Abstract
Bacteria are able to communicate and gene regulation can be mediated through the production of acylated homoserine lactone (AHL) signal molecules. These signals play important roles in several pathogenic and symbiotic bacteria. The following study was undertaken to investigate whether AHLs are produced by bacteria found in food at temperatures and NaCl conditions commercially used for food preservation and storage. A minimum of 116 of 154 psychrotrophic Enterobacteriaceae strains isolated from cold-smoked salmon or vacuum-packed chilled meat produced AHLs. Analysis by thin-layer chromatography indicated that N-3-oxo-hexanoyl homoserine lactone was the major AHL of several of the strains isolated from cold-smoked salmon and meat. AHL-positive strains cultured at 5°C in medium supplemented with 4% NaCl produced detectable amounts of AHL(s) at cell densities of 106 CFU/ml. AHLs were detected in cold-smoked salmon inoculated with strains of Enterobacteriaceae stored at 5°C under an N2 atmosphere when mean cell densities increased to 106 CFU/g and above. Similarly, AHLs were detected in uninoculated samples of commercially produced cold-smoked salmon when the level of indigenous Enterobacteriaceae reached 106 CFU/g. This level of Enterobacteriaceae is often found in lightly preserved foods, and AHL-mediated gene regulation may play a role in bacteria associated with food spoilage or food toxicity.
There is growing evidence that several gram-negative bacteria coordinate their colonization and association with higher organisms by intercellular communication systems that function via small diffusible N-acyl homoserine lactone (AHL) signal molecules (11, 14, 17, 33, 36). These signal molecules are synthesized from cellular precursors by a synthase protein, I, and they interact with a transcriptional activating R protein to induce expression of different target genes (13). Such regulatory systems operate as quorum-sensing mechanisms that allow bacteria to sense population numbers and to synchronize the functions of the entire population. A well-described example is the squid symbiont Vibrio fischeri, which colonizes the light organ of certain types of squid and fish, where the bacteria at high culture density express bioluminescence to the benefit of the host (28). Many other examples are, however, related to expression of pathogenicity traits, and AHL-regulated systems have been described in Vibrio harveyi (3), Pseudomonas aeruginosa (30, 39), Erwinia carotovora (1), and Agrobacterium tumefaciens (43). It has been proposed that this mode of regulating gene expression reflects the need for the invading pathogen to reach a critical population density sufficient to overwhelm host defenses and thus establish colonization and/or infection. Also, antibiotic production in Pseudomonas aureofaciens (32) and swarming behavior of Serratia liquefaciens MG1 (11) are regulated by AHLs. Recent observations suggest that interspecies communication might exist (10, 26, 31). These extracellular signals emerging from some bacterial species may influence the activity of other bacterial species.
The quality of most chilled foods deteriorates due to microbial activity, and an evaluation of the roles of different bacterial species in the spoilage process is important for shelf life prediction and for development of more effective and targeted preservation procedures. Understanding food spoilage requires detailed knowledge of the bacteria involved and their growth kinetics and metabolism within the food environment. In aerobically stored fish, the growth and activity of one specific bacterial group (species) usually causes spoilage when the population reaches a level of approximately 108 to 109 CFU/g (18). However, fresh meat and chicken that are vacuum packed or foods preserved by the addition of salt or by light smoking or which are slightly acidified (by additives) contain a mixture of bacterial species, and in no case has a single species been identified as the cause of spoilage (6, 18). These are often high-value food products, and a better understanding of the spoilage process would allow establishment of scientifically based quality indices as well as enable development of improved preservation methods.
Psychrotrophic members of the family Enterobacteriaceae (e.g., Enterobacter agglomerans, Hafnia alvei, Serratia liquefaciens, and Rahnella aquatilis) are frequent members of the spoilage microflora of vacuum-packed meats and lightly preserved fish products like cold-smoked salmon, where they typically reach numbers of 105 to 107 CFU/g (6, 20, 41). It has been suggested that these Enterobacteriaceae play a role in spoilage. However, levels of less than 106 CFU/g are not believed to be sufficient to cause quality defects. Several members of the Enterobacteriaceae have been found to employ AHL-mediated gene regulation (1, 10, 36, 38). This could also be true for food-borne Enterobacteriaceae and could enable them to boost production of, e.g., enzyme systems at certain cell densities, thereby contributing to quality changes at relatively low cell numbers. AHL-based communication systems regulate expression of hydrolytic exoenzymes (21), toxins (1, 15), and biosurfactant production (24) and mediate bacterial processes, such as surface motility and colonization (9, 11). These processes are potentially important in food spoilage and safety. A general knowledge of the AHL-producing bacteria involved in food quality deterioration and the phenotypes governed by this type of control is therefore of interest.
The purpose of the present study was to evaluate whether common food spoilage bacteria, like psychrotrophic Enterobacteriaceae, produce AHL molecules and if such a production takes place at bacterial levels and physical-chemical conditions (temperature, NaCl-levels, and atmospheric conditions) relevant to the preservation and storage of food products.
MATERIALS AND METHODS
Identification, growth, and enumeration of bacteria.
Strains of Enterobacteriaceae present in vacuum-packed and CO2-packed cold-smoked salmon stored at 5°C were enumerated on Trypticase soy agar (TSA) (catalog no. CM131; Oxoid, Basingstoke, England) overlaid with double-strength violet red bile glucose agar (VRBG) (catalog no. CM485; Oxoid). The strains were isolated from VRBG plates and cultured on TSA plates (29) and in Luria-Bertani (LB) broth, Miller (catalog no. 0446-17-3; Difco Laboratories, Detroit, Mich.). The strains were identified as Enterobacteriaceae by standard biochemical techniques (2) and by testing for DNase production (catalog no. 0220-01; Difco), gelatin hydrolysis, decarboxylation of lysine or ornithine, deamination of arginine (12), fermentation of sorbitol, and gas production from glucose in Hugh and Leifson’s (19) medium and testing by the API 20E system (bioMérieux, Marcy, France).
Enterobacteriaceae strains from vacuum-packed meats stored at −1.5 or 4°C were received from E. Borch and Y. Blixt of the Swedish Meat Research Institute, where they had been identified by the API system (5a).
Screening for AHL production.
The presence of AHLs was detected with a LuxR bioluminescence-monitoring system present on pSB403 in Escherichia coli (11, 38). One hundred microliters of the monitor strain at an optical density at 450 nm (OD450) of 0.8 to 1.0 was mixed with each 100-μl sample (sterile filtered culture supernatant or salmon extract) in microtiter wells (MicroBeta 1450-405/511; Wallac). Bioluminescence was measured for up to 6 h on a MicroBeta 1450 TriLux scintillation and luminescence counter (Wallac). Positive controls included supernatants from V. fischeri MJ1 (28) and S. liquefaciens MG1 (11) producing N-oxohexanoyl-l-homoserinelactone (OHHL) and N-butanoyl-l-homoserinelactone (BHL) respectively, as major AHLs. Negative controls included sterile growth media and supernatants from an AHL-negative S. liquefaciens swrI mutant strain, MG4.4 (11). The ability of the organisms to produce AHL was tested after culturing them in LB broth for 24 h at 25°C.
AHL production was also detected by AHL-regulated violacin production with Chromobacterium violaceum CV026 as an indicator (25). Enterobacteriaceae strains were streaked on LB plates in parallel with a lane of strain CV026. The same positive and negative control strains employed in the LuxR bioluminescence assay were used.
Extraction of culture supernatants and analytical TLC.
The procedure described by Shaw et al. (35) for thin-layer chromatography (TLC) was used. In brief, extracts for analytical TLC were prepared from 100-ml cultures grown in AB minimal medium (8) with 0.5% glucose and 0.5% casamino acids at 30°C. The early-stationary-phase culture was mixed with 100 ml of ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness. The AHLs were dissolved in 500 μl of high-performance liquid chromatography-grade ethyl acetate. Samples in volumes of 1 to 3 μl were applied to reversed-phase TLC plates (20 by 20 cm2; 200-μm layer; RP-18F254S [Merck article no. 1.05559]), and chromatograms were developed with methanol-water (60:40 [vol/vol]). After development, the solvent was evaporated and the dried plates were overlaid with 5 mm of LB growth medium (5) containing 1.4% agar and 0.4 ml of outgrown culture of the LuxR bioluminescence indicator bacterium. After the agar solidified, the coated plates were incubated at 30°C for approximately 16 h. Bioluminescence was detected and captured with a Hamamatsu charge-coupled device camera connected via a Hamamatsu M4314 controller to a Hamamatsu Argus-50 image processor.
Kinetics of AHL production in laboratory substrates.
Three strains representing the AHL-positive species were grown in LB broth, Miller and in a defined medium with M9 salts (34) supplemented with 0.4% glucose and 0.3% casamino acids (M9GC). Two strains (S. liquefaciens and E. agglomerans) isolated from cold-smoked salmon were tested at 5°C in media supplemented with 4% NaCl to reflect the salt level in lightly preserved food products. One strain (H. alvei) isolated from vacuum-packed meat was tested in LB broth at 4°C. The strains were precultured in the appropriate medium for 5 to 7 days at 5°C and inoculated at a cell density of 102 to 103 CFU/ml. Samples were withdrawn daily for determination of cell density by spread plating and AHL content. When light emissions exceeding 150,000 luminescence counts per second (LCPS) were found, the sample was diluted appropriately in sterile medium prior to measurement.
Kinetics of AHL production in cold-smoked salmon. (i) Sterile muscle blocks.
Cold-smoked, dry-salted salmon sides produced from a Norwegian farmed salmon were obtained from a Danish smokehouse. The sides were vacuum packed immediately after smoking and stored frozen at −20°C until use. The sides were thawed at 2°C for 1 to 2 days, and the upper 1-cm layer of the meat was removed under aseptic conditions. Blocks of assumed sterile meat were excised under aseptic conditions and stored at 0°C until use (maximum, 72 h).
Inoculation and withdrawal of samples.
An E. agglomerans strain and an S. liquefaciens strain isolated from cold-smoked salmon were precultured at 10°C for 3 days in LB broth supplemented with 4% NaCl and diluted in chilled physiological saline with 0.1% peptone to 105 to 106 CFU/ml. Blocks of salmon were each inoculated with 10 μl of this suspension per g, yielding a starting level of 103 to 104 CFU/g. The muscle blocks were placed individually in 4.5-cm-diameter petri dishes. The samples were stored at 5°C in anaerobic jars, from which atmospheric gas was removed and replaced with an N2 atmosphere (simulating the low-oxygen conditions of vacuum packing). At regular intervals, three blocks inoculated with each strain were withdrawn and analyzed separately. Bacterial cell densities were followed by spread plating on TSA. AHL(s) were extracted by mixing 2 g of muscle with 2 ml of sterile water, removing food debris and bacterial cells by centrifugation 16,000 × g for 10 min), and filtration (0.45-μm pore size) of the liquid. The extracts were tested for the presence of AHLs by the LuxR bioluminescence system.
Presence of AHL(s) in commercial samples of cold-smoked salmon.
Ten samples of cold-smoked salmon produced by different Danish smokehouses were bought at retail markets and stored at 5°C. When the samples were rejected by a sensory panel for the appearance of objectionable odors and flavors (21a), sterile filtered extracts were tested as described above. Counts of Enterobacteriaceae were estimated by the TSA-VRBG procedure.
RESULTS
Relationship between concentration of AHL and bioluminescence emission.
A standard curve relating light emission to concentration of OHHL was determined with the pSB403 E. coli monitor strain grown in LB broth or in LB broth with 4% NaCl. The bioluminescence response was linear until it reached a light emission of 150,000 to 200,000 LCPS covering from 0.1 to 10 nM OHHL in LB broth and from 0.1 to 62.5 nM OHHL in LB broth with NaCl (Fig. 1). The result of this standard curve led us to dilute samples exceeding 150,000 LCPS.
FIG. 1.
Light emission from an E. coli harboring the pSB403 LuxR monitor plasmid as a function of increasing concentrations of OHHL.
AHL production and identification of psychrotrophic strains of Enterobacteriaceae.
Of 96 strains isolated from cold-smoked salmon, 33 elicited a response in both the LuxR bioluminescence and the C. violaceum monitors (Table 1). Twenty-seven E. agglomerans strains were positive in the C. violaceum assay but caused no reaction in the LuxR bioluminescence system. In contrast, 17 strains, identified as S. liquefaciens, did not cause violacin production in C. violaceum but gave a positive reaction with the LuxR bioluminescence system. The majority of AHL-positive strains from cold-smoked salmon were identified as E. agglomerans or S. liquefaciens. Several strains produced exoproteases, as judged from digestion of gelatin; however, neither this activity nor any other of the phenotypic traits tested correlated with AHL production as determined by the LuxR bioluminescence and violacin-based monitoring systems.
TABLE 1.
Characterization and AHL production of 96 psychrotrophic Enterobacteriaceae strains isolated from cold-smoked salmon
| Total no. | AHL productiona
|
Hydrolysis
|
Decarboxylase
|
Fermentation of sorbitol | Gas from glucose | Tentative identification | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| pSB403 | C. violaceum | DNA | Gelatin | Arginine | Ornithin | Lysin | ||||
| 38 | 27 | 33 | − | −b | − | − | − | − | + | E. agglomerans |
| 21 | 0 | 21 | − | − | − | − | − | + | − | E. agglomerans |
| 9 | 0 | 0 | − | − | − | − | − | + | + | R. aquatilisc |
| 2 | 2 | 2 | − | + | − | − | − | − | + | Not identified |
| 3 | 0 | 0 | + | + | − | − | + | + | + | Serratia sp. |
| 23 | 21 | 4 | + | + | − | + | + | + | + | S. liquefaciens |
| 96 | 50 | 60 | ||||||||
Production of AHL measured by light emission from pSB403 or by violacin production by C. violaceum. The column gives the number of positive strains.
One strain positive.
Identified by API 20E (bioMérieux).
Fifty-eight Enterobacteriaceae strains from vacuum-packed chilled meats were identified as S. liquefaciens (21 strains) and H. alvei (37 strains) (Table 2) (5a). All H. alvei strains elicited a strong V. fisheri-like response from the LuxR bioluminescence monitor plasmid, while only two S. liquefaciens strains produced AHL(s) detectable by the monitoring systems employed (Table 2).
TABLE 2.
Characterization and AHL production of 58 psychrotrophic Enterobacteriaceae strains isolated from chilled, vacuum-packed meat
| Total no. | AHL productiona
|
Hydrolysis
|
Decarboxylase
|
Fermentation of sorbitol | Tentative identification | ||||
|---|---|---|---|---|---|---|---|---|---|
| pSB403 | C. violaceum | DNA | Gelatin | Arginine | Ornithin | Lysin | |||
| 37 | 37 | 37 | − | − | − | + | + | − | H. alvei |
| 21 | 2 | 0 | + | + | − | + | + | + | S. liquefaciens |
| 58 | 39 | 37 | |||||||
Production of AHL measured by light emission from a LuxR-based monitoring system or by violacin production by C. violaceum.
TLC analysis of culture supernatants.
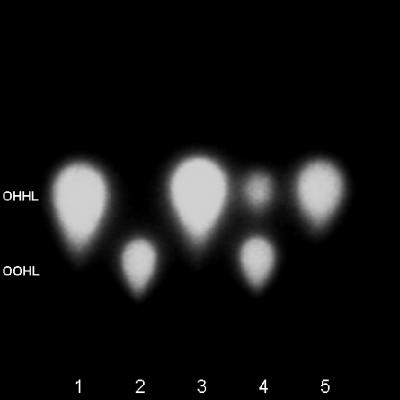
The TLC analysis performed on ethyl acetate extracts from the three selected strains, H. alvei, S. liquefaciens, and E. agglomerans, indicated the presence of OHHL in all extracts. However, the major AHL product in extracts from E. agglomerans appeared to be N-3-oxooctanoyl-l-homoserine lactone, OOHL (Fig. 2).
FIG. 2.
TLC of extracted supernatants from H. alvei (lane 3), E. agglomerans (lane 4), and S. liquefaciens (lane 5). Standards included 10 pmol of OHHL (lane 1) and 30 pmol of OOHL (lane 2). The TLC chromatogram was developed with 60%/40% methanol-water and overlaid with LB agar containing the LuxR bioluminescence-based monitoring system, pSB403, in E. coli.
Kinetics of AHL production in laboratory substrates.
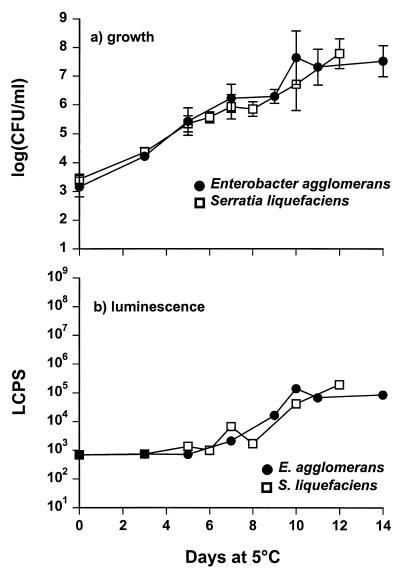
S. liquefaciens and E. agglomerans grew well at low temperatures in both LB broth and defined medium, reaching cell densities of 109 and 108 CFU/ml, respectively (Fig. 3a). Generation times in both media were 8 to 9 h (data not shown). Addition of 4% NaCl increased the generation time to 11 to 12 h (Fig. 3a). Sterile filtered supernatants from the two AHL-positive strains caused detectable stimulation of the LuxR bioluminescence-monitoring system when cell counts of the producer strain reached 106 CFU/ml and above (Fig. 3b). The response from the monitoring system increased at the same rate as the increase in cell numbers (Fig. 3). In a similar manner, an H. alvei strain isolated from vacuum-packed beef also produced compounds that stimulated the LuxR bioluminescence system (Fig. 3).
FIG. 3.
Growth (a) and AHL production (b) of S. liquefaciens, E. agglomerans, and H. alvei. S. liquefaciens and E. agglomerans were grown in LB broth with a total of 4% NaCl at 5°C, whereas H. alvei was grown in LB broth at 4°C. AHL(s) were detected by the LuxR bioluminescence monitoring system and measured as log (LCPS). The points are the averages of duplicate samples, and the error bars indicate standard deviations.
Kinetics of AHL production in cold-smoked salmon.
The strains grew rapidly in cold-smoked salmon and reached a maximum mean cell density of 108 CFU/g after 2 weeks at 5°C (Fig. 4). A 1:1 water extract of the salmon inoculated with AHL-positive strains produced a two- to fivefold increase in bioluminescence of the LuxR monitoring system when cell numbers on the salmon reached mean values of 105 to 106 CFU/g (Fig. 5). A 200-fold increase in bioluminescence was recorded when mean cell counts reached 108 CFU/g (Fig. 4). Thus, no increase in specific activity (bioluminescence per cell unit) was seen.
FIG. 4.
Growth (a) and AHL production (b) of S. liquefaciens and E. agglomerans in cold-smoked salmon stored under N2 atmosphere at 5°C. The points are the averages of triplicate samples, and the error bars indicate standard deviations.
FIG. 5.
Comparison of bacterial cell density (CFU/g) and AHL production measured as LCPS from a LuxR bioluminescence monitoring system, pSB403. The points are from individual samples of cold-smoked salmon (with less than 107 CFU/g) inoculated with either E. agglomerans or S. liquefaciens and incubated under an N2 atmosphere at 5°C.
Presence of AHLs in commercial cold-smoked salmon.
Four of the 10 samples of retail cold-smoked salmon tested at the end of shelf life caused a detectable response in the LuxR bioluminescence-monitoring system (Table 3). All four samples contained a mean of 106 to 107 Enterobacteriaceae per g, whereas the level of Enterobacteriaceae in samples where AHLs were not detected was below 105 CFU/g.
TABLE 3.
Levels of Enterobacteriaceae and presence of AHL(s) in commercial noninoculated samples of cold-smoked salmon when rejected by a sensory panela
| Sample | AHL(s) presentb | Enterobacteriaceae count (CFU/g)c |
|---|---|---|
| a1 | − | 1.4 × 105 |
| a2 | − | 1.0 × 103 |
| b1 | − | <103 |
| c1 | − | 1.8 × 104 |
| 4a | + | 1.2 × 106 |
| 4b | ++ | 1.9 × 106 |
| 4c | ++ | 1.2 × 107 |
| 5c | − | <103 |
| 6a | ++ | 1.0 × 106 |
| 6c | − | 1.0 × 103 |
The salmon was vacuum packed and stored at 5°C.
Two- to fivefold (+) or 10-fold (++) increase of bioluminescence from a LuxR-based monitoring system (pSB403) compared to a negative control.
Counts of Enterobacteriaceae were done by the TSA-VRBG procedure.
DISCUSSION
AHLs have been identified in many gram-negative pathogenic and symbiotic bacteria, and they are recognized as a primary way of regulating gene expression in relation to cell density. The majority of studies have focused on one or a few strains of a particular species, e.g., V. fisheri MJ1 (23), P. aeruginosa PAO1 (30), or S. liquefaciens MG1 (11, 16). However, more comprehensive studies of bacteria and bacterial populations must be undertaken to reveal the importance of this type of regulation in the environment and thereby understand its possible ecological significance. Cha et al. (7) screened 106 bacterial strains representing seven gram-negative plant-associated genera and found more than half to be AHL-positive. Our study demonstrates that the production of AHLs is a common feature in psychrotrophic Enterobacteriaceae found in food products.
The detection ranges and sensitivities of the applied AHL-monitoring systems define which AHL(s) can be detected. It is therefore possible that more than 116 of the 154 strains tested in the present study can produce AHLs, and our work can only be seen as a preliminary screening. Nevertheless, based on the TLC analysis and the magnitude of response elicited from a LuxR-based AHL-monitoring system, we anticipate that a large fraction of the strains investigated in this study produce OHHL or one of its closest analogues, e.g., OOHL. OHHL has been identified as the major regulator of bioluminescence in V. fischeri (23). To date, OHHL is the most common signal molecule found in Enterobacteriaceae, regulating carbapenem and exoprotease production in E. carotovora (1, 21) and exopolysaccharide production in Pantoea (Erwinia) stewartii (4). AHLs have also been found in other Enterobacteriaceae strains, including Yersinia enterocolitica (40), E. agglomerans (38), and H. alvei (38). The phenotypes regulated in these strains, however, were not determined (38). In contrast to Swift et al. (38), we did not detect AHL from R. aquatilis strains by either of the two AHL-monitoring systems. A few S. liquefaciens strains caused a response from the LuxR bioluminescence system similar to the response caused by the BHL- and N-hexanoyl homoserine lactone (HHL)-producing S. liquefaciens strain MG1, indicating that BHL or another nonsubstituted homoserine lactone was produced by these strains. The detection of OOHL in the TLC analysis confirmed that other types of AHLs were produced. Although most strains (70 of 116) caused responses in both the LuxR bioluminescence and the Chromobacterium assays, several strains were positive in only one of the assays, showing that different strains had different AHL profiles. Since Chromobacterium detects BHL and HHL much more efficiently than the LuxR system, several strains (Tables 2 and 3) probably produce BHL and related compounds.
AHLs are generally thought to accumulate in media throughout the growth cycle, and expression of target genes shows a “burst” at a certain threshold level. This induction is believed to be caused by an autoinducing circuit (11, 23, 38). The strains of Enterobacteriaceae examined under food-relevant conditions in vivo did not appear to exhibit an autoinducible production at the cell densities studied (Fig. 3 and 4), as the amount of AHL (measured as luminescence response) per unit of cell density did not increase. Preliminary experiments done with the strains in LB broth at 25°C indicated that the strains used in our study exhibited autoinduction at higher cell densities above an OD450 of 3.0, equivalent to 108 to 109 CFU/ml (data not shown). The cell counts of cold-smoked salmon are mean values and do not take into account the spatial distribution, and discrete areas (e.g., on the surface) could have higher local cell densities. It might be speculated that the bacterial population reaches quorum size in such local areas, leading to AHL production. Investigations of this possibility are in progress in our laboratories.
Assessment of the AHL content was performed by measuring bioluminescence emission from an E. coli strain harboring the LuxR-based pSB403 monitor following exposure to a sterile filtered extract. Although the light response was linearly related to the concentration of OHHL over a certain range (Fig. 1), it may not be as accurate as directly measuring the phenotype regulated by the AHLs. However, the system is simple and allows for rapid screening of a large number of strains. Similar to our findings, a linear relationship between AHL concentration and response from an AHL-monitoring system has been reported (35, 41). The LuxR bioluminescence-based system responded to concentrations of OHHL in the nanomolar range (Fig. 1), as reported in other studies (42).
While many studies have shown that gram-negative bacteria under laboratory conditions are capable of producing AHLs, fewer have evaluated the possible production and potential importance under in vivo conditions. This requires that the growth conditions be similar to those of the real environment and that the kinetics be evaluated; i.e., at what cell densities are AHLs produced and are these densities ever reached in real life? AHL production by E. agglomerans, H. alvei, R. aquatilis was seen at an OD600 of 0.3 to 0.9 (38) and by Aeromonas hydrophila, similarly, at an OD600 of approximately 1.0 (37). These absorbance levels are approximately equivalent to 107 to 108 CFU/ml. Production of AHLs in our study was detected at cell densities that were 1 to 2 orders of magnitude lower (106 CFU/ml). Differences in culture conditions may explain the lower cell density at which AHL production took place in our experiments. Our studies were carried out under food storage conditions (5°C; limited oxygen; NaCl), whereas other studies (37, 38) have been carried out at higher temperatures (30°C) under highly aerated conditions in laboratory media. In the latter, maximum densities of 1010 CFU/ml are typically reached, whereas maximum densities were 108 to 109 CFU/ml in our study.
AHLs were detected in naturally contaminated (noninoculated) samples of vacuum-packed cold-smoked salmon containing 105 to 107 Enterobacteriaceae/g (Table 3). These data, together with the pure-culture studies, indicated that in cold-smoked salmon, Enterobacteriaceae are the main producers of AHLs, as AHLs could not be detected in samples with low numbers of Enterobacteriaceae. The actual limit in terms of CFU per gram at which AHLs can be detected is of course also influenced by the lower detection limit of the monitoring system.
There are several studies that have failed to identify target genes or determine the phenotypes regulated by identified AHL-based signalling systems (27, 37). Construction of AHL-negative mutants followed by secondary mutagenesis with a reporter transposon as described by Lindum et al. (24) may be a strategy for identifying target genes and phenotypes (11, 24), and we have recently started identifying the AHL synthetase and receptor genes in the psychrotrophic Enterobacteriaceae. However, the construction of AHL-negative mutants of E. agglomerans (38) or Vibrio anguillarum (27) did not identify target genes nor did it unravel the phenotypes regulated. Given the large array of factors regulated by AHL, it may be speculated that either hydrolytic enzymes or biosurfactants or other lipopeptides could be regulated by the AHLs. If the AHLs regulate factors of importance in the deterioration of foods, the specific blockage of the AHL molecule (17, 22) could give rise to a new approach to food preservation.
In conclusion we have shown that many strains of Enterobacteriaceae isolated from foods produce AHLs. Production is detectable from naturally contaminated foods and from samples to which pure cultures have been added when levels of Enterobacteriaceae reach 105 to 107 CFU/g. These levels are not uncommon in foods, which indicates that AHLs could be implicated in regulating phenotypes important in food spoilage and thus possibly play a role in food quality deterioration.
ACKNOWLEDGMENTS
This work was financed by grants from the Danish Technical Research Council and the Danish Research Council for Natural Research.
The excellent technical assistance of Jette Melchiorsen and Linda Stabell is highly appreciated.
REFERENCES
- 1.Bainton N J, Stead P, Chhabra S R, Bycroft B W, Salmond G P C, Stewart G S A B, Williams P. N-(3-oxohexanoyl)-l-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992;288:997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow G I, Feltham R K A, editors. Cowan and Steel’s manual for the identification of medical bacteria. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 3.Bassler L B, Wright M, Silverman M R. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 4.Beck von Bodman S, Farrand S K. Capsular polysaccharide synthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J Bacteriol. 1995;177:5000–5008. doi: 10.1128/jb.177.17.5000-5008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertani G. Studies on lysogenesis. 1. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Blixt, Y. Personal communication.
- 6.Borch E, Kant-Muermans M-L, Blixt Y. Bacterial spoilage of meat and cured meat products. Int J Food Microbiol. 1996;33:103–120. doi: 10.1016/0168-1605(96)01135-x. [DOI] [PubMed] [Google Scholar]
- 7.Cha C, Gao P, Chen Y-C, Shaw P D, Farrand S K. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 8.Clark J D, Maaloe O. DNA replication and the cell cycle in Escherichia coli. J Mol Bacteriol. 1967;23:99–112. [Google Scholar]
- 9.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 10.Eberl L, Molin S, Givskov M. Surface motility of Serratia liquefaciens MG1. J Bacteriol. 1999;181:1703–1712. doi: 10.1128/jb.181.6.1703-1712.1999. . (Minireview.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberl L, Winson M K, Sternberg C, Stewart G S A B, Christiansen G, Chhabra S R, Daykin M, Williams P, Molin S, Givskov M. Involvement of N-acyl-l-homoserine lactone autoinducers in control of multicellular behavior of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 12.Falkow S. Activity of lysine decarboxylase as an aid in the identification of Salmonellae and Shigellae. Am J Clin Pathol. 1958;29:598. doi: 10.1093/ajcp/29.6_ts.598. [DOI] [PubMed] [Google Scholar]
- 13.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 14.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambello M J, Kaye S, Iglewski B H. LasR of Pseudomonas aeruginosa is a transcriptional activator of elastase expression. Infect Immun. 1991;61:1180–1184. doi: 10.1128/iai.61.4.1180-1184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givskov M, Eberl L, Molin S. Control of exoenzyme production, motility and cell differentiation in Serratia liquefaciens. FEMS Microbiol. 1997;148:115–122. [Google Scholar]
- 17.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P D, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated procaryotic signalling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gram L, Huss H H. Microbiological spoilage of fish and fish products. Int J Food Microbiol. 1996;33:121–138. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- 19.Hugh R, Leifson E. The taxonomic significance of fermentative versus oxidative gram-negative bacteria. J Bacteriol. 1953;66:24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiménez S M, Salsi M S, Tiburzi M C, Rafaghelli R C, Tessi M A, Coutaz V R. Spoilage microflora in fresh chicken breast stored at 4°C: influence of packaging methods. J Appl Microbiol. 1997;83:613–618. doi: 10.1046/j.1365-2672.1997.00276.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Bhhabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Jørgensen, L. V. Unpublished data.
- 22.Kjelleberg S, Steinberg P D, Givskov M, Gram L, Manefield M, de Nys R. Do marine products interfere with procaryotic AHL regulatory systems? Aquat Microbiol Ecol. 1997;13:85–93. [Google Scholar]
- 23.Kuo A, Blough N V, Dunlap P V. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994;176:7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindum P W, Anthoni U, Christophersen C, Eberl L, Molin S, Givskov M. N-Acyl-l-homoserine lactone autoinducers control production of an extracellular lipopeptide biosurfactant required for swarming motility of Serratia liquefaciens MG1. J Bacteriol. 1998;180:6384–6388. doi: 10.1128/jb.180.23.6384-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClean K H, Winson M K, Fish L, Taylor A, Chhabra S R, Camara M, Daykin M, Lamb J H, Swift S, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 26.McKenny D, Borwn K E, Allison D G. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: evidence of interspecies communication. J Bacteriol. 1995;177:6989–6992. doi: 10.1128/jb.177.23.6989-6992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milton D L, Hardman A, Camara M, Chhabra S R, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing in Vibrio anguillarum: characterization of the vanI/vanR locus and identification of the autoinducer N-(3-oxodecanoyl)-l-homoserine lactone. J Bacteriol. 1997;179:3004–3012. doi: 10.1128/jb.179.9.3004-3012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nealson K H. Autoinduction of bacterial luciferase: occurrence, mechanisms and significance. Arch Microbiol. 1977;122:73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- 29.Paludan-Müller C, Dalgaard P, Huss H H, Gram L. Evaluation of the role of lactic acid bacteria in the spoilage of cold-smoked salmon. Int J Food Microbiol. 1998;39:155–166. doi: 10.1016/s0168-1605(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 30.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 31.Pierson E A, Wood D W, Cannon J A, Blachere F M, Pierson L S., III Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol Plant-Microbe Interact. 1998;11:1076–1084. [Google Scholar]
- 32.Pierson L S, III, Wood D W, Pierson E A. Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu Rev Phytopathol. 1998;36:207–225. doi: 10.1146/annurev.phyto.36.1.207. [DOI] [PubMed] [Google Scholar]
- 33.Salmond G P C, Bycroft B W, Stewart G S A B, Williams P. The bacterial enigma: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swift S, Throup J P, Williams P, Salmond G P C, Stewart G S A B. Quorum sensing: a population density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 37.Swift S, Karlyshev A V, Fish L, Durant E L, Winson M K, Chhabra S R, Williams P, MacIntyre S, Stewart G S A B. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J Bacteriol. 1997;179:5271–5281. doi: 10.1128/jb.179.17.5271-5281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swift S, Winson M K, Chan P F, Bainton N J, Birdsall M, Reeves P J, Rees C E D, Chhabra S R, Hill P J, Throup J P, Bycroft B W, Salmond G P C, Williams P, Stewart G S A B. A novel strategy for the isolation of luxI homologues: evidence for the widespread distribution of a LuxR:LuxI superfamily in enteric bacteria. Mol Microbiol. 1993;10:511–520. doi: 10.1111/j.1365-2958.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 39.Tang H B, DiMango D, Bryan R, Gambello M, Iglewski B H, Goldberg J B, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Throup J P, Camara M, Briggs G S, Winson M K, Chhabra S R, et al. Characterisation of the yenI/yenR locus from Yersinia enterocolitica mediating the synthesis of two N-acylhomoserine lactone signal molecules. Mol Microbiol. 1995;17:345–356. doi: 10.1111/j.1365-2958.1995.mmi_17020345.x. [DOI] [PubMed] [Google Scholar]
- 41.Truelstrup Hansen L, Gill T, Huss H H. Effects of salt and storage temperature on chemical, microbiological and sensory changes in cold-smoked salmon. Food Res Int. 1995;23:17–34. [Google Scholar]
- 42.Winson M K, Swift S, Fish L, Throup J P, Jørgensen F, Chhabra S R, Bycroft B W, Williams P, Stewart G S A B. Construction and analysis of luxCDABE-based plasmid-sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett. 1998;163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-homoserine lactones. Nature (London) 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]