SUMMARY
Influenza surveillance in Danish intensive care units (ICUs) was performed during the 2009/10 and 2010/11 influenza seasons to monitor the burden on ICUs. All 44 Danish ICUs reported aggregate data for incidence and point prevalence, and case-based demographical and clinical parameters. Additional data on microbiological testing, vaccination and death were obtained from national registers. Ninety-six patients with influenza A(H1N1)pdm09 were recorded in 2009/10; 106 with influenza A and 42 with influenza B in 2010/11. The mean age of influenza A patients was higher in 2010/11 than in 2009/10, 53 vs. 44 years (P = 0·004). No differences in other demographic and clinical parameters were detected between influenza A and B patients. In conclusion, the number of patients with severe influenza was higher in Denmark during the 2010/11 than the 2009/10 season with a shift towards older age groups in influenza A patients. Influenza B caused severe illness and needs consideration in clinical and public health policy.
Key words: Influenza, public health, surveillance
INTRODUCTION
Since 1994 influenza in Denmark has been monitored using a sentinel surveillance system by general practitioners (GPs), which consists of reporting of influenza-like illness (ILI) by GPs and laboratory testing of a random sample of nasal swabs. In 2006 an additional surveillance system based on primary healthcare consultations was established in collaboration with the Danish medical on-call service [1]. The Danish influenza surveillance was intensified during the pandemic season of 2009/10 with an assessment of influenza-related hospitalizations [2] and by active reporting of influenza patients in intensive care units (ICUs) [3] in order to inform healthcare authorities about the severity of disease. The latter system had the additional aim of timely detection of shortages in ICU bed capacity.
In August 2010 the World Health Organization declared the start of the post-pandemic phase [4]. From previous influenza pandemics it has been observed in the USA and England and Wales that several years following a pandemic the numbers of patients with ILI and influenza-related mortality remained high before returning to a seasonal pattern [5–7]. However, each pandemic and post-pandemic phase is influenced by various factors such as natural immunity and vaccination campaigns. High numbers of influenza patients were not particularly expected in Europe for 2010/11 [8].
In the first week of December 2010, the UK announced an early warning concerning a large number of patients with influenza in ICUs, while the influenza activity in the general population was still relatively low [9]. In Denmark the sentinel surveillance by GPs and the surveillance system for on-call GPs were in place. In addition, ICU surveillance was continued in Denmark, with a few logistical changes.
We describe the methodology used for the surveillance in ICUs and present the burden of influenza on the ICUs during the influenza season of 2010/11 compared to the 2009/10 season. In addition, demographical and clinical parameters were compared for patients with severe influenza A in the two seasons and between patients with severe influenza A and B in the 2010/11 season. The objectives were to gain insight in severe clinical illness associated with influenza A and B in the post-pandemic phase and to place the findings from the pandemic phase in perspective.
MATERIALS AND METHODS
Active reporting by ICUs
All 44 Danish ICUs, including general ICUs, neurosurgical and paediatric ICUs actively reported aggregate and case-based data. The system ran from week 46 (2009) (starting 9 November 2009) to week 11 (2010) (ending 21 March 2010) and from week 49 (2010) (starting 6 December 2010) to week 14 (2011) (ending 10 April 2011), covering the periods of increased circulation of influenza virus in both seasons. ILI incidence did not increase in Denmark during the summer of 2009 and this period was not included in the surveillance system. Once a week, the number of new patients with influenza admitted during the previous week was reported. In addition, the ICUs provided a point estimate of the bed capacity used for influenza patients; the number of influenza patients present in ICUs on Monday 08:00 hours divided by the total number of patients present in the ICUs at that time [3]. It is important to note that this is not a measure of the potential bed capacity, but of the number of beds occupied.
During the 2009/10 season the ICUs reported directly to the Statens Serum Institut (SSI). The case definition was a patient in a Danish ICU with laboratory-confirmed influenza A(H1N1)pdm09 or with ILI after close contact with a person with laboratory-confirmed influenza A(H1N1)pdm09. Case-based data were reported on a separate form at a later date and included the unique personal identification number (CPR number) from the Danish Civil Registration System, information on demographics, antiviral treatment, interventions, such as ventilation, dialysis and extracorporeal membrane oxygenation (ECMO), and clinical outcome.
A few changes were made for the 2010/11 season to adapt to the post-pandemic situation and improve the efficiency of the system. The ICUs reported to one of the five regional coordinators for emergency disaster preparedness who in turn reported to SSI. The case definition was a patient in a Danish ICU with laboratory-confirmed infection with influenza A or B. Case-based data were reported at the same time as the aggregate data. The case-based data were less detailed to reduce the workload of ICUs; however, the data still included the CPR number, information on demographics, underlying medical conditions and ECMO treatment.
A consequence of simplifying the case-based data was a different assessment of the presence or absence of underlying medical conditions. During the 2009/10 season the ICUs were asked to indicate presence or absence of underlying medical conditions from a given list, and height and weight to calculate the body mass index. During the 2010/11 season the ICUs were given a free-text field to report the presence or absence of underlying medical conditions.
Laboratory data
During the pandemic season all microbiology laboratories in Denmark were by law required to send samples from suspected influenza patients to the SSI Department of Virology for confirmation by polymerase chain reaction (PCR) and for subtyping. In the 2010/11 season many clinical laboratories performed their own PCR tests, but samples were still sent to SSI for subtyping. Since January 2010 laboratory data from all microbiology laboratories in Denmark have been collected in one national microbiology database (MiBa) [10]. Linkage of surveillance data to MiBa, using the CPR number, allowed verification of laboratory confirmation and collection of further details on subtyping of influenza A strains and lineage determination of influenza B strains. Patients from before 2010 were verified in the database of the SSI Department of Virology.
Vaccination data
The pandemic vaccine, which only included the influenza A(H1N1)pdm09 strain, was available in Denmark from week 45 in 2009. In the 2010/11 season the seasonal influenza vaccine, including strains of influenza A(H1N1)pdm09, influenza A(H3N2) and influenza B Victoria lineage was available from week 39 in 2010. Information on pandemic and seasonal vaccination status was obtained from the Danish Vaccination Register.
Vital status
Information on death was obtained from the Civil Registration System. Mortality due to influenza was defined as death within 30 and within 90 days of ICU admission.
Burden of influenza on ICUs
The burden of influenza on ICUs was assessed as the total number of influenza patients in ICUs during the two seasons and the proportion of influenza patients among the total number of patients in ICUs.
Influenza in the general population
To relate the burden of influenza in ICUs with patterns in the general population, numbers of new ICU admissions were compared to data from the sentinel surveillance, which showed the percentage of patients with ILI among all patients consulting their GPs.
Data on typing and subtyping of samples from ICU patients were also compared to samples tested at the SSI Department of Virology. These samples included diagnostic samples from GPs and hospitals, surveillance samples from general practices in the sentinel surveillance and positive samples from hospitals which sent samples to SSI for reference.
Statistical analysis
Differences in mean age, mean length of stay and the mean proportion of beds used for influenza patients were tested using the Mann–Whitney test. Differences in presence of underlying illness were analysed using the z test. Levels of significance were set at 0·05.
RESULTS
All 44 Danish ICUs participated in the surveillance in both seasons. During the 2009/10 season some ICUs reported as a group, resulting in 36 reporting ICUs. The proportion of reporting ICUs varied per week between 61% and 80%, and decreased to around 43% for the last 2 weeks [3]. During the 2010/11 season reporting by the 44 ICUs was consistently high, varying between 91% and 100%.
Virological information
Ninety-six patients with influenza A(H1N1)pdm09 were reported in the 2009/10 season [3]. During the 2010/11 season 148 influenza patients were recorded; 106 (72%) with influenza A and 42 (28%) with influenza B. Of the 106 patients with influenza A, 91 (86%) were subtyped and all had influenza A(H1N1)pdm09. For 17/42 influenza B patients the lineage was determined, showing nine patients with Victoria lineage and eight with Yamagata lineage.
Comparison with diagnostic and surveillance samples tested at the SSI Department of Virology in the 2009/10 season showed 1801 (99%) samples with influenza A(H1N1)pdm09, one with influenza A(H3N2), four with seasonal influenza A(H1N1) from before 2009 and 16 with influenza B. In the 2010/11 season 473 (51%) samples showed influenza A(H1N1)pdm09, seven influenza A(H3N2) and 442 (48%) influenza B. For 416 influenza B samples where the lineage was determined: 329 (79%) had Victoria lineage and 87 (21%) Yamagata lineage.
The burden of influenza in ICUs in 2009/10 and 2010/11 seasons
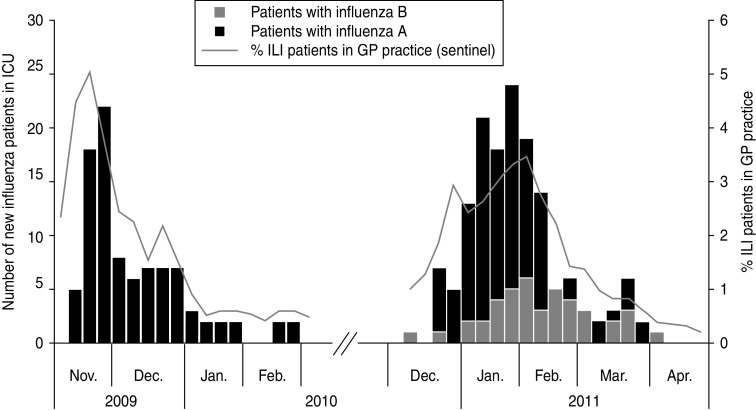
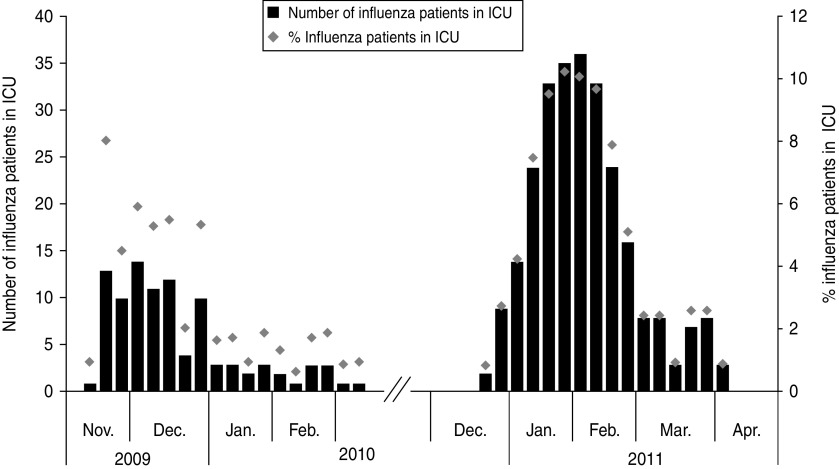
Figure 1 shows the number of influenza-related admissions for both seasons combined with sentinel data from GPs. Figure 2 shows that the proportion of ICU beds used for influenza patients was higher in the 2010/11 season than during the 2009/10 season. The percentage of beds used for influenza patients in the 2009/10 season varied between 0·7% and 6% with one outlier at 8% and in the 2010/11 season between 0·8% and 10%. The mean proportion was 2·8 in the 2009/10 season and 5·0 in the 2010/11 season (Mann–Whitney test: P = 0·15). The denominator in these calculations (the total number of patients in an ICU) was on average 172 beds (range 103–237) in the 2009/10 season and 319 beds (range 256–359) in the 2010/11 season.
Fig. 1.
Number of new patients with influenza A and B by week of admission to intensive care unit (ICU) during the 2009/10 and 2010/11 seasons (bars, left axis), projected against the percentage of influenza-like illness (ILI) in patients in general practitioner (GP) practice (line, right axis).
Fig. 2.
Number of influenza patients in Danish intensive care units (ICUs) at 08:00 hours (bars, left axis) and the percentage of beds in use for influenza patients on Monday 08:00 hours (diamonds, right axis) during the 2009/10 and 2010/11 seasons.
Characteristics of patients with influenza A infection in 2009/10 and 2010/11 seasons
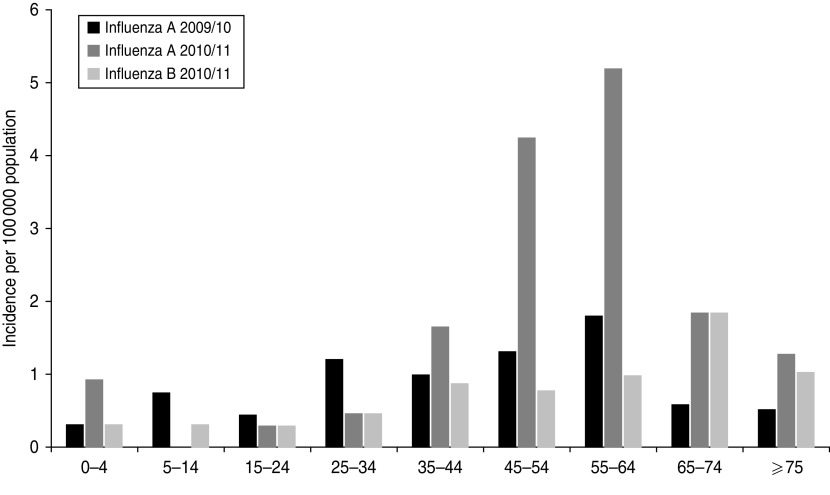
Case-based data from the 2009/10 season was available for 53 influenza A patients and was compared to the 106 patients with influenza A in the 2010/11 season. The gender distribution was similar (male:female ratio of 1:0·7 and 1:0·8, respectively). The mean age was lower in the 2009/10 season at 44 years (range 3–80 years) than the mean age of 53 years (range 1 week–83 years) in the 2010/11 season (Mann–Whitney test: P = 0·004). Figure 3 presents the incidence of influenza patients by age group. While the incidence was relatively high in children and young adults in the 2009/10 season, the incidence of influenza A in the 2010/11 season was relatively higher in very young children aged 0–4 years and persons aged >45 years.
Fig. 3.
Incidence of admissions to intensive care units associated with influenza A and B per 100 000 population by age group in Denmark during the 2009/10 and 2010/11 seasons.
Table 1 shows underlying conditions during both seasons. There was no underlying illness in 11 (21%) of 52 patients in the 2009/10 season nor in 14 (18%) of 80 influenza A patients, for whom information was available, in the 2010/11 season. Neurological disease, obesity and cardiovascular disease were more prevalent in patients in the 2009/10 season than the 2010/11 season (z test: P < 0·001, P = 0·02, P = 0·03, respectively).
Table 1.
Underlying conditions in patients with influenza A (2009/10 and 2010/11) and influenza B (2010/11)
| Underlying condition | Influenza A 2009/10 | Influenza A 2010/11 | Influenza B 2010/11 | Comparison seasons† P value | Comparison influenza types‡ P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N* | % | n | N * | % | n | N* | % | |||
| None | 11 | 52 | 21 | 14 | 80 | 18 | 10 | 35 | 29 | 0·60 | 0·18 |
| Chronic renal failure | 3 | 49 | 6 | 4 | 80 | 5 | 0 | 35 | 0 | 0·79 | 0·18 |
| Cancer | 9 | 48 | 19 | 16 | 80 | 20 | 8 | 35 | 23 | 0·87 | 0·72 |
| Immunocompromised condition | 9 | 47 | 19 | 6 | 80 | 8 | 1 | 35 | 3 | 0·05 | 0·34 |
| Neurological disease | 9 | 47 | 19 | 0 | 80 | 0 | 1 | 35 | 3 | <0·001 | 0·13 |
| Diabetes | 10 | 52 | 19 | 7 | 80 | 9 | 5 | 35 | 14 | 0·08 | 0·38 |
| Chronic pulmonary disease | 11 | 49 | 22 | 14 | 80 | 18 | 7 | 35 | 20 | 0·49 | 0·75 |
| Obesity | 10 | 41 | 24 | 7 | 80 | 9 | 1 | 35 | 3 | 0·02 | 0·25 |
| Cardiovascular disease | 12 | 49 | 25 | 8 | 80 | 10 | 4 | 35 | 11 | 0·03 | 0·82 |
| Other underlying conditions | 14 | 47 | 30 | 19 | 80 | 24 | 3 | 35 | 8 | 0·46 | 0·06 |
| Pregnancy | 1 | 22 | 5 | 0 | 47 | 0 | 1 | 20 | 5 | 0·14 | 0·12 |
| Post-partum <42 days | 1 | 22 | 5 | 0 | 47 | 0 | 0 | 20 | 0 | 0·14 | NA |
Denominator showing those patients for which information on underlying illness was available.
z test comparing influenza A patients from seasons 2009/10 and 2010/11.
z test comparing influenza A and B patients from the 2010/11 season.
The length of stay in the ICU was calculated for 40 patients in the 2009/10 season and also for 40 patients with influenza A in the 2010/11 season. The mean number of days was similar with 13 days (range <1–65 days) in the 2009/10 season and 12 days (range <1–49 days) in the following season.
Six (11%) of the 53 influenza A patients received ECMO in the 2009/10 season as did 10 (12%) of 86 influenza A patients, for whom this information was available, in the 2010/11 season.
The proportion of patients who died within 30 days of ICU admission was 28% (15/53) in the 2009/10 season and 27% (29/106) in the 2010/11 season. In 2009/10 three more patients died between 30 and 90 days after ICU admission, leading to a 90-day mortality of 34% (18/53). In the 2010/11 season an additional seven patients died between 30 and 90 days, also resulting in a 90-day mortality of 34% (36/106).
Three of the 15 patients who died in the 2009/10 season were vaccinated; all of them less than a week before ICU admission. Of the 29 patients who died in the 2010/11 season four (14%) were vaccinated, all more than 2 weeks before ICU admission. For three of the four patients information on underlying medical condition was available and showed haematological malignancies.
Characteristics of patients with influenza A and B infection in 2010/11
The 106 influenza A patients of the 2010/11 season were also compared to the 42 influenza B patients. The male:female ratio was similar with 1:0·9 for influenza B patients. The mean age was 52 years for both patient groups (range: influenza B patients 1–83 years). The influenza B incidence, as shown in Figure 3, was relatively lower in patients aged 35–64 years compared to influenza A patients.
As shown in Table 1, diabetes was more prevalent in influenza B patients, whereas immunocompromised conditions and obesity were more prevalent in influenza A patients. These findings were, however, not statistically significant. The majority of cancer patients had haematological malignancies: 15/16 influenza A patients and 7/8 influenza B patients. In the category ‘other’ the conditions mentioned more than once were pneumonia (four influenza A patients), alcohol abuse (three influenza A patients, one influenza B patient) and drug abuse (two influenza A patients).
For 40 influenza A and 20 influenza B patients admission and discharge dates were available. Of these, influenza A patients stayed on average 12 days (range <1–49 days) in the ICU and influenza B patients 7 days (range <1–35 days). This difference was not statistically significant.
Ten (12%) of 86 influenza A patients and three (7%) of 41 influenza B patients received ECMO treatment in the 2010/11 season. The proportion of patients with influenza B who died within 30 days of ICU admission was 26% (11/42) compared to 27% in influenza A patients. Of the 11 influenza B patients, five had a strain of the Victoria lineage and one of the Yamagata lineage. An additional three patients died between 30 and 90 days after ICU admission, leading to a 90-day mortality of 33% (14/42).
The Danish Vaccination Register showed that 16 (11%) of 106 influenza A patients and 12 (29%) of 42 influenza B patients received the 2010/2011 seasonal influenza vaccine between weeks 39 and 50 in 2010. All were vaccinated more than 2 weeks before ICU admission. The lineage was determined in only one of the 12 vaccinated influenza B patients, and found to be Yamagata lineage.
DISCUSSION
During the 2009/10 and 2010/11 influenza seasons surveillance was performed in which Danish ICUs reported the number of influenza patients in their units. The system proved highly flexible, and sufficiently sustainable to be initiated at short notice. It provided timely information to assess a potential increased burden on ICUs that the UK had warned about [9]. With a different case definition the system may be useful for other emerging infectious diseases.
The response rate during the 2009/10 season was high for most of the surveillance period, but was consistently higher during the 2010/11 season. From personal contact with the ICUs, we know that when ICUs did not report during the 2009/10 season, they generally did not have any new influenza patients. Therefore we assumed that the numbers of new patients and patients in ICUs on Mondays at 08:00 hours which were reported in the 2009/10 season reflect real numbers and are comparable with the numbers during the 2010/11 season. However, the total number of patients in ICUs was affected by underreporting during the 2009/10 season. The proportion of beds used for influenza patients may therefore be overestimated, especially for the 2009/10 season.
We compared characteristics of patients with influenza A(H1N1)pdm09 infection from the 2009/10 season with characteristics of patients with laboratory-confirmed influenza A infection from the 2010/11 season. Since all 91 patients who were subtyped during 2010/11 had influenza A(H1N1)pdm09 and very few other influenza A strains were found to be circulating in the Danish population, we consider it realistic to assume that all 106 influenza A patients had an infection with influenza A(H1N1)pdm09 and can be compared to the patients from the 2009/10 season.
The number of influenza patients in ICUs was higher during the 2010/11 season than during the pandemic season. The proportion of beds used for influenza patients seemed to have increased accordingly, although this difference was not statistically significant. As mentioned, there may be an overestimation of the proportion of beds used for influenza patients during the 2009/10 season, which suggests that the difference was even larger. The length of stay did not differ between the two seasons and can therefore not be accountable for the additional increase in burden on ICUs. It can be argued that the larger number of patients in the 2010/11 season was a result of more testing, but that is not necessarily the case. In fact, the opposite may be true considering the fact that awareness of the need to test for influenza was probably higher during the pandemic season of 2009/10. Surveillance during more consecutive influenza seasons is needed to better understand the dynamics behind this measure.
The comparison between influenza A patients showed a shift to older age groups from the 2009/10 to the 2010/11 seasons. Theoretically, geographical differences may contribute to different age groups being affected from one season to another. However, given the fact that Denmark is a small, fairly densely populated country with a well-developed transport infrastructure, we do not believe that geography played a substantial role in this observation. Moreover, surveillance data of severe influenza in the UK and mortality data from The Netherlands also show an age shift [11, 12]. Based on data of the three pandemics in the 20th century it was estimated that it may take 3–10 years after a pandemic before the population has adapted immunologically to the new virus [13]. An age shift in excess mortality towards older age groups in the influenza waves of 1919/20 was suggested to be indirect evidence of the emergence of an early influenza A(H1N1) drift variant in the years after the 1918 pandemic [13]. The age shift observed in our data may be an early indication that such a development is taking place.
Obesity, neurological disease and cardiovascular disease were significantly less prevalent during the 2010/11 season. The finding of obesity may be an artifact, due to the different way of reporting. However, the findings of neurological illnesses and cardiovascular diseases may be real differences, which will need to be studied more closely in the coming seasons. Neurological diseases were specifically reported as a risk factor for severe disease in children during the pandemic [14–16]. Our observation of an age shift may therefore explain why neurological illness was less prevalent in influenza patients during the 2010/11 season. In this respect cardiovascular disease would be expected to be more prevalent in patients during the 2010/11 season, since it is more common in older age groups. This was indeed found to be the case in mortality surveillance in The Netherlands [12]. In both seasons cancer was reported frequently. The information on the 2010/11 season showed that most of the cancer patients had haematological malignancies, which generally cause severely depressed immune function. The severity of illness between the two seasons was similar in terms of ECMO treatment and proportion of deaths.
Influenza B was not included in the case definition of 2009/10. However, we assumed that we did not miss substantial numbers of influenza B patients, as diagnostic and surveillance samples in the SSI laboratory showed that influenza B did not circulate to any significant extent in the 2009/10 season.
Influenza B infection has been described in a small number of patients, mostly through case reports, as a cause of severe illness in children and adults aged <65 years [17–21]. We describe the largest group of patients with influenza B in an ICU to date. Age and gender distribution and severity in terms of ECMO treatment and death were comparable between patients with influenza A and B.
A substantial proportion of influenza B samples were of the Yamagata lineage, even though this lineage was found to a much lesser extent in diagnostic and surveillance samples. The seasonal vaccine in 2010/11 included the Victoria lineage but not the Yamagata lineage. This may be the reason why one-third of influenza B patients in ICUs had received the seasonal vaccination more than 14 days before admission and still developed severe influenza. We cannot confirm this hypothesis as only one of the vaccinated influenza B patients had B lineage determined. However, it may be a reason to consider including both the Yamagata and Victoria lineages in the seasonal vaccine. This possibility has recently been explored by Reed et al. who showed that introduction of a quadrivalent vaccine containing both influenza B strains could lead to a reduction in influenza-associated health outcomes, although moderate [22].
In conclusion, the total number of influenza patients in ICUs was higher in the 2010/11 season than in the 2009/10 season. The percentage of ICU beds occupied with influenza patients was also higher in 2010/11 than in 2009/10. The observed age shift indicates that the burden of influenza may be moving towards the usual seasonal pattern. It is important to closely monitor this development over the coming years and take it into account when making decisions regarding vaccination and mitigation strategies. The profile of underlying conditions in influenza patients developing severe illness also needs further study as it may have important implications for vaccination strategies and recommendations for antiviral treatment. Finally, the role of influenza B in severe disease may have been underestimated until now. Our data show that influenza B can cause severe illness in all age groups and that it needs to be considered in the setting of an ICU as well as in public health policy.
ACKNOWLEDGEMENTS
We thank all those in the ICUs who reported the data during the influenza seasons. In particular we thank the regional contact persons, Sille Arildsen (Capital Region of Denmark, Copenhagen, Denmark), Liv Nørregaard Skøtt (Zealand Region, Sorø, Denmark), Martin Grum-Nymann and Malene Jeppesen (Region of Southern Denmark, Vejle, Denmark), Mathias Paul Goldinger and Susanne Ilkjær (Central Denmark Region, Skejby Hospital, Århus, Denmark) and Mette Mølvadgaard and Henrik Nielsen (North Denmark Region, Aalborg Hospital, Aalborg, Denmark) for collecting the data, and Hanne-Dorthe Emborg (Department of Infectious Disease Epidemiology, SSI, Copenhagen, Denmark) for providing the data from the Danish Vaccination Register. This work was funded by the Department of Infectious Disease Epidemiology of Statens Serum Institut.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Harder KM, et al. Electronic real-time surveillance for influenza-like illness: experience from the 2009 influenza A(H1N1) pandemic in Denmark. Eurosurveillance 2011; 16(3): pp. ii = 19767. [PubMed] [Google Scholar]
- 2.Widgren K, Nielsen J, Mølbak K. Registry-based surveillance of influenza-associated hospitalisations during the 2009 influenza pandemic in Denmark: the hidden burden on the young. PLoS One 2010; 5: e13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubbels S, et al. National surveillance of pandemic influenza A(H1N1) infection-related admissions to intensive care units during the 2009–10 winter peak in Denmark: two complementary approaches. Eurosurveillance 2010; 15(49): pp. ii = 19743. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. H1N1 in post-pandemic period (http://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/index.html). Accessed 18 July 2011.
- 5.Simonsen L, et al. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. Journal of Infectious Diseases 1998; 178: 53–60. [DOI] [PubMed] [Google Scholar]
- 6.Elliot AJ, Fleming DM. Surveillance of influenza-like illness in England and Wales during 1966–2006. Eurosurveillance 2006; 11(10): pp. ii = 651. [PubMed] [Google Scholar]
- 7.Fleming DM, Elliot AJ. Lessons from 40 years' surveillance of influenza in England and Wales. Epidemiology and Infection 2008; 136: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoll A, Sprenger M. The end of the pandemic – what will be the pattern of influenza in the 2010–11 European winter and beyond? Eurosurveillance 2010; 15(32): pp. ii = 19637. [PubMed] [Google Scholar]
- 9.Health Protection Agency. HPA weekly national influenza report – week 49, 2010 (http://www.ncbi.nlm.nih.gov/pubmed/20532237).
- 10.Den danske mikrobiologidatabase. (http://miba.ssi.dk/graphics/miba/index.html). Accessed 22 October 2010.
- 11.Bolotin S, et al. A new sentinel surveillance system for severe influenza in England shows a shift in age distribution of hospitalised cases in the post-pandemic period. PLoS One 2012; 7: e30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Gageldonk-Lafeber RA, et al. Case-based reported mortality associated with laboratory-confirmed influenza A(H1N1) 2009 virus infection in the Netherlands: the 2009–2010 pandemic season versus the 2010–2011 influenza season. BMC Public Health 2011; 11: 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saglanmak N, et al. Gradual changes in the age distribution of excess deaths in the years following the 1918 influenza pandemic in Copenhagen: using epidemiological evidence to detect antigenic drift. Vaccine 2011; 29 (Suppl. 2): B42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagdure D, et al. Hospitalized children with 2009 pandemic influenza A (H1N1): comparison to seasonal influenza and risk factors for admission to the ICU. PLoS One 2010; 5: e15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciftçi E, et al. Clinical and epidemiological features of Turkish children with 2009 pandemic influenza A (H1N1) infection: experience from multiple tertiary paediatric centres in Turkey. Scandinavian Journal of Infectious Diseases 2011; 43: 923–929. [DOI] [PubMed] [Google Scholar]
- 16.Del Rosal T, et al. Pandemic H1N1 influenza-associated hospitalizations in children in Madrid, Spain. Influenza and Other Respiratory Viruses 2011; 5: e544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabbutt S, et al. Severe influenza B myocarditis and myositis. Pediatric Critical Care Medicine 2004; 5: 403–406. [DOI] [PubMed] [Google Scholar]
- 18.Moore DL, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004. Pediatrics 2006; 118: e610–619. [DOI] [PubMed] [Google Scholar]
- 19.Hon KL, et al. Influenza and parainfluenza associated pediatric ICU morbidity. Indian Journal of Pediatrics 2010; 77: 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aebi T, et al. Co-infection of influenza B and streptococci causing severe pneumonia and septic shock in healthy women. BMC Infectious Diseases 2010; 10: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulakou G, et al. First influenza season after the 2009 pandemic influenza: characteristics of intensive care unit admissions in adults and children in Vall d'Hebron Hospital. Clinical Microbiology and Infection 2012; 18: 374–380. [DOI] [PubMed] [Google Scholar]
- 22.Reed C, et al. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30: 1993–1998. [DOI] [PubMed] [Google Scholar]