SUMMARY
Since the implementation of Streptococcus pneumoniae (SPn) conjugate vaccination (PCV), non-vaccine types have prevailed in invasive pneumococcal disease (IPD), and an increase in Staphylococcus aureus (SA) burden has been suggested. Here, we assess the epidemiology of SA and SPn nasal carriage in 620 children at day-care centres; 141 of these children had received 1–4 PCV7 doses. A higher vaccine dosage was associated with non-vaccine-type SPn carriage. Of all SPn isolates, 45% were PCV7 types, 1% were additional PCV10 types and 22% were the three additional PCV13 types. SA carriage was inversely associated with vaccine-type SPn carriage. SPn serotype 19A showed higher SA co-carriage rates compared to other SPn serotypes. PCV7 implementation does not prevent children from being part of the IPD-related SPn transmission chain. These results contribute to the monitoring of SA- and SPn-related disease and add to the debate on the current national vaccination policy that recently included a change from PCV7 to PCV10.
Key words: Antibiotic resistance, children, day-care centres, Staphylococcus aureus, Streptococcus pneumoniae, vaccination
INTRODUCTION
Streptococcus pneumoniae (SPn) causes about 11% of all deaths in children aged <5 years and is a leading cause of bacterial pneumonia, meningitis, and sepsis in children worldwide [1]. Several countries, including The Netherlands, have implemented the pneumococcal conjugate vaccine (PCV), which has proved effective in preventing invasive diseases with SPn in young children [1–6]. However, the incidence of invasive pneumococcal disease (IPD) caused by non-vaccine SPn serotypes is substantially increasing. The most common types found in IPD isolates since the introduction of PCV7 in Belgium, France, Germany, Greece, Norway, Portugal, Spain, UK and The Netherlands are the non-PCV7 serotypes 1, 3, 6A, 7F, and 19A; these serotypes are all included in PCV13 [6, 7]. Serotype 19A currently poses a major concern as its carriage rates have rapidly increased. This serotype has emerged as the major cause of otitis media in children, and in the USA its strains are frequently multiresistant to antibiotics [8, 9]. In the pre-vaccination era, the most common serotypes found in IPD isolates were PCV7 types 14, 6B, 19F, and 23F [7, 10].
Invasive disease is preceded by the establishment of a bacterial population in the nasal passages and the competition of this population with other bacteria in the host. Several epidemiological studies have demonstrated lower SPn carriage rates in the presence of Staphylococcus aureus (SA) nasal carriage [11–15]. In young children, SA is the main cause of impetigo contagiosa, which can lead to otitis media, and the most common cause of severe pneumonia after an influenza virus infection [16]. Epidemiological studies in the pre-PCV era demonstrated an inverse relationship between SA and vaccine SPn serotypes but not for non-vaccine SPn serotypes. One study conducted after vaccine implementation also showed such inverse association for vaccine serotypes [15]. Two other studies could not demonstrate an inverse effect for either vaccine or non-vaccine types [17] or overall SPn carriage, without analysing the serotypes separately [18]. With widespread vaccination, non-vaccine types replace vaccine types and the carriage rate of non-vaccine types increases [19]. This mechanism may lead to a rise in SA carriage rates [12, 15]. The changing epidemiology of SPn and possibly SA in the vaccine era requires further monitoring for a better understanding of the carriage of these bacteria and related invasive bacterial diseases. This understanding is relevant not only to the case of PCV7 vaccination but also to developing strategies for using higher valent vaccines and preventing serotype replacement [20].
The current cross-sectional study was conducted in PCV7-vaccinated and non-vaccinated young children. Our aim is to assess nasal carriage and determinants of SPn and SA carriage. Therefore, we focus on their interrelationship and explore the role of serotypes, age and number of PCV7 doses in SPn and SA carriage.
MATERIALS AND METHODS
Procedures and study population
In The Netherlands, neonates have been vaccinated with PCV7 since April 2006 with a four-dose schedule administered at ages 2, 3, 4, and 11 months; vaccinations are registered, and there is no catch-up vaccination programme for children born before April 2006.
The current cross-sectional study was conducted in a convenience sample of children (aged 6 weeks to 4 years) who attended 48 randomly chosen day-care centres out of a total of 150 in the province of Limburg, The Netherlands. After obtaining written informed consent from at least one of the parents/guardians, a nasal swab was taken from the participating child and a standardized short questionnaire was completed by the parent. The study population comprised 620 children. Approval for this study was obtained from the Medical Ethical Committee of Maastricht University (no. 104112).
Laboratory testing
On the same day of collection, the nasal swabs were forwarded to the Microbiology Laboratory of University Hospital Maastricht for testing on SA and SPn using standard biochemical tests. Serotyping was performed as previously described, using antiserum from Statens Serum Institute (Denmark) [21]. Of all the SPn isolates, 20 were unavailable for typing and six were non-typable.
Variables and statistical analyses
Univariate and multivariate logistic regression analyses were performed to identify independent determinants for SA and SPn carriage. The determinants of interest included carriage of SA, SPn and specific SPn serotypes. Age as a determinant was presented using polynomials (quintic function) and was tested by using two first-order splines describing monthly trends and by constructing two age groups (cut-off at 15 months). Finally, the number of PCV7 doses was included (continuous variable). Reduced-dose PCV7 schedules have reportedly been effective in reducing vaccine-type carriage, and schedules with more doses have been associated with higher serotype 19A carriage rates [22–24]. Other determinants, including gender, household size, and pet/cattle ownership, were not found to be significantly associated with SA or SPn carriage; thus, these results are not presented.
For the entire group, the correlation between age and number of vaccine doses was relatively high (r = 0·7) as vaccination was largely dependent on a child's age at time of study (only children aged <15 months were eligible for vaccination). Furthermore, both SA and SPn carriage rates are known to change substantially with age in young children [11, 25]. Therefore, when evaluating vaccination as a determinant, only children aged <15 months were included in the analyses (at this age, the correlation between age and number of vaccine doses is r = 0·3). We considered a P value ⩽0·05 as statistically significant. Analyses were performed with SPSS package version 14.0.2 (SPSS Inc., USA).
RESULTS
Of the 620 participants, 453 (73·1%) children were aged ⩾15 months and were born in the pre-PCV7 vaccination era, and although no data on their vaccination status are available, these children were unlikely to have been vaccinated. Of the 167 younger children, 14 had received one or two PCV7 vaccine doses, 86 had received three doses, 41 had completed the four-dose schedule, and 26 were unvaccinated.
Carriage of SA and SPn serotypes
The overall carriage rates were 19·8% (n = 123) for SA and 37·3% (n = 231) for SPn; 45% (n = 95) of the SPn isolates were PCV7 types, two (0·9%) were additional PCV10 types and 46 (21·8%) were the three additional PCV13 types. The carriage rates of the PCV7 types and non-vaccine types were 15·8% and 19·3%, respectively. Vaccine types 6B, 14, 19F, and 23F, and non-vaccine types 6A and 19A were the most common (Table 1).
Table 1.
Nasal carriage rates for heptavalent pneumococcal conjugate vaccine (PCV7) serotypes and a selection of non-PCV7 types in 620 children aged between 0 and 4 years attending day-care centres
| PCV7 | Non-PCV7 | ||||
|---|---|---|---|---|---|
| Serotype | n | (%)* | Serotype | n | (%)* |
| 4 | 2 | (0·3) | 1† | 2 | (0·3) |
| 6B | 29 | (4·8) | 3‡ | 1 | (0·2) |
| 9V | 0 | (0) | 6A‡ | 29 | (4·8) |
| 14 | 19 | (3·2) | 5†, 7F† | 0 | (0) |
| 18C | 5 | (0·8) | 11 | 11 | (1·8) |
| 19F | 18 | (3·0) | 15 | 12 | (2·0) |
| 23F | 22 | (3·7) | 19A‡ | 16 | (2·7) |
| Other§ | 45 | ||||
Denominator excludes 20 participants with no isolates available for typing.
Serotypes additionally included in PCV10.
Serotypes additionally included in PCV13.
Number of isolates was <15 for all serotypes, including six untypable isolates.
Age-related carriage
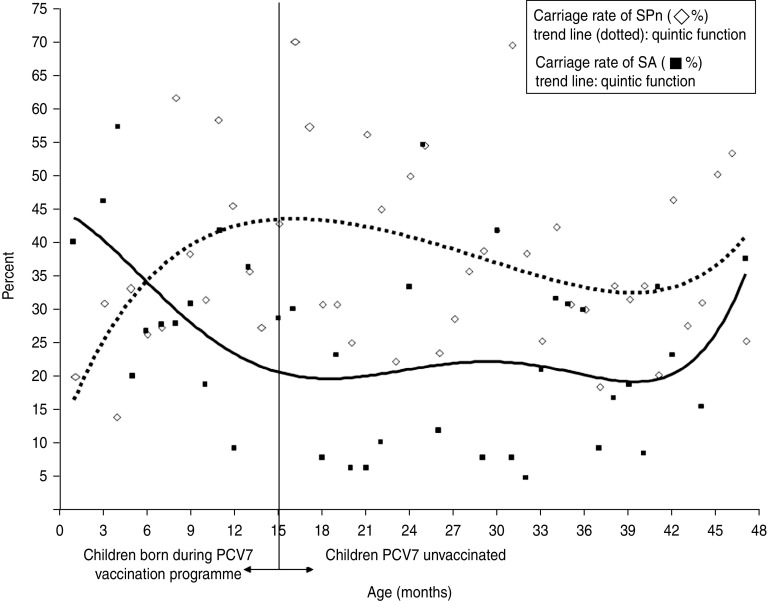
Age-related carriage of SA and SPn showed divergent curvilinear trends (Fig. 1). A significant decrease in SA carriage was observed in children aged <15 months [odds ratio (OR) per month 0·9, 95% confidence interval (CI) 0·6–1·0]. Compared to the older children, children aged <15 months (Table 2) had higher SA carriage rates (OR 1·8, 95% CI 1·2–2·8), higher co-carriage rates of SA and non-vaccine SPn types (OR 4·1, 95% CI 1·5–10·8), and lower carriage rates of SPn vaccine types (OR 0·6, 95% CI 0·3–1·0).
Fig. 1.
Age-related Streptococcus pneumoniae (SPn) and Staphylococcus aureus (SA) carriage in 620 children aged between 0 and 4 years attending day-care centres.
Table 2.
Carriage rates of Streptococcus pneumoniae (SPn) and Staphylococcus aureus (SA) serotypes in relation to the number of received doses of heptavalent pneumococcal conjugate vaccine (PCV7) and to age in 620 children aged between 0 and 4 years attending day-care centres
| (Co-)carriage rate, n (%)† | Age <15 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Unvaccinated | 1 or 2 PCV7 doses | 3 PCV7 doses | 4 PCV7 doses | P value‡ | Age <15 months | Age 15–48 months (all unvaccinated) | P value§ | |
| (n = 26) | (n = 14) | (n = 87) | (n = 42) | (n = 169) | (n = 457) | |||
| SA | 3 (11·5) | 7 (50·0) | 26 (30·2) | 10 (24·4) | 46 (27·5) | 77 (17·0) | ** | |
| SPn (all types) | 9 (34·6) | 3 (21·4) | 30 (34·9) | 19 (46·3) | 61 (36·5) | 170 (37·5) | ||
| SPn vaccine types | 5 (20·0) | 1 (7·1) | 9 (10·7) | 3 (7·7) | 18 (11·1) | 77 (17·6) | * | |
| SPn non-vaccine types | 3 (12·0) | 2 (14·3) | 19 (22·6) | 14 (35·9) | ** | 38 (23·5) | 78 (17·8) | |
| SPn vaccine type 6B | 1 (4·0) | 0 | 3 (3·6) | 1 (2·6) | 5 (3·1) | 24 (5·5) | ||
| SPn vaccine type 14 | 0 | 0 | 2 (2·4) | 1 (2·6) | 3 (1·9) | 16 (3·7) | ||
| SPn vaccine type 19F | 1 (4·0) | 0 | 2 (2·4) | 0 | 3 (1·9) | 15 (3·4) | ||
| SPn vaccine type 23F | 1 (4·0) | 1 (7·1) | 2 (2·4) | 1 (2·6) | 5 (3·1) | 17 (3·7) | ||
| SPn non-vaccine type 6A | 2 (8·0) | 1 (7·1) | 2 (2·4) | 2 (5·1) | 7 (4·2) | 22 (5·0) | ||
| SPn non-vaccine type 19A | 0 | 0 | 2 (2·4) | 1 (2·6) | 3 (1·9) | 13 (3·0) | ||
| SA and SPn | 1 (3·8) | 0 | 7 (8·1) | 4 (9·8) | 12 (7·2) | 18 (4·0) | ||
| SA and SPn vaccine types | 0 | 0 | 1 (1·2) | 0 | 1 (0·6) | 11 (2·5) | ||
| SA and SPn non-vaccine types | 0 | 0 | 6 (7·1) | 4 (10·3) | # | 10 (6·2) | 7 (1·6) | ** |
Denominator excludes the 20 isolates that were not typed.
χ2 test for linear trend in the association between number of PCV7 doses and carriage rate.
χ2 test of the association between carriage rate and age group.
P ⩽ 0·10, * P ⩽ 0·05, ** p ⩽ 0·01.
Vaccination and carriage in children aged <15 months
The number of vaccine doses (Table 2) was not associated with SA carriage, SPn carriage or vaccine-type carriage. However, an increase in non-vaccine-type carriage (OR per dose 1·5, 95% CI 1·0–2·1) and in co-carriage of SA and non-vaccine types (OR per dose 2·3, 95% CI 0·9–5·8) was observed, although the latter association had only borderline significance (P = 0·07). Adjusting for age did not substantially change the association estimates for SPn, although for SA, the association with number of vaccine doses became somewhat stronger (adjusted OR 1·44, 95% CI 0·98–2·11, P = 0·06).
Interrelationship between SA and SPn carriage
Of all the children, 32·4% (n = 201) exhibited only SPn carriage, 15% (n = 93) had only SA carriage, and 4·8% (n = 30) had SA-SPn co-carriage. An inverse association was present between SA and SPn carriage, between SA and vaccine-type SPn carriage, and between SA and non-vaccine-type SPn carriage, although this last association had only borderline significance (Table 3). Risk estimates were similar for the younger and older children (interaction terms P > 0·1) and when adjusting for PCV7 dose or age.
Table 3.
Univariate associations between Streptococcus pneumoniae (SPn) and Staphylococcus aureus carriage in 620 children aged between 0 and 4 years attending day-care centres
| Total group (n = 620) OR (95% CI) | |
|---|---|
| SPn (all types)† | 0·48 (0·30–0·74)** |
| SPn vaccine types | 0·52 (0·27–0·98)* |
| SPn non-vaccine types | 0·62 (0·36–1·08)# |
OR, Odds ratio; CI, confidence interval.
Of all 233 SPn isolates, 20 were not serotyped.
P ⩽ 0·10, * P ⩽ 0·05, ** P ⩽ 0·01.
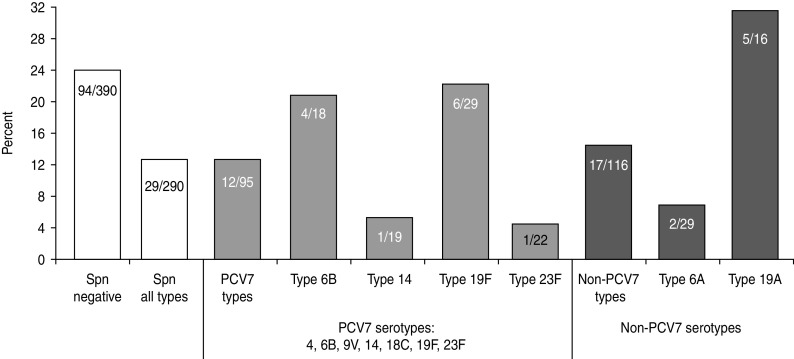
A notable serotype-specific pattern was observed in that the highest SA co-carriage rate occurred with serotype 19A (Fig. 2). Compared to children carrying SPn type 19A, the SA carriage rate was lower in children carrying SPn type 6A (OR 0·2, 95% CI 0·03–1·0), type 23F (OR 0·1, 95% CI 0·01–1·0), and type 14 (OR 0·1, 95% CI 0·01–1·2), although this last serotype had only borderline significance (P = 0·06). Other categories that were non-significant included SPn-negative children and children carrying type 19F, type 14, type 6B, other vaccine types and other non-vaccine types. Adjusting for age did not substantially change the risk estimates, while subsidiary analysis adjusting for PCV7 dose in young children was not possible due to the small number of cases.
Fig. 2.
Carriage rate of Staphylococcus aureus (SA) stratified by co-carriage of Streptococcus pneumoniae (SPn) (by serotype) in 620 children aged between 0 and 4 years attending day-care centres.
DISCUSSION
The current study assessed nasal carriage rates of SA and SPn serotypes and their interrelationship in PCV7-vaccinated and non-vaccinated children attending day-care centres. The carriage of non-vaccine types increased with the number of administered PCV7 doses. The rate of co-carriage between SA and SPn vaccine types was low; however, SA and non-vaccine-type SPn co-carriage was associated with young age and had a tendency to increase with vaccination. The SA carriage rate was remarkably high in children carrying non-vaccine-type 19A.
The observed inverse association between SA and SPn vaccine types agrees with previous studies [11–15]. Our findings did not provide direct evidence for the hypothesis [12, 15] that the overall SA carriage rate would increase with vaccination, although a tendency to increase was noted after correcting for the opposing effect of age. Notably, co-carriage with non-vaccine types tended to occur most frequently in vaccinated children. Marked serotype-specific patterns were also observed. The non-vaccine serotype 19A showed higher SA carriage rates than did several other serotypes. Previous studies have found that in unvaccinated individuals, successful nasal SA colonization is independent of both SA and SPn genotypes [11, 26]. No major differences between SA clones have been found regarding their capacity to compete with SPn types [27] and no association between the levels of IgG and IgA in the child vs. colonization status has been reported [28]. It is possible that the virtual absence of a pilus-like structure (i.e. an adhesin and inflammatory virulence factor) in serotype 19A may have played a role in this result. Some, but not all, studies have shown lower odds for co-colonization with a non-piliated SPn type [26, 29]. The mechanism of the negative association between SPn and SA is likely to be multi-factorial, involving both host immune responses and bacterial characteristics that may inhibit the other pathogen. In addition, the serotype-specific associations observed may reflect an epidemiological association, and further study is needed to address this interesting phenomenon.
Our study further confirmed an increase in non-vaccine types after the implementation of PCV7 vaccination [20]. SPn serotype 19A rates have been reported to increase [21–24, 30], but in our study, the number of cases was too small to demonstrate such serotype-specific associations. In Europe, the non-PCV7 types 1, 3, 6A, 7F and 19A have become the most common IPD isolates, all of which are targeted by PCV13 [6, 7]. Vaccination does not preclude children from becoming part of the IPD-related SPn transmission chain. For example, in the specific case of The Netherlands, a country with high vaccination coverage (∼95%), it was recently reported that 3 years after PCV7 implementation, 23% of infants aged 11 or 24 months carried serotypes targeted by PCV13 [23]. However, in 2011, The Netherlands changed to PCV10. PCV10 additionally targets serotypes 1, 5 and 7F, which show very low carriage rates in Dutch children, but does not include the important PCV13 types 3, 6A and 19A. As advised by the Dutch Health Council [31] PCV13 rather than PCV10 is likely to be the better choice in The Netherlands for preventing IPD [32].
In our study, vaccination was not associated with a reduced overall nasal SPn carriage, which is in agreement with other carriage studies [20]. However, proceeding further into the vaccination era, some reports suggest that the decline in PCV7 types may be starting to outweigh the non-vaccine-type increase, resulting in an overall decrease in SPn carriage [22, 23, 33].
The current study has several limitations. Some colonization with SPn may have been overlooked as the tested samples were taken from the anterior nares and not from the nasopharyngeal site, which is the most common sampling site. However, anterior nares sampling has been previously used to describe the epidemiology of SPn [17, 34] and our sampling method was unlikely to result in a substantial bias of the results. However, the serotype-specific carriage rates may be slightly underestimated due to the fact that the serotype could not be determined for 17 unvaccinated and three vaccinated children. Finally, the assessment of the effect of vaccination was somewhat limited by the small numbers in the young age group; thus, the results for the effect of vaccination should be interpreted with caution.
In conclusion, vaccinated children are still part of the IPD-related transmission chain. Current findings show that, at least on an epidemiological scale, the dynamics of SPn and SA carriage influence each other and are influenced by vaccination, and close monitoring remains essential for designing and evaluating infection prevention strategies.
CONCLUSIONS
-
(1)
Overall SPn carriage and carriage of non-vaccine serotypes commonly found in IPD are not reduced after PCV7 vaccination.
-
(2)
Although co-carriage rates of SA and SPn are generally low, co-carriage rates with SA and SPn non-vaccine types are higher in young children, especially those vaccinated with PCV7.
-
(3)
The rate of SA co-carriage is highest in children carrying SPn serotype 19A.
ACKNOWLEDGEMENTS
This study was supported by an unrestricted grant from the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment (RIVM), The Netherlands. The authors thank Kim Ottenheim of the Public Health Services of North Limburg for collecting samples and organizing logistics, the staff of the Medical Microbiology Laboratory, Maastricht University Centre, and Els Verhaagh, Elisabeth Hospital Tilburg, for conducting laboratory analyses. We thank RIVM for funding the study.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.O'Brien KL, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374: 893–902. [DOI] [PubMed] [Google Scholar]
- 2.Black SB, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatric Infectious Disease Journal 2002; 21: 810–815. [DOI] [PubMed] [Google Scholar]
- 3.Hansen J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatric Infectious Disease Journal 2006; 25: 779–781. [DOI] [PubMed] [Google Scholar]
- 4.Klugman KP, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. New England Journal of Medicine 2003; 349: 1341–1348. [DOI] [PubMed] [Google Scholar]
- 5.Lucero MG, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatric Infectious Disease Journal 2009; 28: 455–462. [DOI] [PubMed] [Google Scholar]
- 6.Rodenburg GD, et al. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerging Infectious Diseases 2010; 16: 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. International Journal of Infectious Diseases 2009; 14: e197–209. [DOI] [PubMed] [Google Scholar]
- 8.Xu Q, et al. Novel type of Streptococcus pneumoniae causing multidrug-resistant acute otitis media in children. Emerging Infectious Diseases 2009; 15: 547– 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan TQ. Antibiotic resistant infections due to Streptococcus pneumoniae: impact on therapeutic options and clinical outcome. Current Opinion in Infectious Diseases 2003; 16: 271–277. [DOI] [PubMed] [Google Scholar]
- 10.Hanage WP, et al. Evidence that pneumococcal serotype replacement in Massachusetts following conjugate vaccination is now complete. Epidemics 2010; 2: 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogaert D, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 2004; 363: 1871–1872. [DOI] [PubMed] [Google Scholar]
- 12.Regev-Yochay G, et al. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in Children. Journal of the American Medical Association 2004; 292: 716–720. [DOI] [PubMed] [Google Scholar]
- 13.Quintero B, et al. Epidemiology of Streptococcus pneumoniae and Staphylococcus aureus colonization in healthy Venezuelan children. European Journal of Clinical Microbiology Infectious Diseases 2011; 30: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNally LM, et al. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected South African children. Journal of Infectious Diseases 2006; 194: 385–390. [DOI] [PubMed] [Google Scholar]
- 15.van Gils EJM, et al. Effect of seven-valent pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLoS ONE 2011; 6: e20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. American Journal of Medicine 2008; 121: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee GM, et al. Epidemiology and risk factors for Staphylococcus aureus colonization in children in the post-PCV7 era. BMC Infectious Diseases 2009; 9: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacoby P, et al. Modelling the co-occurrence of Streptococcus pneumoniae with other bacterial and viral pathogens in the upper respiratory tract. Vaccine 2007;25: 2458–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Gils EJ, et al. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. Journal of the American Medical Association 2009; 302: 159–167. [DOI] [PubMed] [Google Scholar]
- 20.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378: 1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen AG, et al. Invasive pneumococcal disease in the Netherlands: Syndromes, outcome and potential vaccine benefits. Vaccine 2009; 27: 2394–2401. [DOI] [PubMed] [Google Scholar]
- 22.van Gils EJ, et al. Pneumococcal conjugate vaccination and nasopharyngeal acquisition of pneumococcal serotype 19A strains. Journal of the American Medical Association 2010; 304: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 23.Spijkerman J, et al. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, the Netherlands. Emerging Infectious Diseases 2011; 17: 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberger DM, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clinical Infectious Diseases 2010; 51: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elberse KE, et al. Seroprevalence of IgG antibodies against 13 vaccine Streptococcus pneumoniae serotypes in the Netherlands. Vaccine 2010; 29: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 26.Regev-Yochay G, et al. The pneumococcal pilus predicts the absence of Staphylococcus aureus co-colonization in pneumococcal carriers. Clinical Infectious Diseases 2009; 48: 760–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melles DC, et al. Nasopharyngeal co-colonization with Staphylococcus aureus and Streptococcus pneumoniae in children is bacterial genotype independent. Microbiology 2007; 153: 686–692. [DOI] [PubMed] [Google Scholar]
- 28.Lebon A, et al. The inverse correlation between Staphylococcus aureus and Streptococcus pneumoniae colonization in infants is not explained by differences in serum antibody levels in the Generation R Study. Clinical and Vaccine Immunology 2011; 18: 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basset A, et al. Association of the pneumococcal pilus with certain capsular serotypes but not with increased virulence. Journal of Clinical Microbiology 2007; 45: 1684–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberger DM, et al. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathogens 2009; 5: e1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Health Council of The Netherlands. Vaccination of infants against pneumococcal infections (2). (http://www.gezondheidsraad.nl/sites/default/files/Summary%20pneumokokkeninfecties%202.pdf). Health Council of The Netherlands, The Hague, 2010. [Google Scholar]
- 32.Paradiso PR. Advances in pneumococcal disease prevention: 13-valent pneumococcal conjugate vaccine for infants and children. Clinical Infectious Diseases 2011; 52: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 33.van Lier EA, et al. High vaccination coverage of the National Immunization Programme in the Netherlands [in Dutch]. Nederlands Tijdschrift voor Geneeskunde 2009; 153: 950–957. [PubMed] [Google Scholar]
- 34.Miller M, et al. Incidence and persistence of Staphylococcus aureus nasal colonization in a community sample of HIV-infected and -uninfected drug users. Clinical Infectious Diseases 2007; 45: 343–346. [DOI] [PubMed] [Google Scholar]