The Netherlands [21]. This method was developed by the Joint United Nations programme on HIV/AIDS (UNAIDS) and the WHO, with support from the UNAIDS Reference Group on Estimates, Modelling and Projections [22, 24]. Since the method is independent of mode of transmission, it is applicable for similar concentrated infectious diseases like HCV, whose epidemic spread in The Netherlands is, as for HIV, restricted to specific high-risk populations. The method is based on HCV prevalence in populations with high-risk behaviour and populations at low risk, as well as estimates of the sizes of these populations. The target population was restricted to the 15–79 years age group; the target year was 2009. Individuals were classified into the following groups defined as being mutually exclusive:
-
(1)
HIV-positive IDUs;
-
(2)
HIV-negative IDUs;
-
(3)
HIV-positive MSM;
-
(4)
Haemophilia patients treated before 1992;
-
(5)
First-generation migrants originating from HCV-endemic countries (⩾2%);
-
(6)
Individuals at low risk of infection (not in any of the previous groups).
Dutch studies included in the model
Data input for the Workbook Method was obtained from several data sources as described below. We included prevalence rates of antibodies against HCV (anti-HCV, in this report referred to as HCV).
PIENTER-2 serosurvey
From February 2006 to June 2007, this population-based cross-sectional serosurvey included 6386 participants donating blood (response rate 32%, including 646 immigrants) of which 6243 samples were tested for HCV and 6211 questionnaires were administered. The study design is described extensively elsewhere [25, 26]. Presence of antibodies against HCV was assayed using a micro-particle enzyme immunoassay (MEIA, Abbott Laboratories, USA). For estimation of the HCV seroprevalence in the PIENTER-2 dataset, the data was weighted by gender, age and ethnicity. More detailed information about laboratory methods, statistical analyses and population characteristics are described elsewhere [5].
Dutch HIV Monitoring Foundation (SHM)
Longitudinal data of all newly registered HIV-infected individuals are collected by the SHM. The goal of SHM is to monitor HIV-infected individuals registered in the 25 recognized HIV treatment centres in The Netherlands to study changes in the epidemic and the effects of treatment. All HIV-infected individuals registered in this cohort are followed prospectively from the time of reporting for care. In total, out of 16 451 HIV-infected patients aged ⩾18 years at the time of HIV diagnosis, 15 734 (96%) were tested for HBV or HCV co-infection (www.hiv-monitoring.nl [27]).
Amsterdam drug cohort
The Amsterdam cohort study is a longitudinal cohort of drug users that started in 1985. Participants were recruited at methadone outposts, the sexually transmitted infection (STI) clinic for drug-using prostitutes and by word of mouth. HIV-negative and asymptomatic HIV-positive IDUs and non-IDUs were invited to participate. Participants were seen every 4–6 months regardless of HIV status, but many returned more irregularly. Clinical, epidemiological and drug-use related information was collected on each occasion by interviewing participants using a standardized questionnaire. In 2009, the cohort existed of 377 non-IDUs with a known HCV status. Of these, 172 men and 81 women ever injected drugs, having a mean age of 48 years (www.amsterdamcohortstudies.org [8]).
The Hague IDU survey
Between March and July 2000, a serum sample and a questionnaire on risk behaviour were obtained from 217 drug users who have ever injected drugs in The Hague with a mean age of 39 years [28]. Participants were recruited at methadone treatment sites (35%) and low-threshold daytime care projects (65%). Despite the voluntary method of participation the response rate was high, only 10 persons refused. In total 199 samples were tested for the presence of HCV antibodies (181 males, 18 females).
Amsterdam Health Monitor
From April to June 2004, a health monitoring survey was conducted in the general Amsterdam population. A random sample of residents aged ⩾18 years was drawn from the Population Registry of Amsterdam, including 1736 individuals (response rate 42·9%). The sample was stratified by age and ethnicity, with oversampling of people from Turkish and Moroccan origin. In total 1364 (78·6%) donated blood of which 1355 were tested for anti-HCV antibodies. Details on the study design and laboratory methods have been described elsewhere [6].
Van Creveld clinic
The Van Creveld clinic (part of the Haematology department of the University Medical Center Utrecht) is the largest treatment centre in The Netherlands for children and adults with haemophilia and related coagulation disorders. Half of the haemophilia patients in The Netherlands are treated at this clinic including more than 900 patients of which 560 patients aged ⩾18 years have haemophilia type A or B (www.vancreveldkliniek.nl)
Model parameters
From above-mentioned data sources, prevalence rates of specific groups were obtained and used for input in the model. Furthermore, group sizes were estimated.
IDUs
In the model we stratified the population of IDUs by HIV status. The lower estimation was extracted from the SHM. In 2009, 91·6% (95% CI 88·0–94·2) of all HIV-positive drug users who ever injected were positive for HCV (n/N = 294/321). For the upper estimation we used data from the Amsterdam drug cohort; 95·9% (95% CI 86·3–98·9) of HIV-positive IDUs were HCV positive (n/N = 47/49).
For the HIV-negative IDUs, a HCV prevalence of 47·2% (95% CI 40·1–54·4) was used from the The Hague IDU survey (n/N = 94/199) as the lower estimation [28]. As the upper estimation we used data from the Amsterdam drug cohort showing a prevalence of 78·4% (95% CI 72·3–83·5) (n/N = 160/204).
Population sizes were extracted from the study of Van Veen et al. [21], who used the Multi Parameter Evidence Synthesis Method along with other modelling methods to estimate population sizes of high-risk groups and the HIV prevalence in The Netherlands, these were estimated at 603–1017 and 7009–15 263 for HIV-positive and HIV-negative (ever) IDUs, respectively [21].
MSM
In this study we defined HIV-positive MSM, in whom HCV has spread sexually since the introduction of effective HIV therapy [13], as a risk group since currently virtually all acute HCV cases in MSM in The Netherlands are diagnosed among HIV-positive MSM [14, 20]. Of HIV-positive MSM in care in 2009 in The Netherlands (SHM data), 9·8% (n/N = 694/7086) were HCV positive. The 95% CI of 9·1–10·5 was used as input for the model. The low and high estimations of the population size of HIV-positive MSM (8670 and 17 573, respectively) were extracted from the study of Van Veen et al. [21].
For this study, based on recent prevalence data, we assumed that HIV-negative MSM are not at increased risk for HCV (prevalence about 0·2%, excluding IDUs [14]) and were therefore not included as a separate risk group.
Haemophilia patients
Another important but small risk group is patients with haemophilia who received blood (products) before 1992. Data from the Van Creveld clinic reported a HCV prevalence of 46·6% (95% CI 42·5–50·7%) (n/N = 261/560) in treated persons aged ⩾18 years in 2009. In addition, the clinic estimated the number of haemophilia patients treated before 1992 and still alive in 2009 at about 1120.
Migrants
Since a growing number of migrants from HCV-endemic countries are living in Western Europe, including The Netherlands, they become an increasing risk group in low-prevalence countries. In our model we included only first-generation migrants from high-endemic countries (⩾2%) as a separate risk population since the prevalence in this group is notably higher (0·7–2·3%) compared to second-generation non-Western migrants (0–1·6%) and Dutch indigenous inhabitants (0·1–0·3%) [29]. For the low estimation of HCV prevalence in first-generation migrants from HCV-endemic countries, data from the Amsterdam Health Monitor survey was used (0·4%, 95% CI 0·1–1·2) (n/N = 3/706). The PIENTER-2 study estimated a HCV prevalence of 1·6% (95% CI 0·4–2·9) (n/N = 9/391) and was used as the upper estimation.
Population size of first-generation migrants was extracted from Statistics Netherlands (http://statline.cbs.nl); at 1 January 2009 there were 1 178 722 migrants from high-endemic countries aged between 15 and 79 years living in The Netherlands. Subtracting an estimated 4% of MSM and 0·09% of IDUs [21] gives a population size of about 1 130 512.
Individuals at low risk of HCV infection
All persons who were not assigned to a risk group were categorized as individuals at low risk of infection. The PIENTER-2 study provided the lower as well as the upper estimate. After excluding HIV-positive MSM, IDUs and first-generation migrants from high-endemic countries we found a HCV prevalence of 0·05% (95% CI 0·00–0·12) (n/N = 2/4046). Population size was estimated by subtracting the above-mentioned group sizes from the total number of individuals aged 15–79 years and living in The Netherlands in 2009 (N = 12 931 521) (Statistics Netherlands; http://statline.cbs.nl).
RESULTS
Estimating HCV prevalence based on the Workbook Method
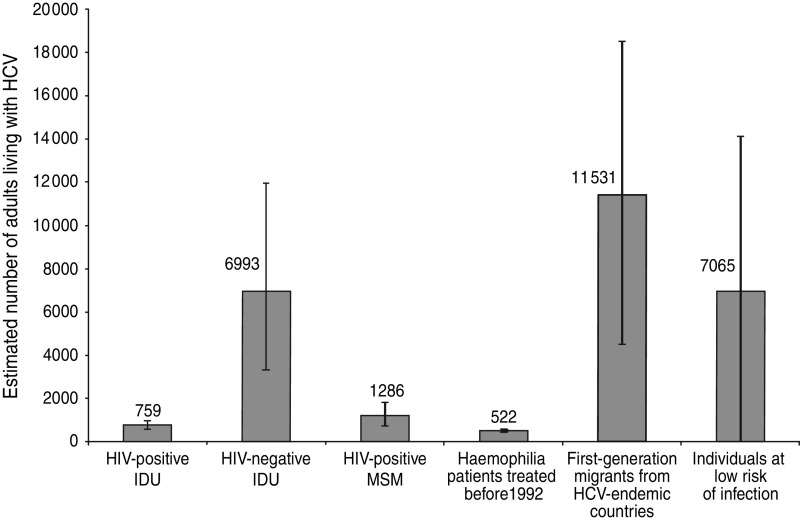
The Workbook Method estimated an overall HCV prevalence of 0·22% (min 0·07%, max 0·37%) corresponding to about 28 100 (min n = 9600, max n = 48 000) HCV-infected persons aged between 15 and 79 years in The Netherlands. The estimated number of infected persons per risk group and the estimated prevalence rates are presented in Table 1. The main risk groups are first-generation migrants, with an estimated 11 500 (min n = 4500, max n = 18 500) infected persons, followed by HIV-positive and HIV-negative (former) IDUs, with an estimated 7800 (min n = 3900, max n = 12 900) infected individuals, about 1300 HIV-positive MSM (min n = 800, max n = 1800) and an estimated 500 haemophilia patients who were treated before 1992 (Fig. 1). The large group of persons at low risk, with an estimated prevalence of 0·05%, contained about 7100 (min n = 0, max n = 14 100) infected individuals.
Table 1.
Low and high population size estimates and estimated national HCV prevalences, by using a modified Workbook Method
| Population groups | Population size estimate | Anti-HCV prevalence estimate (%) | ||
|---|---|---|---|---|
| Low | High | Low | High | |
| HIV-positive IDU | 603 | 1017 | 91·60 | 95·90 |
| HIV-negative IDU | 7009 | 15 263 | 46·70 | 78·40 |
| HIV-positive MSM | 8670 | 17 573 | 9·10 | 10·50 |
| Haemophilia patients treated before 1992 | 1120 | 1120 | 42·50 | 50·70 |
| First-generation migrants from HCV-endemic countries | 1 130 512 | 1 130 512 | 0·40 | 1·64 |
| Individuals at low risk of infection | 11 766 036 | 11 783 607 | 0·00 | 0·12 |
IDU, Injecting drug user; MSM, men who have sex with men.
Fig. 1.
Estimated number (including low and high estimates) of adults (aged 15–79 years) living with HCV antibodies in The Netherlands using a modified version of the Workbook Method per main subgroup.
DISCUSSION
Using the Workbook Method, including recent data, the current HCV seroprevalence in Dutch inhabitants aged 15–79 years was estimated at 0·22% (min 0·07%, max 0·37%). From this we can conclude that about 28 100 (min n = 9600, max n = 48 000) individuals have antibodies against HCV. Our model included data on the changing HCV epidemiology with a growing number of people immigrating from low- and middle-income countries and recent spread of HCV in HIV-positive MSM. First-generation migrants are now the largest risk group for HCV in The Netherlands, followed by IDUs.
The previous modelling of Kretzschmar et al. [15] in 2004 resulted in a prevalence of 0·4% in the Netherlands, but group sizes were uncertain due to a lack of data. In 1997 the Dutch Health Council estimated a seroprevalence of 0·1–0·4% resulting in an estimated infected population of 15 000–60 000 individuals [30]. This estimation was based on limited data of the Dutch donor population from almost 15 years ago. Although the HCV epidemiology has changed, our results lie within the range of these estimations. An explanation of this steady rate could be the decrease in the absolute number of HCV infections in blood donors, haemophilia patients and IDUs, due to a decline in new infections but also due to natural death, accompanied by an increase of new HCV infection in MSM and growing numbers of first-generation migrants.
Our study presents an updated and specific prevalence estimation, based on data from the general population and current data of the main relevant risk groups. The model also gives an overview of the estimated HCV prevalence rates per risk group. First-generation migrants from HCV-endemic countries account for more than half of all HCV-positive persons in The Netherlands, followed by IDUs and HIV-positive MSM. Up to 2004, IDUs were the main risk group in The Netherlands for acquiring an acute HCV infection, but since the last few years there has been a shift towards HIV-positive MSM [20]. Regarding migrants, only individuals born in HCV-endemic countries form a risk group [29]. Our findings support the view that immigration from endemic areas significantly contributes to the changes in HCV in European countries seen in the last 15 years [31].
In this study we used different data sources, leading to different biases which could have influenced the outcome. For example, the data sources had different study designs, such as random sampling or testing the total population, and were based on different regions. Data used for prevalence parameters per risk group in the model were partly derived from local studies from the capital city Amsterdam. The population composition of this city is not representative for the total population of The Netherlands, although no consequences are expected for first-generation migrants as they acquired the infection in their country of birth. The estimates for IDUs could be slightly overestimated compared to the general Dutch IDU population. Furthermore, although based on a robust Bayesian model, population sizes like the number of IDUs and MSM and their HIV status are difficult to estimate. We used the confidence intervals of these groups for input in our model; however, bias cannot be fully excluded. Furthermore, it should be noted that haemodialysis patients and patients who (sporadically) received blood or blood products before 1992, were not included as a risk group but are captured in the last group of individuals at low risk of infection. For these groups no recent Dutch data were available, but due to small numbers of haemodialysis patients treated before 1992 and high death rates within this population no effect is expected of this specific group on the overall prevalence. Furthermore, for patients who received blood (products) before 1992, it is expected that a large proportion of those who frequently received blood (products), and therefore had a higher risk of becoming infected with HCV, have already deceased since they were probably less healthy compared to persons who sporadically received blood (products). There are no recent data available for this population, but we expect the group size to be small. Since there is no reason to expect that this specific group was underrepresented in PIENTER-2 we have grouped them with the overall low-risk group. Despite these limitations, we expect that our anti-HCV prevalence estimate gives a more accurate range of HCV prevalence in The Netherlands than previous studies.
Based on the results, screening of individuals living or migrating to The Netherlands from HCV-endemic countries could be considered, since infections are often silent and undiagnosed. Early diagnosis may prevent adverse events from HCV infection if treatment is indicated. About 75% of patients with acute HCV will progress to chronic infection. Of all chronically infected patients 10–20% develop liver cirrhosis within 20 years [32]. In The Netherlands cirrhosis is the primary cause of 4% (4–8 patients per year) of all (split) liver transplants (Dutch Transplant Foundation). Hepatocellular carcinoma (HCC) develops solely in patients with cirrhosis with a risk of 0–3% each year [33]. Offering antiviral HCV treatment can suppress the virus and reduce the chance of developing these complications and further transmission. Unfortunately, not all HCV genotypes have good treatment results with the current standard of care treatment, but a direct acting antiviral (DAA) drug has recently been approved by the U.S. Food and Drug Administration [32]. Information about the cost-effectiveness of preventing the development of complications by screening and treating HCV exists but is lacking for the targeted screening of migrants. Therefore, future research should focus on the cost-effectiveness of preventing complications of HCV by screening migrants born in HCV-endemic countries. MSM and drug users who are in care for their HIV infection and/or drug dependence are currently being screened for HCV infections.
CONCLUSION
Using recent data on HCV epidemiology, we estimated that the HCV seroprevalence in the Dutch population aged 15–79 years is 0·22%. The majority of these are first-generation migrants born in HCV-endemic countries. An assessment seems appropriate to determine whether screening of this specific subgroup would be feasible and cost-effective in order to reduce adverse events due to HCV infection and to improve quality of life of those infected with HCV.
ACKNOWLEDGEMENTS
The authors thank the PIENTER study group, especially Liesbeth Mollema and Fiona van der Klis, for collecting and providing data for estimation of the national HCV prevalence. Furthermore, initial data analyses on HIV-positive IDU and MSM were undertaken by Colette Smit who is an employee of the Dutch HIV Monitoring Foundation, initial data analyses on HIV-positive and HIV-negative IDUs in the Amsterdam drug cohort were undertaken by Bart Grady, employee of the Public Health Service of Amsterdam, and Joop Arends, employee of the Van Creveld clinic which is part of the University Medical Center of Utrecht, undertook initial data analyses on haemophilia patients. All above-mentioned studies were mainly funded by the Dutch Ministry of Health, Sports and Welfare.
We also gratefully acknowledge the following persons for their valuable contributions to the design or execution of the study: Priscilla de Graaff, Jeroen Cremer, Thijs van de Laar, Marianne van der Sande and Maarten Schipper.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.World Health Organization. Hepatitis C: Factsheet no. 164, 2011. (http://www.who.int/mediacentre/factsheets/fs164/en/). Accessed 22 February 2012.
- 2.Muhlberger N, et al. HCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health 2009; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rantala M, van de Laar MJ. Surveillance and epidemiology of hepatitis B and C in Europe – a review. Eurosurveillance 2008; 13. [DOI] [PubMed] [Google Scholar]
- 4.Veldhuijzen IK, Conyn-van Spaendonck MAE, Dorigo-Zetsma JW. Seroprevalence of hepatitis B and C in the Dutch population [in Dutch]. Infectieziekten Bulletin 1999; 10: 182–184. [Google Scholar]
- 5.Vriend HJ, et al. Hepatitis C virus seroprevalence in The Netherlands. European Journal of Public Health. Published online: 29 March 2012. doi: 10.1093/eurpub/cks013. [DOI] [PubMed] [Google Scholar]
- 6.Baaten GGG, et al. Population-based study on the seroprevalence of hepatitis A, B, and C virus infection in Amsterdam, 2004. Journal of Medical Virology 2007; 79: 1802–1810. [DOI] [PubMed] [Google Scholar]
- 7.Veldhuijzen IK, et al. Viral hepatitis in a multi-ethnic neighborhood in the Netherlands: results of a community-based study in a low prevalence country. International Journal of Infectious Diseases 2009; 13: e9–e13. [DOI] [PubMed] [Google Scholar]
- 8.van den Berg CH, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. European Journal of Epidemiology 2007; 22: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Laar TJW, et al. Changes in risk behavior and dynamics of hepatitis C virus infections among young drug users in Amsterdam, the Netherlands. Journal of Medical Virology 2005; 77: 509–518. [DOI] [PubMed] [Google Scholar]
- 10.Posthouwer D, et al. Hepatitis C infection among Dutch haemophilia patients: a nationwide cross-sectional study of prevalence and antiviral treatment. Haemophilia 2005; 11: 270–275. [DOI] [PubMed] [Google Scholar]
- 11.Schneeberger PM, et al. The prevalence and incidence of hepatitis C virus infections among dialysis patients in The Netherlands: a nationwide prospective study. Journal of Infectious Diseases 2000; 182: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 12.Schreuder I, et al. Seroprevalence of HIV, hepatitis B, and hepatitis C among opioid drug users on methadone treatment in the Netherlands. Harm Reduction Journal 2010; 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Laar TJW, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. Journal of Infectious Diseases 2007; 196: 230–238. [DOI] [PubMed] [Google Scholar]
- 14.Urbanus AT, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS 2009; 23: F1–F7. [DOI] [PubMed] [Google Scholar]
- 15.Kretzschmar M. The prevalence of hepatitis C in The Netherlands. Notice VWS-IGZ 2004/02. Project V/210041 [in Dutch]. Bilthoven: RIVM, 2004. [Google Scholar]
- 16.De Angelis D, et al. An evidence synthesis approach to estimating hepatitis C Prevalence in England and Wales. Statistical Methods in Medical Research 2009; 18: 361–379. [DOI] [PubMed] [Google Scholar]
- 17.Razali K, et al. Modelling and calibration of the hepatitis C epidemic in Australia. Statistical Methods in Medical Research 2009; 18: 253–270. [DOI] [PubMed] [Google Scholar]
- 18.Remis RS. Modelling the incidence and prevalence of hepatitis C infection and its sequelae in Canada, 2007. Public Health Agency of Canada. [Google Scholar]
- 19.Van Den Berg C, et al. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction 2007; 102: 1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vriend HJ, et al. Sexually transmitted infections, including HIV, in the Netherlands in 2009. Bilthoven: RIVM, 2010. Report No.: 210261007. [Google Scholar]
- 21.van Veen MG, et al. National estimate of HIV prevalence in the Netherlands: comparison and applicability of different estimation tools. AIDS 2011; 25: 229–237. [DOI] [PubMed] [Google Scholar]
- 22.UNAIDS Reference Group on Estimates MaP. Improved methods and assumptions for estimation of the HIV/AIDS epidemic and its impact: recommendations of the UNAIDS Reference Group on Estimates, Modelling and Projections. AIDS 2002; 16: W1–14. [DOI] [PubMed] [Google Scholar]
- 23.Lyerla R, et al. The 2005 Workbook: an improved tool for estimating HIV prevalence in countries with low level and concentrated epidemics. Sexually Transmitted Infections 2006; 82 (Suppl. 3): iii41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker N, et al. The workbook approach to making estimates and projecting future scenarios of HIV/AIDS in countries with low level and concentrated epidemics. Sexually Transmitted Infections 2004; 80 (Suppl. 1): i10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Klis FR, et al. Second national serum bank for population-based seroprevalence studies in the Netherlands. Netherlands Journal of Medicine 2009; 67: 301–308. [PubMed] [Google Scholar]
- 26.Mollema L, et al. PIENTER 2-project: second research project on the protection against infectious diseases offered by the national immunization programme in the Netherlands. Bilthoven: RIVM, 2009. [Google Scholar]
- 27.Van Sighem A, et al. Monitoring report 2011, human immunodeficiency virus (HIV) infection in the Netherlands. Amsterdam: Stichting HIV Monitoring, 2011. [Google Scholar]
- 28.Beuker RJ, et al. Surveillance of HIV infection among injecting drug users in the Netherlands: results Den Haag 2000 [in Dutch]. Bilthoven: RIVM, 2001. Report No.: 441100015. [Google Scholar]
- 29.Urbanus AT, et al. Hepatitis C in the general population of various ethnic origins living in the Netherlands: should non-Western migrants be screened? Journal of Hepatology 2011; 55: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 30.Health Council of the Netherlands. Committee on Hepatitis C. Detection and treatment of people with hepatitis C. Rijswijk: Health Council of the Netherlands, 1997. Report No.: 1997/19. [Google Scholar]
- 31.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. Journal of Hepatology 2008; 48: 148–162. [DOI] [PubMed] [Google Scholar]
- 32.Mauss S, et al. (eds). Short Guide to Hepatitis C. Flying Publisher, 2012. (www.flyingpublisher.com). [Google Scholar]
- 33.Chopra S. Clinical features and natural history of hepatitis C virus infection. In: UpToDate, Waltham, MA, 2012. (www.uptodate.com). [Google Scholar]