Abstract
Phylogenetic analysis carried out in several Brazilian regions shows the circulation of the Asian and East-Central South African (ECSA) Chikungunya virus (CHIKV) genotypes in the country. Until now, there are no genetic studies about CHIKV strains circulating in the South region. In this study, we sequenced 5 new partial sequences of the CHIKV Envelope 1 gene from strains detected in Paraná state during the years 2016–2017. Maximum likelihood and neighbor-joining trees grouped all sequences in Brazilian branches within ECSA genotype and comparative analysis did not show E1-A226V mutation. However, we identified E1-K211T amino acid substitution in a sample demonstrating the dispersion of mutant strains in the country.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-022-00680-x.
Keywords: Chikungunya, Sequence analysis, Paraná
Introduction
Chikungunya virus (CHIKV) is a re-emerging alphavirus belonging to the Togaviridae family, with from person-to-person transmission through the bite of female mosquitoes of the genus Aedes [1]. First described in Tanzania in 1952, CHIKV has since been causing outbreaks in Africa, Asia, Europe, and the Americas and it represents yet a major public health problem in tropical and subtropical countries, infecting millions of people worldwide [2, 3]. Although the mortality rate is small, the rapid dissemination of CHIKV and prolonged morbidity should be considered [4].
The first autochthonous cases of CHIKV in the Americas occurred at the end of 2013 on the islands of San Martin, Caribe [5], and soon spread to several countries in South America [6]. In Brazil, the first imported cases of CHIKV occurred in Rio de Janeiro in 2010, but the virus was not isolated or demonstrated by polymerase chain reaction (PCR) [7]. Based on the notification of the Brazilian surveillance systems, the Chikungunya virus (CHIKV) was re-introduced in Brazil in 2014, with the first autochthonous cases occurring in the state of Amapá, followed by cases in Bahia and other regions of the country [3, 8]. However, in 2019, Souza et al. suggested, through phylogenomic analyses, that CHIKV ECSA genotype was introduced into Brazil in 2013, up to 1 year before estimates, most probably in Bahia. Currently, Brazil presents a scenario marked by the coexistence of arboviruses such as dengue, zika, and chikungunya [9].
Phylogenetic analyses have shown the occurrence of three major CHIKV genotypes, according to the geographic region of their isolation: West African (WA), the East-Central-South African (ECSA), which originated the Indian Ocean lineage; and the Asian, which was the genotype introduced in the Americas [2, 10]. As far as we know, the CHIKV virus genotype circulating in Brazil is ECSA, this being the first time that this genotype has been reported in the Americas [3, 6]. Since then, CHIKV has been spreading throughout several regions of the country, affecting mainly the Northeast region [3]. Although the Asian genotype was introduced in Northern Brazil in 2014, it did not establish a cycle here. On the other hand, the ECSA genotype not only persisted, but also spread from Feira de Santana, Bahia, to other regions, inside and outside the country, as demonstrated by Nunes et al. [3]. Here, using CHIKV sequences from strains that circulated in Paraná, Brazil, we presented a phylogenetic analysis based on the E gene.
Material and methods
Serum samples from 5 patients with positive molecular diagnosis for CHIKV were provided by Laboratório Central do Estado do Paraná (LACEN-PR) and sent to the Clinical Virology laboratory of the Universidade Estadual de Maringá. The samples were collected in Paraná state during arboviruses epidemics (CHIKV and/or DENGV and/or ZIKV) that occurred in 2016 and 2017. Total RNA was extracted using the commercial kit QIAmp® Viral RNA Mini Handbook extraction kit (QIAGEN, Courtaboeuf, France), according to the manufacturer’s instructions. The RNA extracted from 140 µl of the sample was immediately used for cDNA synthesis with Superscript III (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. This study was approved by the Permanent Research Ethics Committee of the State University of Maringá (protocol no. 1.618.106).
In order to sequence representative fragments of the CHIKV envelope E1, primer pairs were designed (Table S1). A RT-PCR reaction was standardized for amplifying approximately 748 nucleotides along the E1 gene. The reactions were prepared to a final volume of 20 μl, with 1% 10 × buffer, 0.062 mM of each dNTP (10 mM dNTP mix), 1.5 mM MgCl2, 0.5 μM of the specific primers (forward and Reverse), 0.2 U of Taq DNA Polymerase (Invitrogen, Carlsbad, CA), and 2 μl of CHIKV cDNA. The thermocycling conditions were as follows: initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C for 1 min, 60 °C for 35 s, 72 °C for 45 s, followed by a final extension at 72 °C for 10 min. The amplified products were analyzed by 2% agarose gel electrophoresis.
The amplicons were sequenced by ACT Gene Análises Moleculares Ltda. (Centro de Biotecnologia, UFRGS, Porto Alegre, RS, Brazil) using the BigDye Terminator Cycle Sequencing Ready Reaction version 3.1 kit (Applied Biosystems®). Sequencing was performed on automatic sequencer AB 3500 Genetic Analyzer armed with 50 cm capillaries and POP7 polymer (Applied Biosystems).
Sequence quality analysis was performed by PHPH tool, a web-based tool, available at the website http://asparagin.cenargen.embrapa.br/phph/reference.html [11]. It provided us the contigs from sequences with acceptable quality, which had their identity assessed using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). After editing, sequences were aligned among them using CLUSTAL OMEGA (https://www.ebi.ac.uk/Tools/msa/clustalo/) and they were aligned with sequences representative of each genotype (Asian, ECSA, and West African) previously deposited on GenBank (Table S2) by ClustalW implemented in MEGA X (https://www.megasoftware.net/) [12]. After the alignments, phylogenetic trees were constructed using the MEGA X (http://www.megasoftware.net/), by both neighbor-joining and maximum likelihood methods using the Kimura-2 parameter model (K2), with a bootstrap of 1000 replications, as confirmation of the results. All sequences were deposited in GenBank under the accession numbers MK972832 to MK972836 (Envelope gene).
Results and discussion
Each one of the five samples corresponded to a distinct patient, and although all of them were collected in the state of Paraná, Brazil, in two of the cases, patients had reported a history of traveling to epidemic regions in the days preceding the onset of symptoms (Recife and Bahia), and one reported residing in Rio de Janeiro state (Table 1). This illustrates the scenario experienced by the State of Paraná during the period studied, where most of the confirmed cases of CHIKV were imported.
Table 1.
Epidemiological data for the sequenced samples
| ID of the sample | Age | Sex | Origen | Travel history |
|---|---|---|---|---|
| CHIKV_E1_amostra1 | 46 | Male | Curitiba, PR | Bahia |
| CHIKV_E1_amostra2 | 76 | Female | Curitiba, PR | Rio de Janeiro |
| CHIKV_E1_amostra3 | 39 | Male | Medianeira, PR | Not reported |
| CHIKV_E1_amostra4 | 43 | Female | Curitiba, PR | Recife, PE |
| CHIKV_E1_amostra5 | 20 | Female | Curitiba, PR | Not reported |
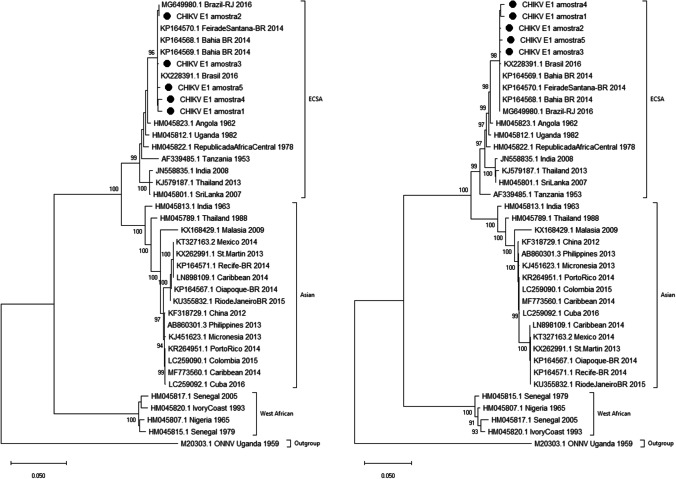
In agreement to Nunes et al. (2015), our results showed that all sequences obtained in this study were within ECSA genotype, clustered with Rio de Janeiro and Bahia samples (Fig. 1A, B). Interestingly, this genotype, as previously stated, was first reported in Bahia, in the northeast region of Brazil, and philographic studies showed that it is originated from Angola, being this the first time that this genotype was described in the Americas [2, 13].
Fig. 1.
Genotyping of CHIKV strains (n = 5) identified in Paraná, Brazil, during 2016 and 2017. Neighbor-joining method (A) and maximum likelihood (B), both K2 parameters model, bootstrap of 1,000 replications. The CHIKV sequences analyzed were named CHIKV_E1_amostra1 to CHIKV_E1_amostra5, and they are represented by black circles
Souza et al., in a surveillance study conducted in 2016, reported, for the first time, the ECSA genotype circulating in Rio de Janeiro, in the southeast region of country [14]. In this same year, Cunha et al. described an outbreak in Sergipe, Brazil, where the ECSA genotype was responsible [15]. More recently, the ECSA genotype also caused a new outbreak of CHIKV in the Southeast region, and it was still found circulating in Maranhão [16, 17].
Our study adds to this data the south region of Brazil, specifically the state of Paraná, demonstrating that also in this region only the ECSA genotype was found in serum samples from patients with CHIKV. In addition, the dispersion of samples among different Brazilian strains suggests multiple introductions of the ECSA genotype of CHIKV in the state of Paraná. However, we cannot affirm this with certainty, due to the high conservation of the genome and the small fragment size. This corroborates the observation through molecular tests in other regions of the country that only the ECSA genotype was able to establish its replication cycle in Brazil, and suggests a greater potential of transmission of this strain [18].
The high density of vector mosquitos, the presence of susceptible individuals, and the constant movement of people can explain the spread of CHIKV in Brazil, which together with the co-circulation of other arboviruses (dengue and zika virus), demonstrates the serious public health problem that these diseases represent [14]. In addition, these results highlight the importance of this genotype in the persistence and dispersion of CHIKV in the country and warn about the need of establishing a surveillance routine, since this is one of the genotypes responsible for triggering not only typical manifestations, but also neurological ones [19].
This study also demonstrated that the A226V mutation, involved in the adaptation and infectivity to A. albopictus, was absent in all samples analyzed. Thus, it is expected that mutations which confer high viral fitness in A. albopictus have not been fixed at regions where the A. aegypti is the main circulating mosquito strain, such as Brazil [17]. Interestingly, in one of the samples analyzed, a K211T amino acid substitution was observed (Fig. 2). This sample belonged to an older female patient from Rio de Janeiro. This mutation was described for the first time in Rio de Janeiro by a molecular characterization of the E1 gene fragment. The same study did not show the A226V mutation; however, it revealed a K211T amino acid substitution in all samples analyzed and a V156A substitution in two sequences [14]. Until now, the consequences of those mutations are unknown, but other studies showed that other mutations such as E1-K211E and E2-V264A were reported to impact the fitness of A. aegypti mosquitoes in India during the 2006–2010 epidemics [20, 21]. More robust studies are needed in order to have a better understanding of these mutations and their consequences on mosquitoes’ fitness and in the human immune system.
Fig. 2.
Analysis of the amino acids substitutions (positions 203 to 265) based on partial sequencing of the envelope 1 (E1) gene of the CHIKV ECSA genotype identified during the years 2016 and 2017 in Paraná, Brazil. V, valine; T, threonine; K, lysine; A, alanine
There were some limitations in our study. Due to the low viral load of CHIKV in peripheral blood, we had difficulty in obtaining good PCR products to sequence, limiting our number of samples and making E2 gene analysis impossible. In addition, we analyzed a partial sequence of the CHIKV E1 gene, which had some impact on our interpretations. The sequencing and analysis of the entire genome of CHIKV from different regions of Brazil and the Americas would bring more precise information on the circulation and mutations of this virus. However, even with those limitations, our results are not only reliable, since the E1 gene has a good phylogenetic signal, but also are interesting; once, as far as we know, it is the first report involving phylogenetic aspects of CHIKV circulating in Paraná.
Conclusion
In conclusion, the strains of CHIKV obtained in this study were clustered within the ECSA genotype. Thus, this study provides, as far as goes our knowledge, the first genotype analysis of CHIKV cases in Paraná, during the epidemic period of arboviruses (CHIKV, DENV, and ZIKV) in Brazil, and warns about the constant need to monitor this virus, since it continues to circulate throughout the country, carrying mutations of functions still unknown. Therefore, new studies are necessary to verify possible mutations and their implications.
Supplementary Information
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Conceptualization, D.C.M and D.A.B.; methodology, F.F.D.J and J.R.P.J; software, D.C.M and F.S.R.; validation, D.A.B and M.V.T; resources, M.M.P; I.N.R; data curation, M.A.F.; writing—original draft preparation, D.C.M.; writing—review and editing, D.A.B, F.A.J, and M.V.T..; supervision, D.A.B.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) – Finance Code 001. Funding was also provided by Fundação Araucária.
Availability of data and material
Supplementary file available.
Declarations
Ethics approval
This study was approved by the Permanent Research Ethics Committee of the State University of Maringá (protocol no. 1.618.106).
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sreekumar E, Issac A, Nair S, Hariharan R, Janki MB, Arathy DS, Regu R, Mathew T, Anoop M, Niyas KP, Pillai MR. Genetic characterization of 2006–2008 isolates of Chikungunya virus from Kerala, South India, by whole genome sequence analysis. Virus Genes. 2010;40(1):14–27. doi: 10.1007/s11262-009-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of Chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81(Pt 2):471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 3.Nunes, M. R. T.; Faria, N. R.; de Vasconcelos, J. M.; Golding, N.; Kraemer, M. U. G.; de Oliveira, L. F.; Azevedo, R. d. S. d. S.; da Silva, D. E. A.; da Silva, E. V. P.; da Silva, S. P.; Carvalho, V. L.; Coelho, G. E.; Cruz, A. C. R.; Rodrigues, S. G.; da Silva Gonçalves Vianez, J. L.; Nunes, B. T. D.; Cardoso, J. F.; Tesh, R. B.; Hay, S. I.; Pybus, O. G.; da Costa Vasconcelos, P. F., Emergence and potential for spread of Chikungunya virus in Brazil. BMC Medicine2015,13 (1), 102. [DOI] [PMC free article] [PubMed]
- 4.Chen KC, Kam YW, Lin RT, Ng MM, Ng LF, Chu JJ. Comparative analysis of the genome sequences and replication profiles of chikungunya virus isolates within the East, Central and South African (ECSA) lineage. Virol J. 2013;10:169. doi: 10.1186/1743-422X-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical Evaluation & Disease | Chikungunya virus | CDC. 2014. Disponível em: < https://www.cdc.gov/chikungunya/hc/clinicalevaluation.html >. Acesso em: 18 December 2018
- 6.Conteville LC, Zanella L, Marin MA, Filippis AM, Nogueira RM, Vicente AC, Mendonca MC. Phylogenetic analyses of chikungunya virus among travelers in Rio de Janeiro, Brazil, 2014–2015. Mem Inst Oswaldo Cruz. 2016;111(5):347–348. doi: 10.1590/0074-02760160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albuquerque IG, Marandino R, Mendonca AP, Nogueira RM, Vasconcelos PF, Guerra LR, Brandao BC, Aguiar GR, Bacco PA. Chikungunya virus infection: report of the first case diagnosed in Rio de Janeiro. Brazil Rev Soc Bras Med Trop. 2012;45(1):128–129. doi: 10.1590/S0037-86822012000100026. [DOI] [PubMed] [Google Scholar]
- 8.de Souza Costa M C, Siqueira Maia LM, Costa de Souza V, Gonzaga AM, Correa de Azevedo V, Ramos Martins L, Chavez Pavoni JH, Gomes Naveca F, Dezengrini Slhessarenko R, Arbovirus investigation in patients from Mato Grosso during Zika and Chikungunya virus introdution in Brazil 2015–2016. Acta Trop 2019 190 395 402. [DOI] [PubMed]
- 9.Souza TML, Vieira YR, Delatorre E, Barbosa-Lima G, Luiz RLF, Vizzoni A, et al. Emergence of the East-Central-South-African genotype of Chikungunya virus in Brazil and the city of Rio de Janeiro may have occurred years before surveillance detection. Sci Rep. 2019;9(1):2760. doi: 10.1038/s41598-019-39406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, Nasar F, Schuh AJ, Holmes EC, Higgs S, Maharaj PD, Brault AC, Weaver SC. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84(13):6497–6504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togawa RC, Brigido MM, Santos CMR, Júnior MT. The use of the PHPH tool to assembly the gene sequences that are candidate to the biotic and abiotic stress in Musa acumitana. XXXV Annual Meeting of the Brazilian Society of Biochemistry and Molecular Biology (SBBq) - Aguas de Lindoia - SP - Brazil – 2006.
- 12.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souza TM, Azeredo EL, Badolato-Correa J, Damasco PV, Santos C, Petitinga-Paiva F, Nunes PC, Barbosa LS, Cipitelli MC, Chouin-Carneiro T, Faria NR, Nogueira RM, de Bruycker-Nogueira F, Dos Santos FB, First Report of the East-Central South African genotype of chikungunya virus in Rio de Janeiro Brazil. PLoS Curr 2017 9. [DOI] [PMC free article] [PubMed]
- 14.Teixeira MG, Andrade AM, Costa Mda C, Castro JN, Oliveira FL, Goes CS, Maia M, Santana EB, Nunes BT, Vasconcelos PF. East/Central/South African genotype chikungunya virus, Brazil, 2014. Emerg Infect Dis. 2015;21(5):906–907. doi: 10.3201/eid2105.141727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessa-Aquino C, Trinta KS, Pestana CP, Ribeiro MO, Sucupira MVF, Boia MN, Baptista PA, Cunha RV, Medeiros MA. Detection of East/Central/South African genotype Chikungunya virus during an outbreak in a southeastern state of Brazil. Epidemiol Infect. 2018;146(16):2056–2058. doi: 10.1017/S0950268818002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aragao CF, Cruz ACR, Nunes Neto JP, Monteiro HAO, da Silva EVP, da Silva SP, Andrade A, Tadei WP, Pinheiro VCS. Circulation of chikungunya virus in Aedes aegypti in Maranhao. Northeast Brazil Acta Trop. 2018;186:1–4. doi: 10.1016/j.actatropica.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Cunha MDP, Santos CAD, Neto DFL, Schanoski AS, Pour SZ, Passos SD, Souza MSF, Costa DD, Zanotto PMA. Outbreak of chikungunya virus in a vulnerable population of Sergipe, Brazil-A molecular and serological survey. J Clin Virol. 2017;97:44–49. doi: 10.1016/j.jcv.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Cunha MS, Costa PAG, Correa IA, de Souza MRM, Calil PT, da Silva GPD, Costa SM, Fonseca VWP, da Costa LJ, Chikungunya virus:an emergent Arbovirus to the South American continent and a continuous threat to the world Frontiers in Microbiology 2020 1297 11. [DOI] [PMC free article] [PubMed]
- 19.Cunha RVD, Trinta KS. Chikungunya virus: clinical aspects and treatment - a review. Mem Inst Oswaldo Cruz. 2017;112(8):523–531. doi: 10.1590/0074-02760170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal A, Sharma AK, Sukumaran D, Parida M, Dash PK. Two novel epistatic mutations (E1:K211E and E2:V264A) in structural proteins of Chikungunya virus enhance fitness in Aedes aegypti. Virology. 2016;497:59–68. doi: 10.1016/j.virol.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Sumathy K, Ella KM. Genetic diversity of Chikungunya virus, India 2006–2010: evolutionary dynamics and serotype analyses. J Med Virol. 2012;84(3):462–470. doi: 10.1002/jmv.23187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary file available.