Abstract
Salmonella Typhimurium is a pathogen of clinical relevance and a model of study in host–pathogen interactions. The virulence and stress-related periplasmic protein VisP is important during S. Typhimurium pathogenesis. It supports bacteria invading host cells, surviving inside macrophages, swimming, and succeeding in murine colitis model, O-antigen assembly, and responding to cationic antimicrobial peptides. This study aimed to investigate the role of the O-antigen molecular ruler WzzST and the periplasmic protein VisP in swarming motility and osmotic stress response. Lambda red mutagenesis was performed to generate single and double mutants, followed by swarming motility, qRT-PCR, Western blot, and growth curves. Here we demonstrate that the deletion of visP affects swarming under osmotic stress and changes the expression levels of genes responsible for chemotaxis, flagella assembly, and general stress response. The deletion of the gene encoding for the O-antigen co-polymerase wzzST increases swarming motility but not under osmotic stress. A second mutation in O-antigen co-polymerase wzzST in a ΔvisP background affected gene expression levels. The ΔvisP growth was affected by sodium and magnesium levels on N-minimum media. These data indicate that WzzST has a role in swarming the motility of S. Typhimurium, as the VisP is involved in chemotaxis and osmotic stress, specifically in response to MgCl2 and NaCl.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-022-00701-9.
Keywords: VisP, O-antigen; Chemotaxis; Osmolarity; Motility
Introduction
Salmonella enterica is a major foodborne pathogen that comprises more than 2500 typhoidal and non-typhoidal serovars [1]. S. Typhimurium and S. Enteritidis are serovars of clinical interest, causing 10 million cases of salmonellosis and thousands of deaths every year [2]. Moreover, S. Typhimurium is a great model for host–pathogen interactions since it invades the gut epithelium and promotes inflammatory diarrhea [1, 3].
The bacterial membrane has a crucial role in the pathogenic process inside the host and protects the cell against different environmental conditions. It harbors several proteins, including fimbriae, flagella, and porins, which allow bacteria to detect and respond to the environment [3]. Salmonella has the lipopolysaccharide (LPS) layer composed of the lipid-A, or the endotoxin portion; the oligosaccharide core; and the O-antigen (O-Ag), distributed in a tri-modal manner [4]. The immune system recognizes the lipid-A, and the O-Ag is detected by the Toll-like receptors 4, resulting in the secretion of TNF-α and IL-1β [5].
The periplasmic space connects the outer membrane to the inner membrane and has several proteins of importance for bacteria. The virulence and stress-related periplasmic protein (VisP) is conserved among Enterobacteria and predicted to have an oligonucleotide/oligosaccharide-binding fold [6]. A transcriptome released with environmental Escherichia coli revealed that visP (former ygiW) was one of the most upregulated genes during stress response [7]. Our group previously described that VisP is essential during S. Typhimurium pathogenesis. It helps bacteria survive inside macrophages, perform swimming motility, succeed in the murine colitis model, and respond to CAMPs by interacting with the lipid-A enzyme LpxO [6]. VisP is known to regulate the stress response to cadmium chloride, hydrochloric acid, and hydrogen peroxide [6]. We also have found that VisP participates in O-Ag assembly, affects the transcription of SPI2-related effectors, and impairs the intramacrophage survival and replication process [8].
The flagellum is a macromolecular structure assembled via its secretion system [9]. Flagella are essential for virulence and allow bacteria to explore new niches through their movement in liquid and semi-solid media (swimming) and surfaces (swarming) [10]. Swarming motility works with chemotaxis, in which bacteria sense the environment via methyl-accepting chemotaxis proteins (MCP), followed by phosphorylation of kinase proteins like CheA and CheY, which are responsible for controlling flagella direction (clockwise and counterclockwise) [11, 12]. Swarming helps in community establishment and survival and contributes to bacterial virulence [11].
A correct setup and fine-tuning of motility, chemotaxis, and O-Ag assembly systems depend on membrane integrity and the interaction in its surroundings. During the infection, Salmonella faces high osmolarity in the intestinal lumen, reducing as bacteria reach the epithelium [13]. It is essential that S. Typhimurium gain the epithelium and start the invasion process since the bacteria that stay in the lumen are killed by neutrophils and cannot colonize the host [14]. Herein, we aimed to investigate the role of VisP and the O-Ag co-polymerase WzzST in swarming motility and their participation in osmotic stress response.
Material and methods
Bacterial strains and cell cultures
All bacterial strains are listed in Table 1 and the plasmids used are listed in Table 2. The bacterial strains were grown in Luria–Bertani (LB) base broth (Invitrogen), containing 10 g/l NaCl. The 2% NaCl media were made by adding NaCl to the same broth, totalizing 20 g/l NaCl, as indicated.
Table 1.
Strains used in this study
| Strain | Relevant genotype or description | Reference or source |
|---|---|---|
| SL1344 | Salmonella enterica serovar Typhimurium prototype | [15] |
| ΔvisP | SL1344 visP mutant | [6] |
| visP + | SL1344 visP complemented strain | [6] |
| ΔwzzST | SL1344 wzzST mutant | [8] |
| wzzST + | SL1344 wzzST complemented strain | This study |
| ΔvisP/wzzST | SL1344 visP and wzzST double mutant | [8] |
| ΔvisP/wzzST /visP + | SL1344 visP and wzzST double mutant, visP complemented | [8] |
Table 2.
Plasmids used in this study
Recombinant DNA and molecular biology techniques were performed as previously described [18]. All oligonucleotides used in this study are cited in Table 3.
Table 3.
Oligonucleotides used in this study
| Primer | Forward sequence | Reverse sequence | Reference or source |
|---|---|---|---|
| wzzST_lambda_red | ATGACAGTGGATAGTAATACGTCTTCCGGGCGTGGGAACGATCCGGAACAGTGTAGGCTGGAGCTGCTTC | TTACAAGGCTTTTGGCTTATAGCTACGTAGCGCATTGCGTCCCAGCACAATCCATATGAATATCCTCCTTA | [8] |
| visP_lambda_red | AAGGGAAAAGTAATCATGAAAAAATTAGCTGCAATCGTTGCGTGTAGGCTGGGAGCTGCTTC | TTACGGATTCACTTTACGAATCTGTTTTACGTCGATTTCGACATATGAATATCCTCCTTA | [6] |
| wzzST_external | CGTAAGCGTCATCAATAAGC | CTATCCACTGTCATAGATA | [8] |
| visP_external | GAATAAGCCGCGCTGATCAG | GCAACGCGAGTTACCGCAAG | [6] |
| cheA | TGGTAATGAGATCGCCGTGG | AAACCTGGTCGGCGAGTTAG | This study |
| cheZ | ACATCCCTGAGAAACTGGCG | ATCCGATCGAGCTTTCCGAC | This study |
| envZ | GGTCATCGGCCAATTGCTTC | GACCCGGGCGTTTAACCATA | This study |
| rpoS | TGTCCAGCAACGCTTTTTCG | TCAGCCGTATGCTTCGTCTC | This study |
| flgM | TAACGTTAAGCGACGCGCA | ATGCTTCGACGCGTTCCATA | This study |
Construction of isogenic mutants
Construction of isogenic nonpolar S. Typhimurium SL1344 mutants ΔvisP and ΔvisP/wzzST was achieved by lambda red recombination [16]. The pKD46 plasmid with Lambda recombinant system was inserted in WT strain to construct single mutants and in ΔvisP background to construct the ΔvisP/wzzST strain. The PCR containing the chloramphenicol cassette was amplified using primers with homology sequences in the target genes, under the following conditions: 94 °C for 5 s, 94 °C for 1 min, 55 °C for 30 s, 68 °C for 1 min (high-fidelity DNA polymerase), 68 °C for 10 min, and 4 °C hold. The PCR products were electroporated in the strain harboring the pKD46 plasmid. Mutants were selected for CmR (20 μg/ml) in LB agar plates and confirmed by sequencing [16]. All strains were complemented with respective genes cloned into the vector pBADMycHisA (KpnI and EcoRI) [6] to generate strains visP + , wzzST + , and ΔvisP/wzzST/visP + . The single mutant strain ΔvisP was resolved via electroporation of the pCP20 plasmid, followed by incubation at 30 °C for 3 h, and plated in LB ampicillin (Amp) agar. Single colonies were chosen and grown in LB at 42 °C overnight and then plated on LB streptomycin (Sm) at 37 °C. Single colonies were patched in Amp, Sm, and Cm and incubated at 37 °C. The resolved clones were chosen based on SmR (100 μg/ml), AmpS (100 μg/ml), and CmS (20 μg/ml) [16]. All mutants were previously constructed at the UT Southwestern Medical Center in the USA under NIH Biosafety regulations [6, 8].
Motility assays
The swarming motility assay was performed in LB agar 0.5% as previously described [19], containing 1% or 2% NaCl. A total inoculum of 1 × 106 CFU in a volume of 5 μl bacteria was added to the surface of agar plates. The plates were incubated at 37 °C, and the motility halos were measured after 12, 24, and 48 h of incubation at 37 °C. Two independent experiments of five replicates were performed.
qRT-PCR
Quantitative real-time reverse transcription-PCR (qRT-PCR) was performed as previously described [20]. Cells were harvested to O.D.600 of 1.0, and RNA was extracted using the RiboPure® Bacteria Purification kit (Thermo Fisher). Each 20 μl reaction contained the SYBR® Master Mix, the Multi-scribe® Reverse Transcriptase, and an RNAse Inhibitor (Thermo Fisher). The reactions were normalized by the rpoA endogenous control, and RNA was used to a final concentration of 100 ng. All reactions were performed in QuantStudio 3 (Thermo Fisher), and data were analyzed via comparative critical threshold (ΔΔCt).
Western blot assay
Culture supernatants were obtained from strains grown statically in LB medium at 37 °C overnight. The medium was removed by centrifugation, and the cells were resuspended in 10 ml of phosphate-buffered saline (PBS) plus 100 μl of phenylmethylsulfonyl fluoride (100 mM). Bacteria were lysed by sonication, pelleted by centrifugation (4200 × g, 10 min, 4 °C), and analyzed by SDS-PAGE. The total proteins were quantified by the Bradford method, and 6 μg of protein was loaded into each lane. The proteins were transferred to nitrocellulose membranes using a semi-dry transfer cell (Bio-Rad) for 1 h. The immunoblots were made using monoclonal antibodies anti-FliC (InvivoGen) at 1:1000 and anti-RpoA (Santa Cruz Biotechnology) at 1:5000. The antibodies were detected with the horseradish peroxidase–conjugated secondary antibody anti-mouse IgG (Promega) at 1:2500, followed by chemiluminescence detection (ECL Western blotting) in the ChemiDoc MP imaging system (Bio-Rad).
Growth rates
Bacteria were grown in a 125-ml Erlenmeyer containing 15 ml of LB 2% NaCl, N-minimum media (100 μM MgCl2), N-minimum low MgCl2 (10 μM), or N-minimum plus 2% of NaCl, on a shaker at 250 rpm and 37 °C. Optical densities (O.D.600) were acquired after 30 min, 1 h, 2 h, 3 h, 4 h, 5 h, 7 h, 8 h, and 18 h of incubation.
Statistical analysis
Data were analyzed for statistical significance using GraphPad Prism 8. Results were compared by two-way ANOVA with Bonferroni post hoc test (P-value < 0.05).
Results
VisP and WzzST are needed for swarming on high osmolarity
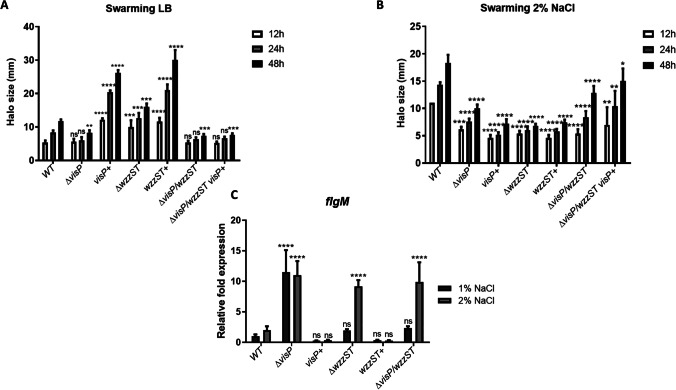
To determine whether the flagellar system was affected in the mutant strains, we assessed the motility swarming phenotype of SL1344 and the isogenic mutants in LB and LB 2% NaCl conditions. In the swarming assays performed in LB (1% NaCl), ΔvisP presented a similar phenotype to WT, whereas ΔvisP/wzzST and ΔvisP/wzzST/visP + had their motility reduced after 48 h of incubation, with 37% and 35% of reduction, respectively (Fig. 1A). The single mutant ΔwzzST showed an increase of 36% in swarming motility compared to WT (Fig. 1A). The complemented strains visP + and wzzST + had an increase in swarming compared to WT: 122% and 154%, respectively. However, when the swarming is performed under osmotic stress (2% NaCl), all strains presented smaller motility halos when compared to WT (Fig. 1B), indicating that disruption of membrane changes the stress response on surface movement. Moreover, the visP + and wzzST + strains did not restore the WT phenotype (Fig. 1B). The ΔwzzST mutant, which had swarming increased in LB, presented less motility than WT in 2% NaCl, around 63% less at the end of 48 h (Fig. 1B). On the other hand, the WT motility increased 55% in osmotic stress, indicating that flagella are working in response to high osmolarity.
Fig. 1.
Swarming motility of Salmonella WT and isogenic mutants. A Swarming motility performed in LB 0.5% agar after 12, 24, and 48 h of incubation. Motility halos of WT, ΔvisP, visP + , ΔwzzST, wzzST + , ΔvisP/wzzST, and ΔvisP/wzzST visP + . ns, non-significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. B Swarming motility perfomed in LB 0.5% agar plus 2% NaCl after 12, 24, and 48 h of incubation. Motility halos of WT, ΔvisP, visP + , ΔwzzST, wzzST + , ΔvisP/wzzST, and ΔvisP/wzzST/visP + . ns, non-significant; *p < 0.05; **p < 0.01; ****p < 0.0001. C qRT-PCR of flgM in LB 1% and 2% NaCl. Relative fold expression of flgM in WT, ΔvisP, visP + , ΔwzzST, wzzST + , and ΔvisP/wzzST strains. ns, non-significant; ****p < 0.0001
Flagella assembly follows a complex cascade of genes encoded by several operons. Here we have evaluated the expression of the anti-sigma factor flgM. The ΔvisP strain presented higher expression levels in both conditions, whereas ΔwzzST and ΔvisP/wzzST presented high levels of flgM gene expression only under stress conditions—around ninefold compared to WT levels (Fig. 1C). The deletion of wzzST in ΔvisP background restored the expression to WT levels in LB 1% NaCl (Fig. 1C). The complemented strains visP + and wzzST + could restore the expression to the WT levels, different from what we have seen for phenotype on Fig. 1A and B.
VisP and WzzST are required for chemotaxis
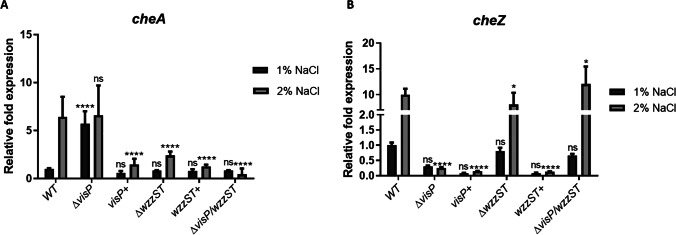
Swarming requires flagella and chemotaxis system expression. Thus, we assessed the expression of cheA (kinase sensor) and cheZ (phosphatase) of two-component systems (TCS) involved in this process. The WT levels were higher under osmotic stress for both genes, around 6- and tenfold, respectively (Fig. 2A and B), which corroborates the increase in swarming in LB 2% NaCl. The ΔvisP presented increased cheA expression in LB 1% NaCl, whereas ΔwzzST and ΔvisP/wzzST strains showed reduced cheA expression of 2- and 0.4-fold, respectively, compared to WT in 1% NaCl (Fig. 2A). These expression levels may indicate that the deletion of wzzST and/or visP compromises the fine-tuning of CheAB TCS. Furthermore, deletion of wzzST in ΔvisP background restored cheA expression to WT levels in 1% but not in 2% NaCl condition (Fig. 2A). The complementation with visP + and wzzST + genes restored expression in 1% NaCl but not in 2% NaCl, with a significant reduction (p < 0.0001). The expression of the phosphatase cheZ was similar to WT in ΔwzzST and ΔvisP/wzzST strains, and ΔvisP presented low levels mainly in 2% NaCl (Fig. 2B). The visP + and wzzST + strains did not restore the WT expression levels in 2% NaCl (Fig. 2B).
Fig. 2.
Expression levels of chemotaxis genes in Salmonella WT and isogenic mutants. A qRT-PCR of cheA in LB 1% and 2% NaCl. Relative fold expression of cheA in WT, ΔvisP, visP + , ΔwzzST, wzzST + , and ΔvisP/wzzST strains. ns, non-significant; ****p < 0.0001. B qRT-PCR of cheZ in LB 1% and 2% of NaCl. Relative fold expression of cheZ in WT, ΔvisP, visP + , ΔwzzST, wzzST + , and ΔvisP/wzzST strains. ns, non-significant; *p < 0.05; ****p < 0.0001
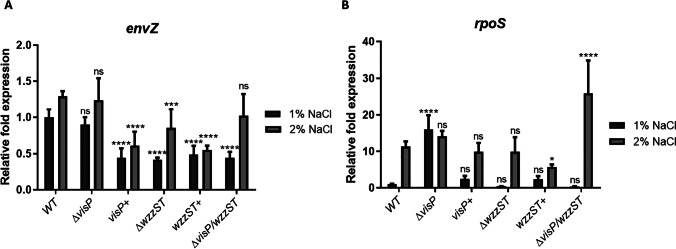
WzzST impacts on envZ-dependent porin expression
The regulation of porins is essential during the osmotic stress response. We checked envZ expression levels, and except for ΔvisP, all strains presented reduced levels compared to WT, mainly in 1% NaCl (Fig. 3A). The complementation with visP + did not restore the envZ expression to WT levels (Fig. 3A). The wzzST + complemented strain did not change the expression compared to ΔwzzST and did not reach the WT expression levels (Fig. 3A). The double mutant ΔvisP/wzzST reduced envZ expression compared to WT at 1% NaCl, which might indicate that envZ expression is WzzST-dependent. Furthermore, the expression levels of the global stress regulator rpoS were checked, and, except for ΔvisP, all strains presented similar expression levels in LB 1% NaCl media (Fig. 3B). Under osmotic stress, WT expression increased tenfold, also observed for ΔwzzST and visP + . The double mutant ΔvisP/wzzST expression increased 25-fold in 2% NaCl compared to the LB condition (Fig. 3B). In this case, the deletion of wzzST in a ΔvisP background changes the stress response and increases rpoS transcription. The complementation with wzzST + did not restore to the WT expression levels in 2% NaCl condition (Fig. 3B).
Fig. 3.
Expression levels of envZ and rpoS genes in Salmonella WT and isogenic mutants. A qRT-PCR of envZ in LB 1% and 2% NaCl. Relative fold expression of envZ in WT, ΔvisP, visP + , ΔwzzST, wzzST + , and ΔvisP/wzzST strains. ns, non-significant; ***p < 0.001; ****p < 0.0001. B qRT-PCR of rpoS in LB 1% and 2% NaCl. Relative fold expression of rpoS in WT, ΔvisP, visP + , ΔwzzST, wzzST + , and ΔvisP/wzzST strains. ns, non-significant; ****p < 0.0001
VisP absence affects growth on minimal media.
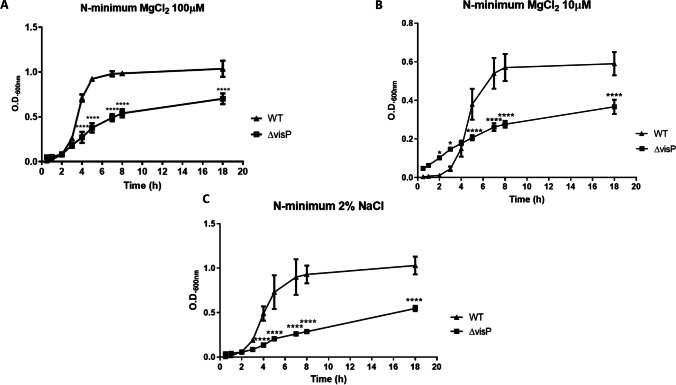
Because of the differences observed in gene expression levels in 1% and 2% NaCl conditions, we performed Western blot targeting the flagellin FliC protein expression. For that, the monoclonal α-FliC antibody and the control α-RpoA were employed. The FliC expression in ΔvisP was lower than WT in both LB 1% NaCl and LB 2% NaCl (Additional File 1), and the complemented strain visP + could not restore WT flagellin levels. Furthermore, no difference was observed in FliC expression between 1 and 2% NaCl (Additional File 1). Lastly, we performed growth curves in N-minimum media to evaluate differences in stress response, such as the influence of magnesium and sodium on growth. Employing regular N-minimum conditions (100 μM MgCl2), ΔvisP growth was defected at 50% less than WT growth, peaking O.D.600 of 0.5 (Fig. 4A). Under MgCl2 starvation (10 μM), both WT and ΔvisP struggled to grow, as the deletion of visP caused a slow growth when the two curves were compared (Fig. 4B). The same pattern was observed after adding 2% NaCl in regular N-minimum, in which the ΔvisP strain presented half of the O.D.600 of WT strain after 18 h (Fig. 4C). We did not observe this difference in LB 2% NaCl, where the ΔvisP strain grows similar to WT levels (Additional File 2). These data indicate that VisP protein is involved in the osmotic response, but mainly in poor media conditions, where Salmonella must change the metabolic routes to survive.
Fig. 4.
Growth curves performed in N-minimum media. A N-minimum 100 μM MgCl2. B N-minimum 10 μM MgCl2. C N-minimum media 2% NaCl. Optical density (O.D.600) of WT and ΔvisP at different time points after 18 h of incubation. *p < 0.05; ****p < 0.0001
Discussion
Swarming requires one or more flagella, surfactant synthesis, and a grouped movement [10]. S. Typhimurium promotes cell elongation and increases torque, but flagella amounts in cells do not change, while some bacteria as Vibrio parahaemolyticus and Aeromonas spp. may increase flagellar synthesis [10]. When compared ΔvisP to the WT during swarming on LB, both have similar motility phenotypes (Fig. 1A). Our previous data showed that swimming motility on LB is reduced in ΔvisP, and flagellar genes were downregulated [8]. All strains had swarming reduced in 2% NaCl compared to WT phenotype (Fig. 1B). The single mutant ΔwzzST had an increase compared to WT, and the complementation with wzzST + increased the motility even more (Fig. 1A). The WzzST protein encodes for the long O-Ag assembly forms (L), while the WzzfepE (which is the remaining co-polymerase in ΔwzzST) encodes for very long O-Ag forms (VL) [21]. Even in the absence of WzzST, the swarming is still facilitated by O-Ag VL forms. After deletion of wzzST in a ΔvisP background, the swarming motility had decreased, and the complemented strain ΔvisP/wzzST visP + did not restore the ΔwzzST phenotype (Fig. 1A). After deletion of wzzST in a ΔvisP background, the swarming motility had decreased, and the complemented strain ΔvisP/wzzST visP + did not restore the ΔwzzST phenotype (Fig. 1A).
The qRT-PCR of flgM was performed and exhibited increased expression levels under osmotic stress, except for ΔvisP, which presented overexpression in both conditions tested (Fig. 1C). The reduced swimming motility in LB previously observed in ΔvisP is related to the low levels of expression in flagellar genes [8]. The overexpression of the flgM can affect the substrate shift from class 2 to class 3 genes since FlgM is secreted via flagellar T3SS. Then, FliA initiates transcription of the FliC or FljB strand [9]. The complemented strains visP + and wzzST + presented a slight decrease compared to WT (Fig. 1C). These data corroborate previous studies and indicate the importance of VisP for membrane homeostasis.
The expression of chemotaxis genes cheA and cheZ exhibited different levels in ΔvisP compared to WT (Fig. 2A and B). The CheA kinase sensor phosphorylates CheY and starts the flagella rotation, while CheZ is responsible for resetting the switch and changing direction [19]. The cheA expression on LB was increased fivefold in ΔvisP when compared to WT levels. However, the cheZ expression in the ΔvisP mutant was reduced in both conditions. In the WT strain, the cheZ expression is higher under osmotic conditions. Because VisP has a role in flagella expression and the chemotaxis genes are part of the flagella cascade [8], the chemotactic system might not work properly during osmotic stress. The cheA expression for both ΔwzzST and ΔvisP/wzzST strains was similar to WT levels in 1% NaCl, but it was reduced compared to WT in 2% NaCl (Fig. 2A). When comparing cheZ expression, the mutant strains presented similar levels to WT, except for ΔvisP (Fig. 2B). Despite these differences observed in all mutant strains, none of them had the same swarming phenotype under osmotic stress as observed in WT (Fig. 1B). For both cheA and cheZ expressions, the complemented strains visP + and wzzST + could not restore the WT levels (Fig. 2A and B). The results have shown that the importance of a functional chemotactic system is reflected in the stress response phenotype.
The regulation of porins in osmotic gradient depends on a critical bacterial TCS: the EnvZ/OmpR system. EnvZ is a kinase sensor and phosphorylates the response regulator OmpR in the presence of high osmolarity [22]. In S. Typhi, EnvZ binds to tviA and activates transcription of the capsule; in contrast, in S. Typhimurium, it regulates SPI-1 and SPI-2 through the SsrAB system [22]. The expression of envZ had decreased in all mutant strains compared to WT in LB, except for ΔvisP (Fig. 3A). OmpR tends to inhibit flhDC expression in Escherichia coli [23]; however, this regulation needs to be more explored in Salmonella. The complementation with visP + and wzzST + could not restore the WT expression levels (Fig. 3A). The ΔvisP strain did not show a significant difference in expression levels; on the other hand, WzzST showed to be essential for envZ expression (Fig. 3A). RpoS is on the top of the stress response cascade and responds to osmotic and oxidative stress, low pH, UV radiation, temperature, ethanol, microaerophilic growth, among others [17, 24, 25]. The rpoS expression in ΔvisP presented an increase compared to WT in LB, but not in LB 2% NaCl (Fig. 3B). The VisP protein is involved in stress response and membrane homeostasis, and its absence could impact rpoS expression. The complementation with visP + restored the expression to WT levels. The ΔwzzST strain presented the same rpoS levels as WT, indicating that the absence of the O-antigen PCP protein did not impact rpoS transcription (Fig. 3B). However, the double mutation ΔvisP/wzzST had a significant increase in expression levels under osmotic stress, about 25-fold (Fig. 3B).
The FliC expression in ΔvisP showed to be lower than WT levels but did not change in 2% NaCl (Additional File 1). The genomic localization of the visP gene suggested that it is part of the qseBC regulon, encoded in an adjacent operon and the opposite direction [6]. Our previous data demonstrated that the ΔvisP/wzzST strain overexpressed FliC protein [8] and presented overexpression of the qseC gene (Manieri, data not shown). The deletion of visP also abrogates bacterial resistance to acid, heavy metals, and oxidative stress [6]. Thus, the growth curves exhibited that ΔvisP grows slow in N-minimum and even slower than WT in MgCl2 starvation and 2% NaCl (Fig. 4A–C). It corroborates with previous data about VisP and includes the osmotic stress response as another stressor agent related to VisP function.
Conclusion
Our findings highlight the direct importance of VisP protein and indirect via WzzST for S. Typhimurium in motility and stress response. VisP and WzzST have a more specific role in swarming, chemotaxis, and osmotic stress response; WzzST is also required for envZ expression, and, consequently, porin regulation. VisP is required for growth in minimal media, specifically in response to MgCl2 and NaCl. These data open new perspectives about swarming and mechanisms involved in flagella-independent motility.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1: (PDF 124 KB) SDS-PAGE of whole cell lysate obtained from WT (lanes 1 and 2), ΔvisP (lanes 3 and 4) and visP+ (lanes 5 and 6) strains, in conventional LB (NaCl -) and LB 2% NaCl (NaCl +). The ladder indicates kDa of bands.
Supplementary file2: (PDF 99 KB) Western blot probing the flagellin FliC and the control RpoA in WT (lanes 1 and 2), ΔvisP (lanes 3 and 4), and visP+ (lanes 5 and 6) strains, in LB (NaCl -) and LB 2% NaCl (NaCl +).
Supplementary file3: (PDF 51 KB) Growth curves performed in LB 2% NaCl media. Optical density (O.D.600) of WT and ΔvisP at different time points after 18 hours of incubation. ***, p<0.001; ****, p<0.0001.
Acknowledgements
We thank all members of the Biological Sciences Department at the Faculty of Pharmaceutical Sciences, São Paulo State University. We are thankful to Dr. Vanessa Sperandio from the UT Southwestern Medical Center for providing the lambda red recombination system.
Author contribution
FZM and CGM designed the study. FZM performed the research. FZM and CGM performed the data analysis. FZM wrote the manuscript. CGM supervised the research.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 2019/03049-7, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant number 441884/2014–8, and financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Data availability
All data and material are available under request.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13(4):191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Who W (2016) Salmonella (non-typhoidal) Fact sheet n°139
- 3.Walch P, et al. Global mapping of Salmonella enterica-host protein-protein interactions during infection. Cell Host Microbe. 2021;29(8):1316–1332.e12. doi: 10.1016/j.chom.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HD, Groisman EA. The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications. Annu Rev Microbiol. 2013;67:83–112. doi: 10.1146/annurev-micro-092412-155751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raetz CR, et al. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira CG, et al. Virulence and stress-related periplasmic protein (VisP) in bacterial/host associations. Proc Natl Acad Sci U S A. 2013;110(4):1470–1475. doi: 10.1073/pnas.1215416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, et al. Identification of stress-related proteins in Escherichia coli using the pollutant cis-dichloroethylene. J Appl Microbiol. 2010;108(6):2088–2102. doi: 10.1111/j.1365-2672.2009.04611.x. [DOI] [PubMed] [Google Scholar]
- 8.da Silva P et al (2018) Novel role of VisP and the Wzz system during O-antigen assembly in Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun 86(8):e00319–18 [DOI] [PMC free article] [PubMed]
- 9.Baker AE, O’Toole GA (2017) Bacteria, rev your engines: stator dynamics regulate flagellar motility. J Bacteriol 199(12):e00088–17 [DOI] [PMC free article] [PubMed]
- 10.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8(9):634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partridge JD, Harshey RM. Swarming: flexible roaming plans. J Bacteriol. 2013;195(5):909–918. doi: 10.1128/JB.02063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salah Ud-Din AI, Roujeinikova A (2017) Methyl-accepting chemotaxis proteins: a core sensing element in prokaryotes and archaea. Cell Mol Life Sci 74(18):3293–3303 [DOI] [PMC free article] [PubMed]
- 13.Winter SE, et al. The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Mol Microbiol. 2009;74(1):175–193. doi: 10.1111/j.1365-2958.2009.06859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keestra-Gounder AM, Tsolis RM, Baumler AJ. Now you see me, now you don’t: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol. 2015;13(4):206–216. doi: 10.1038/nrmicro3428. [DOI] [PubMed] [Google Scholar]
- 15.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 16.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farizano JV, et al. The RcsCDB regulatory system plays a crucial role in the protection of Salmonella enterica serovar Typhimurium against oxidative stress. Microbiology. 2014;160(Pt 10):2190–2199. doi: 10.1099/mic.0.081133-0. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual
- 19.Partridge JD, Harshey RM. More than motility: Salmonella flagella contribute to overriding friction and facilitating colony hydration during swarming. J Bacteriol. 2013;195(5):919–929. doi: 10.1128/JB.02064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellermann M, et al. Endocannabinoids inhibit the induction of virulence in enteric pathogens. Cell. 2020;183(3):650–665.e15. doi: 10.1016/j.cell.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pescaretti MLM, et al. The PmrA/PmrB regulatory system controls the expression of the wzzfepE gene involved in the O-antigen synthesis of Salmonella enterica serovar Typhimurium. Microbiology. 2011;157(Pt9):2515–21. doi: 10.1099/mic.0.050088-0. [DOI] [PubMed] [Google Scholar]
- 22.Perkins TT, et al. ChIP-seq and transcriptome analysis of the OmpR regulon of Salmonella enterica serovars Typhi and Typhimurium reveals accessory genes implicated in host colonization. Mol Microbiol. 2013;87(3):526–538. doi: 10.1111/mmi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osterman IA, et al. Regulation of flagellar gene expression in bacteria. Biochemistry (Mosc) 2015;80(11):1447–1456. doi: 10.1134/S000629791511005X. [DOI] [PubMed] [Google Scholar]
- 24.Battesti A, Majdalani N, Gottesman S (2011) The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. [DOI] [PMC free article] [PubMed]
- 25.Harshey RM, Partridge JD. Shelter in a swarm. J Mol Biol. 2015;427(23):3683–3694. doi: 10.1016/j.jmb.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1: (PDF 124 KB) SDS-PAGE of whole cell lysate obtained from WT (lanes 1 and 2), ΔvisP (lanes 3 and 4) and visP+ (lanes 5 and 6) strains, in conventional LB (NaCl -) and LB 2% NaCl (NaCl +). The ladder indicates kDa of bands.
Supplementary file2: (PDF 99 KB) Western blot probing the flagellin FliC and the control RpoA in WT (lanes 1 and 2), ΔvisP (lanes 3 and 4), and visP+ (lanes 5 and 6) strains, in LB (NaCl -) and LB 2% NaCl (NaCl +).
Supplementary file3: (PDF 51 KB) Growth curves performed in LB 2% NaCl media. Optical density (O.D.600) of WT and ΔvisP at different time points after 18 hours of incubation. ***, p<0.001; ****, p<0.0001.
Data Availability Statement
All data and material are available under request.
Not applicable.