Abstract
Dipteran-specific insecticidal protein Cry4A is produced as a protoxin of 130 kDa in Bacillus thuringiensis subsp. israelensis. Here we performed the in vitro processing of Cry4A and showed that the 130-kDa protoxin of Cry4A was processed into the two protease-resistant fragments of 20 and 45 kDa through the intramolecular cleavage of a 60-kDa intermediate. The processing into these two fragments was also observed in vivo. To investigate functional properties of the two fragments, GST (glutathione S-transferase) fusion proteins of the 60-kDa intermediate and the 20- and 45-kDa fragments were constructed. Neither the GST–20-kDa fusion protein (GST-20) nor the GST–45-kDa fusion protein (GST-45) was actively toxic against mosquito larvae of Culex pipiens, whereas the GST–60-kDa intermediate fusion protein (GST-60) exhibited significant toxicity. However, when the two fusion proteins GST-20 and GST-45 coexisted, significant toxicity was observed. The coprecipitation experiment demonstrated that the two fragments associated with each other. Therefore, it is strongly suggested that the two fragments formed an active complex of apparently 60 kDa. A mutant of the 60-kDa protein which was apparently resistant to the intramolecular cleavage with the midgut extract of C. pipiens larvae had toxicity slightly lower than that of GST-60.
Bacillus thuringiensis is a gram-positive soil bacterium that produces crystalline inclusions consisting of highly specific insecticidal proteins called δ-endotoxins during sporulation, which are toxic to the larvae of lepidopteran, dipteran, and coleopteran insects (11, 15). During sporulation of B. thuringiensis, intensive production of the δ-endotoxin in the mother cell compartment results in intracellular accumulation of the protein, accounting for 25% of the dry weight of the cell (1). The δ-endotoxins released as protoxins are ingested by susceptible insect larvae, dissolved in the midgut, and processed by gut proteases into the active forms (21). The activated toxins bind to a receptor in the midgut epithelium (13, 14), and the conformational change in the toxin molecules triggers the insertion of their pore-forming domain into the membrane (9, 10). Colloid-osmotic swelling and lysis of the cell result in the death of the larvae (17).
The three-dimensional structures of Cry3A and Cry1Aa have been analyzed (12, 20). These two toxin molecules have a similar three-dimensional structure comprising three domains. The activated Cry3A and Cry1Aa share about 33% amino acid sequence identity, and the level of identity shared between Cry4A and Cry1Aa (or Cry3A) is approximately 25%. However, the five highly conserved amino acid sequences among many δ-endotoxins called blocks 1 to 5 are located at the centers of domains or at the interfaces between domains. It is therefore expected that the activated Cry4A, which also possesses the five conserved blocks, would have a similar three-dimensional structure. Generally, domain I has been assumed to be involved in membrane partitioning and ion channel regulation. Domain II is proposed to be involved in the determination of insect specificity and in recognition of receptor molecules on midgut epithelial cells of the target insects. The function of domain III remains obscure. It is now believed that domain III is involved in ion channel formation, receptor binding, and insect specificity (24).
B. thuringiensis produces different δ-endotoxins among subspecies. B. thuringiensis subsp. israelensis produces dipteran-specific δ-endotoxins Cry4A, Cry4B, and Cry11A and nonspecifically cytotoxic Cyt1A (4, 15, 16).
It is generally believed that lepidopteran-specific δ-endotoxins of the 130-kDa type, exemplified by Cry1A, give the 60- to 70-kDa active forms through processing by the gut proteases. In contrast, the processing mechanism and the mode of action of dipteran-specific δ-endotoxins are rather poorly elucidated. Angsuthanasombat et al. reported that the 130-kDa protoxin of Cry4A was processed with midgut extracts of some mosquito larvae into a major product of 48 kDa (2). They also reported that the 130-kDa protoxin of Cry4B was converted into the 46- to 48-kDa and 16- to 18-kDa fragments. Therefore it seems that dipteran-specific δ-endotoxins have a processing pattern different from the lepidopteran-specific toxins of the 130-kDa type. In this paper, we demonstrated an unique processing pattern of Cry4A both in vitro and in vivo and analyzed the functional properties and the toxicity of the processing products of Cry4A against Culex pipiens. Functional analysis demonstrated that the two Cry4A fragments of 20 and 45 kDa were produced by the intramolecular cleavage of the 60-kDa intermediate at the loop between the α5 and α6 helices and that the two fragments associated with each other. The functional role of the association between the two segments and the intramolecular cleavage is also discussed. In addition, the processing pattern of Cry4A was compared with that of Cry4B.
MATERIALS AND METHODS
In vitro processing of Cry4A.
Purification of Cry4A crystal was performed as described previously (23). Cry4A crystal was solubilized in 100 mM Na2CO3 (pH 10.5)–10 mM dithiothreitol (DTT) for 30 min at 4°C, and 50-μg aliquots of the solubilized Cry4A were treated with 5 μg of the gut extract of C. pipiens at 30°C in a volume of 100 μl of 100 mM Na2CO3 (pH 10.5)–10 mM DTT. At appropriate times during the treatment, each sample was taken and immediately frozen at −80°C after addition of the serine protease inhibitor (p-amidinophenyl)methanesulfonyl fluoride hydrochloride (p-APMSF). The gut extract was prepared as follows. A 0.5-g sample of C. pipiens larvae was sonicated in 2.5 ml of 100 mM Na2CO3 (pH 10.5). After centrifugation, the supernatant was filtered through a 0.20-μm-pore-size filter, and the 20-μl aliquots were stored at −80°C. The protein concentration of the gut extract was 1.06 μg/μl.
In vivo processing of Cry4A.
One hundred larvae of C. pipiens were collected and fasted for 4 h in 10 ml of distilled water. Twenty micrograms of Cry4A crystal was added, and all of the larvae were collected after the appropriate time and washed three times with ice-cold phosphate-buffered saline (PBS) containing Complete (Boehringer Mannheim), a protease inhibitor cocktail. All of the larvae were suspended in 300 μl of ice-cold PBS containing Complete, sonicated, and separated into the pellet and supernatant fractions by centrifugation.
Site-directed mutagenesis.
The recombinant filamentous phage vector M13sH4 carries a fragment of the cry4A gene as an insert. M13sH4 was constructed by recloning the EcoRI fragment (1.9 kb) of pBS4 into the corresponding enzyme-treated M13mp18. The recombinant plasmid vector pBS4 was constructed by inserting the XmnI fragment (3.84 kb) of pIS422 (23) into the SmaI site of the vector pBluescriptSKII+. The following oligonucleotides were used for site-directed mutagenesis: (i) 5′-AACAATCGAGCCTTCGATTACTTAGAGCC-3′ for Q236A, (ii) 5′-CAATCGATAATTCGATTATTCAGAGCC-3′ for Q236X, and (iii) 5′-AAACAACGCAGCCTTCGATTACTTAGAGC-3′ for RQA. The Q236X mutation creates a stop codon at the Q236 position, and the RQA mutation is a double mutant carrying both of the R235A and Q236A mutations. The site-directed mutagenesis was performed with the single-stranded DNA from M13sH4 by using the oligonucleotide-directed in vitro mutagenesis system (version 2.1; Amersham) following the manufacturer’s instructions. All mutations were confirmed by DNA sequencing.
Construction of plasmids.
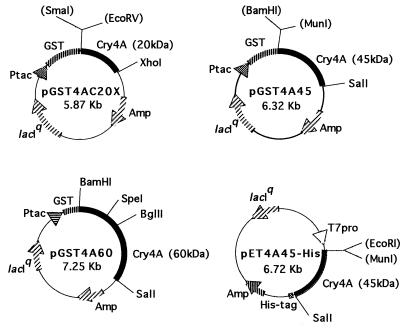
The plasmid pGST4AC20X (Fig. 1) was obtained by inserting the 0.895-kb EcoRV-XhoI fragment from M13sH4 Q236X into the SmaI-XhoI site of pGEX-4T-3 (Pharmacia Biotech). This plasmid encoded GST-20, the fusion protein of glutathione S-transferase (GST) linked to the segment spanning from Pro30 to Arg235 of Cry4A. The plasmid pGST4A45 (Fig. 1) was constructed by subcloning the 1.35-kb MunI-SalI fragment of pLH4-B2-Sal, which was obtained by inserting the SalI linker into the SacI site of pLH4-B2 (28), into the BamHI-SalI site of pGEX-4T-3. The plasmid pGST4A45 encoded GST-45, the fusion protein of GST linked to the segment spanning from Ile247 to Lys695 of Cry4A. Similarly, the plasmid pGST4A60 (Fig. 1) was designed to express GST-60, the fusion protein of GST linked to the segment spanning from Met1 to Lys695 of Cry4A. The plasmid pGST4A60RQA was constructed by using the SpeI-BglII fragment (300 bp) of M13sH4RQA to replace that of pGST60. The plasmid pET4A45-His (Fig. 1) was obtained by subcloning the 1.35-kb MunI-SalI fragment from pLH4-B2-Sal into the EcoRI-SalI site of pET21c (Novagen) to express 45His, the 6×His-tagged segment that spanned from Ile247 to Lys695 of Cry4A.
FIG. 1.
Physical maps of the plasmids. The plasmid pGST4AC20X was obtained by inserting the 0.895-kb EcoRV-XhoI fragment of cry4A into the SmaI-XhoI site of pGEX-4T-3 (Pharmacia Biotech). This plasmid encoded GST-20, the fusion protein of GST linked to the segment spanning Pro30 to Arg235 of Cry4A. The plasmid pGST4A45 was constructed by subcloning the 1.35-kb MunI-SalI fragment of pLH4-B2-Sal, which was obtained by inserting the SalI linker into the SacI site of pLH4-B2 (28), into the BamHI-SalI site of pGEX-4T-3. The plasmid pGST4A45 encoded GST-45, the fusion protein of GST linked to the segment spanning Ile247 to Lys695 of Cry4A. The plasmid pGST4A60 was constructed to express GST-60, the fusion protein of GST linked to the segment spanning Met1 to Lys695 of Cry4A, by inserting the 2.03-kb cry4A fragment into BamHI-SalI site of pGEX-4T-3. The plasmid pET4A45-His was obtained by inserting the 1.35-kb MunI-SalI fragment from pLH4-B2-Sal into the EcoRI-SalI site of pET21c (Novagen) to express 45His, the 6×His-tagged segment spanning Ile247 to Lys695 of Cry4A.
Coprecipitation experiment.
GST-Cry4A fusion protein was expressed upon induction for 2 h with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) in Escherichia coli BL21 cells harboring a pertinent expression vector in 200 ml of 2YT medium containing ampicillin. 2YT medium contains (per liter) 16 g of tryptone, 10 g of yeast extract, and 5 g of NaCl, and the pH is adjusted to 7.0 with NaOH. The cells were collected by centrifugation, resuspended in 20 ml of 100 mM cyclohexylaminopropanesulfonic acid (CAPS) (pH 10.5) containing 100 μg of p-APMSF per ml, and disrupted by sonication. After centrifugation, the supernatant was obtained. The GST-Cry4A fusion protein was purified from the supernatant by using glutathione-Sepharose 4B (Pharmacia Biotech). The recombinant protein 45His was expressed upon induction with 1 mM IPTG in E. coli BL21(DE3) cells harboring the pertinent expression vector in 200 ml of 2YT medium containing ampicillin. The cells were collected by centrifugation, resuspended in 20 ml of 100 mM CAPS (pH 10.5)–2 M urea–20 mM imidazole containing 100 μg of p-APMSF per ml, and disrupted by sonication followed by centrifugation to obtain the supernatant. To the supernatant was added 300 μl of a 50% slurry of Ni-resin (Qiagen). After 2 h of rotation at 4°C, the beads were washed three times with 100 mM CAPS (pH 10.5)–2 M urea–20 mM imidazole containing 100 μg of p-APMSF per ml. The beads were resuspended in 150 μl of the buffer presented above and added to 30 ml of the binding buffer (100 mM CAPS [pH 10.5], 20% glycerol, 500 mM NaCl, 10 mM β-mercaptoethanol, 20 mM imidazole containing 100 μg of p-APMSF per ml). After rotation overnight at 4°C, the purified GST-Cry4A fusion proteins were added, and this mixture was then rotated for another 2 h. After centrifugation, the resin was washed five times with the binding buffer, and the supernatant was concentrated with a microcon30 centrifugal filter device (Amicon). The resulting resin (bound fraction) and the concentrated supernatant (not bound fraction) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (14% polyacrylamide) and subjected to Western blotting with the anti-GST antibody (Pharmacia Biotech).
Bioassay of the mosquitocidal activities of GST-Cry4A fusion proteins.
GST-Cry4A fusion proteins were expressed in E. coli BL21 cells in 200 ml of 2YT medium as described above. The cells were harvested, resuspended in 20 ml of 100 mM Na2CO3 (pH 10.5)–20 mM β-mercaptoethanol containing 100 μg of p-APMSF per ml, and disrupted by sonication followed by centrifugation to obtain the supernatant. To the supernatant was added 200 μl of a 50% slurry of glutathione-Sepharose 4B (Pharmacia Biotech). After 2 h of rotation at 4°C, the beads were washed three times with the buffer described above. GST-Cry4A fusion proteins were eluted with 100 μl of 0.2 M Tris-HCl (pH 8.8) containing 20 mM reduced glutathione. The concentration of the purified proteins was determined with the Bio-Rad protein assay with bovine serum albumin (Sigma) as a standard. The bioassay of GST-Cry4A fusion proteins was performed essentially by the methods of Schnell et al. (25). The proteins were added to 1 ml of 0.1 M Tris (pH 7.5)–0.1% latex beads (Sigma) 0.8 μm in diameter to give a 0.1-μg/ml final protein concentration. After a brief vortex, the samples were rotated for 1 h at room temperature. The mosquitocidal activities were assayed on 4th instar larvae of C. pipiens. The mosquito larvae were grown in a container (35 by 25 by 3 cm) at 25°C. Before the assays, each larva was transferred to 200 μl of distilled water in each well of a 96-well plate. After 8 h, the GST-Cry4A fusion proteins adsorbed to latex beads were added. In each experiment, 96 larvae were tested at a protein concentration of 0.5 μg/ml, and the assay was performed more than three times. Mortality was scored after a 12-h incubation at 25°C. The efficiency of adsorption of protein to the latex beads was almost 100% in a preliminary experiment.
Protein sequencing.
The processed toxin was fractionated by SDS-PAGE (14% polyacrylamide) and transferred to polyvinylidene difluoride membrane (Bio-Rad). The N-terminal amino acid of each fragment band was sequenced with an Applied Biosystems model 476 pulsed-liquid sequencer.
RESULTS
Activation process of Cry4A.
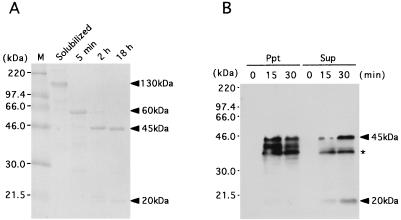
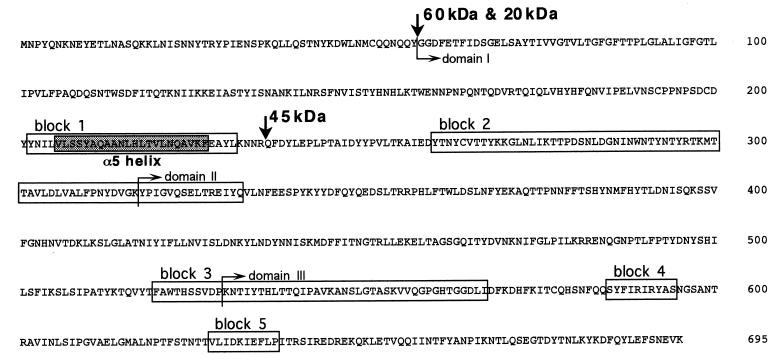
To investigate the activation process of Cry4A, the solubilized 130-kDa protoxin of Cry4A was processed in vitro with the midgut extract of C. pipiens larvae. The 130-kDa protoxin of Cry4A was converted into a 60-kDa fragment and subsequently into protease-resistant 20- and 45-kDa fragments (Fig. 2A). The N-terminal sequence analysis of these fragments demonstrated that the 60- and 20-kDa fragments had Gly58 at the N terminus, while the N terminus of the 45-kDa fragment was Gln236. Gln236 was located between the predicted α5 and α6 helices within the stretch of the 60-kDa fragment. Therefore, the 20- and 45-kDa fragments were generated through the intramolecular cleavage at the loop between the predicted α5 and α6 helices of the 60-kDa intermediate (Fig. 3). In the in vivo processing experiment, Cry4A crystal was given to C. pipiens larvae, and they were collected after 15 and 30 min and washed with ice-cold PBS containing the protease inhibitor cocktail. After they were sonicated and centrifuged, the supernatant and the pellet were analyzed by Western blotting with anti-B. thuringiensis subsp. israelensis crystal antibody. The 20- and 45-kDa fragments were detected in the supernatant fraction (Fig. 2B). It was clearly shown that Cry4A was processed in vivo into the 20- and 45-kDa fragments in C. pipiens, similar to the in vitro processing shown in Fig. 2A. Neither the 60-kDa intermediate nor the 130-kDa crystal could be detected even at 15 min after ingestion. This implied that the processing of 130-kDa protoxin into the 20- and 45-kDa fragments was so fast in vivo that the 60-kDa intermediate was not detectable.
FIG. 2.
The in vitro and in vivo activation processes of Cry4A. (A) In vitro processing of Cry4A was performed as follows. Fifty micrograms of the solubilized Cry4A was treated with 5 μg of the gut extract of C. pipiens at 30°C in a volume of 100 μl of 100 mM Na2CO3 (pH 10.5)–10 mM DTT. At appropriate times during the treatment, each sample was taken and immediately frozen at −80°C after addition of p-APMSF. One microgram of each sample was analyzed by SDS-PAGE (14% polyacrylamide) with Coomassie brilliant blue staining. (B) For in vivo processing of Cry4A, 100 larvae of C. pipiens were collected and fasted for 4 h in 10 ml of distilled water. To these was added 20 μg of Cry4A crystal, and the larvae were collected at appropriate times (15 and 30 min) and washed three times with ice-cold PBS containing Complete (Boehringer Mannheim), a protease inhibitor cocktail. After sonication, the solutions were centrifuged. The fraction of the pellet and the supernatant were analyzed by SDS-PAGE (14% polyacrylamide) followed by Western blotting with anti-B. thuringiensis subsp. israelensis crystal antibody. Ppt, the pellet and larvae debris; Sup, supernatant containing the brush border membrane of midgut. An asterisk indicates nonspecific bands.
FIG. 3.
Amino acid sequence of the N-terminal moiety of Cry4A. The five blocks, which contain amino acid sequences highly conserved among many δ-endotoxins, are depicted in boxes. The cleavage sites with gut extract are indicated by the vertical arrows. Rectangular arrows indicate boundaries that separate domains.
Most of the 20-kDa fragment was detected in the supernatant fraction that contained the brush border membrane fraction. Since the 20-kDa fragment is the possible channel-forming domain (Fig. 3), it is likely that it binds to and was inserted into the brush border membrane of the midgut. The pellet contained materials insoluble in the midgut and the debris of larvae. Therefore, the 45-kDa fragments detected in the pellet fraction might be the aggregated 45-kDa polypeptides that failed to associate with the 20-kDa fragment that was inserted into the brush border membrane.
Toxicity of the Cry4A fragments produced by processing.
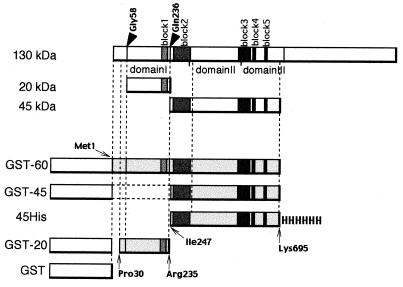
Several GST fusion proteins of the Cry4A fragments were constructed to investigate the function of the 20-, 45-, and 60-kDa fragments. GST-20 is the fusion protein consisting of GST linked to the segment from Pro30 to Arg235 of Cry4A, corresponding to the 20-kDa fragment (Fig. 4). Yoshida et al. reported that the region of Cry4A determining the insecticidal activity against C. pipiens was within the stretch from Pro30 to Lys695 (28). Therefore, we assumed that the C terminus of the 45- and 60-kDa fragment was Lys695. GST-45 is the fusion protein of GST and the segment from Ile247 to Lys695 of Cry4A. Structures of the GST fusion proteins were summarized in Fig. 4.
FIG. 4.
Fusion proteins of the fragments from Cry4A. A schematic representation of the fusion protein structures is shown. The arrowheads indicate the cleavage sites with gut extract. GST-60 is the fusion protein of GST and the Met1 to ∼Lys695 fragment of Cry4A. In GST-45, the Ile247 to ∼Lys695 segment of Cry4A is fused to the C terminus of GST. GST-20 is the fusion protein of GST and the Pro30 to ∼Arg235 region of Cry4A. 45His is the polypeptide ranging along the Ile247 to ∼Lys695 segment of Cry4A tagged with histidine hexamer. The putative domains and blocks are also depicted.
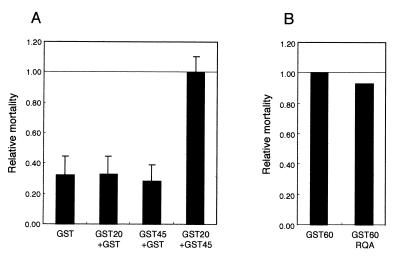
Purified GST fusion proteins were subjected to bioassay for the insecticidal activity against C. pipiens larvae. The proteins were adsorbed to latex beads and assayed at a working concentration of 0.5 μg/ml. The mortality after 12 h was calculated by subtracting the mortality when latex beads alone were given. Neither GST-20 nor GST-45 was toxic. However, when the two fusion proteins coexisted, a significant insecticidal activity was observed (Fig. 5A). Therefore, it is suggested that the concerted action of the 20- and 45-kDa fragments is necessary to exhibit the toxicity against C. pipiens.
FIG. 5.
Toxicity of GST fusion proteins of the processed fragments of Cry4A. The GST-Cry4A fusion proteins were adsorbed to the latex beads to give a final protein concentration of 0.1 μg/ml. The mosquitocidal activities were assayed on the 4th instar larvae of C. pipiens. Before the assays, each larva was transferred to 200 μl of distilled water in each well of a 96-well plate. After 8 h, the GST-Cry4A fusion proteins adsorbed to latex beads were added. In each experiment, 96 larvae were tested at the protein concentration of 0.5 μg/ml, and the assay was performed more than three times. The mortality of mosquito larvae in the presence of each GST fusion protein was scored after a 12-h incubation at 25°C by subtracting the mortality with latex beads alone. The error bars denote standard errors of the mean. (A) The relative mortalities with GST-Cry4A fusion proteins are expressed as a proportion of the mortality with GST-20 and GST-45. (B) The mortality with GST-60 and GST-60RQA is shown.
Association of the processed fragments of Cry4A.
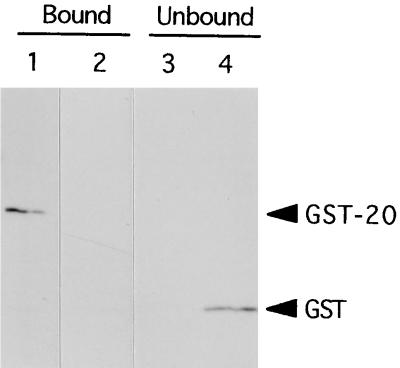
The 20- and 45-kDa fragments of Cry4A cannot be separated from each other by gel filtration, because both the two fragments behave together with the 60-kDa fragment (data not shown). Therefore, we propose that the 20- and 45-kDa fragments associate with each other to form a complex with an apparent molecular mass of 60 kDa. To confirm whether the two fragments associate together, in vitro coprecipitation experiments were performed. We constructed another fusion protein, 45His, with the 45-kDa processed fragment holding a histidine hexamer attached to the C-terminus (Fig. 4). Purified GST-20 or GST was incubated with Ni-resin matrix to which the purified 45His had been attached, and then the Ni-resin-bound fraction was analyzed by Western blotting with anti-GST antibody. As shown in Fig. 6, GST-20 was coprecipitated with 45His. However, GST itself was not detected in the Ni-resin-bound fraction, but in the unbound fraction (Fig. 6). Thus, we conclude that the 20- and 45-kDa fragments associate with each other and will form a complex of apparently 60 kDa that is actively toxic to larvae of mosquito C. pipiens.
FIG. 6.
Coprecipitation of GST-20 with 45His. 45His was purified under denaturing conditions (100 mM CAPS [pH 10.5], 2 M urea, 20 mM imidazole containing 100 μg of p-APMSF per ml). For refolding, 300 μl of a 50% slurry of Ni-resin (Qiagen) that bound 45His was added to 30 ml of the binding buffer (100 mM CAPS [pH 10.5], 20% glycerol, 500 mM NaCl, 10 mM β-mercaptoethanol, 20 mM imidazole containing 100 μg of p-APMSF per ml) and rotated overnight at 4°C. The purified GST-Cry4A fusion proteins were then added, and the mixture was rotated for another 2 h. After centrifugation, the resin was washed five times with the binding buffer, and the supernatant was concentrated with microcon30 centrifugal filter device (Amicon). The resulting resin (bound fraction) and the concentrated supernatant (unbound fraction) were analyzed by SDS-PAGE (14% polyacrylamide) followed by Western blotting with the anti-GST antibody (Pharmacia Biotech). (A) GST-20 coprecipitated with 45His, but GST did not. (B) GST was detected in the supernatant. Lanes: 1, GST-20 and 45His; 2, GST and 45His.
The role of the intramolecular cleavage.
GST-60 is the fusion protein consisting of GST and the 60-kDa intermediate of Cry4A. It is the polypeptide from Met1 to Lys695 of Cry4A linked at the C terminus of GST. To investigate the role of the intramolecular cleavage of the 60-kDa intermediate in the process of producing the active form of Cry4A, a mutant of GST-60, the GST fusion protein of the Cry4A 60-kDa fragment, was constructed by site-directed mutagenesis in which both Arg235 and Gln236 were replaced by Ala. This mutant, designated as GST-60RQA, was virtually resistant to the intramolecular cleavage in vitro with the gut extract of the mosquito larvae, resulting in neither the 20-kDa fragment nor the 45-kDa fragment upon treatment (data not shown). The toxicity of GST-60 and GST-60RQA was assayed against C. pipiens larvae at a concentration of 0.5 μg/ml. The mortality after 12 h was calculated by subtracting the mortality when latex beads alone were given. GST-60RQA had toxicity 90% of that of GST-60 (Fig. 5B).
DISCUSSION
It is generally believed that the 130-kDa type of lepidopteran-specific δ-endotoxins is processed into the activated toxins of 60 to 70 kDa. However, the activation process of the dipteran-specific toxins of 130-kDa type is obscure. Here, we investigated the activation process of Cry4A, a 130-kDa type of dipteran-specific δ-endotoxin, and demonstrated that the 130-kDa protoxin of Cry4A was processed into 20- and 45-kDa fragments by the intramolecular cleavage of the 60-kDa intermediate in vitro (Fig. 2A). The in vivo processing was also analyzed, and we successfully showed that the 20- and 45-kDa processed fragments were generated by midgut proteases of C. pipiens (Fig. 2B). The 20-kDa fragment consisted of five helices (α1 to ∼α5) of domain I, and the 45-kDa fragment involved domains II and III and the α6 and α7 helices of domain I. To investigate the functions of the processed fragments, we constructed GST fusion proteins of them (Fig. 4). This is a novel and powerful approach with which to analyze the mode of action of δ-endotoxins in vitro. All GST fusion proteins (except GST-45) possess the cleavage site (N terminus of Gly58) by processing with gut extract at the junction of GST and the processed fragment of Cry4A. Moreover, the GST gene fusion vector, pGEX-4T-3, has a thrombin recognition site to cleave out GST. Therefore, we consider that, upon ingestion by C. pipiens larvae, the extra GST peptide is removed from the Cry4A-processed fragment and digested with gut proteases. Therefore, in order to know the functions of the processed fragments, it is very significant to assay the toxicities of these GST fusion proteins against C. pipiens larvae. Neither GST-20 nor GST-45 was toxic against C. pipiens (Fig. 5A), suggesting that individual molecular species of the two processed fragments could not exhibit the toxicity and that the channel-forming domain alone could not efficiently interact with the membrane. When GST-20 and GST-45 actively coexisted, the toxicity against C. pipiens was exhibited (Fig. 5A). Therefore, it is suggested that the concerted action of the 20- and 45-kDa fragments is necessary to exhibit the toxicity against C. pipiens. Moreover, the in vitro coprecipitation experiments showed that GST-20 associated with 45His, but GST alone did not (Fig. 6). These data suggest that the 20- and 45-kDa fragments associate to form an active complex, which corresponds to a 60-kDa active fragment of the other lepidopteran-specific 130-kDa type of δ-endotoxins.
In the in vivo processing, 130-kDa crystal of Cry4A was processed into 20- and 45-kDa fragments by 15 min after ingestion, and we could not detect the 130-kDa crystal and 60-kDa intermediate (Fig. 2B). Even with the longer exposure, the bands of 130 and 60 kDa were not detectable (data not shown). Most of the 20-kDa fragment was observed in the supernatant fraction containing the brush border membrane vesicle fraction (Fig. 2B). These data suggest that the solubilization of crystal, proteolytic processing, and insertion into the membrane proceed very rapidly in the midgut.
The intramolecular cleavage of the 60-kDa intermediate of Cry4A into the 20- and 45-kDa fragments occurred at the putative loop between the α5 and α6 helices (Fig. 2). Domain I of δ-endotoxins consists of a seven-helix bundle (12, 20), and the central α4 and α5 helices can be inserted into the membrane to form a channel (9, 26). Apparently, it is reasonable to suppose that the cleavage at the loop between the α5 and α6 helices can facilitate the conformational change of the α-helix bundle and is necessary for the α4 and α5 helices to form a channel. Schwartz et al. reported that channel formation requires domain I to swing away from domains II and III (26). Therefore, a major conformational change in the toxin molecule should occur in the process of forming a channel. Thus the cleavage into the 20- and 45-kDa fragments may lead to swinging away of a channel-forming moiety to be inserted. To examine this, we constructed the GST-60RQA mutant of Cry4A, which was not cleaved in vitro into the 20- and 45-kDa fragments because the intramolecular cleavage site of the 60-kDa intermediate was eliminated. The mutant GST-60RQA had toxicity against C. pipiens larvae slightly lower than that of GST-60 (Fig. 5B), suggesting that the intramolecular cleavage was dispensable for the insecticidal activity at least in the case of Cry4A. However, we have not yet investigated the in vivo processing of GST-60RQA. Therefore, we cannot eliminate the possibility that the intramolecular cleavage of GST-60RQA might occur in vivo. For interpretation of the functional significance of the proteolytic cleavage, further investigation is needed.
The cleavage in domain I is also detected in Cry3A (6), Cry2A (22), Cry4B (3), and Cry9C (19), but the results are contradictory. In the case of Cry3A, Carroll et al. reported that the interhelical proteolytic cleavage in domain I might facilitate its coleopteran toxicity (5). On the contrary, in Cry4B, the blockage of the interhelical proteolytic cleavage in domain I resulted in an increase in toxicity against Aedes aegypti (3). In the case of Cry9C, Lambert et al. reported that 130-kDa protoxin of Cry9C was processed into a 69-kDa fragment and that the digestion to a 55-kDa fragment by the intrahelical cleavage resulted in the loss of toxicity (19). Therefore, the biological significance of the intramolecular cleavage in domain I seems to differ among the target insects. Some unknown factors in the midgut environment of susceptible insects may affect the toxicity.
GST-20 was nontoxic against C. pipiens at the concentration used in the bioassay (Fig. 5A). Apparently, this is not consistent with the observation that activated toxins can form channels in planar lipid bilayers in the absence of their receptor (27). We confirmed, in a preliminary experiment, that the efficiency at which toxins adsorb to the latex beads was significantly decreased at the toxin concentration of 25 μg/ml. Therefore, the assay using latex beads was unsuitable to give toxins at concentrations higher than those we used in the present work. Thus, we failed to perform the bioassay at higher concentrations of toxin. In addition, we cannot rule out the possibility that GST-20 may exhibit a very low level of insecticidal activity.
Komano et al. reported the in vitro processing of Cry4B (18). The 130-kDa protoxin of Cry4B was converted into the 18- and 46-kDa fragments with the gut extract of C. pipiens larvae. No intermediate molecule corresponding to the 60-kDa fragment of Cry4A was observed. We performed gel filtration chromatography with the processed Cry4B fragments and observed that the 18- and 46-kDa fragments of Cry4B associated with each other (data not shown). However, the insecticidal activity of the in vitro-processed Cry4B fragments was almost lost upon processing (18). Therefore, unlike Cry4A, the putative complex of the two fragments of 18 and 46 kDa generated from the 130-kDa protoxin of Cry4B seems not to be an active form. Some unknown factors or events that are not manifested in the in vitro processing may be essential for the activation of Cry4B. In this context, it is considered that the activation process of Cry4B contains a phase(s) different from those in the activation process of Cry4A. This remains for further investigation.
Chungjatupornchai et al. investigated the in vitro processing of Cry4B with the midgut extract of A. aegypti (7): Cry4B was processed rapidly into a 68- to 78-kDa fragment that was actively toxic against A. aegypti and further processed into a nontoxic 45- to 48-kDa fragment. Komano et al. also detected a 70-kDa fragment in the in vitro processing of Cry4B with the midgut extract of C. pipiens. However, they could not detect the toxicity of the 70-kDa fragment (18). This contradiction may be attributable to the difference of the target insect.
Angsuthanasombat et al. performed the in vitro activation experiment with Cry4A and Cry4B by using gut extracts from three kinds of mosquitoes and the assay for cytotoxicity against three mosquito cell lines (2). Cry4A was digested to the 48-kDa fragment with Aedes, Anopheles, or Culex gut extracts, and Cry4B was processed to the 46- to 48-kDa and 16- to 18-kDa fragments. However, their cytotoxicity against mosquito cell lines was not coincident with the toxicity of the purified inclusions. Moreover, they failed to detect the 20-kDa processed fragment in the in vitro processing of Cry4A and to analyze the digestion process with gut proteases.
In this paper, we analyzed the activation process of dipteran-specific Cry4A and suggested the concerted action of the processed fragments of Cry4A. In many experiments with site-directed mutagenesis, the role of each domain of δ-endotoxins turned out to be more complex than expected (8). Therefore, the concerted action of each domain may be important for the functional activity of δ-endotoxins. The functional analysis of the processed fragments of Cry4A will clarify the mode of action of the Cry4A toxin.
ACKNOWLEDGMENTS
We are grateful to the Dainihon Jochugiku Co., Ltd., for providing us with C. pipiens eggs.
This work was supported by a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), Tokyo, Japan, to H.S. This work was supported in part by the Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (M.Y.).
REFERENCES
- 1.Agaisse H, Lereclus D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J Bacteriol. 1995;177:6027–6032. doi: 10.1128/jb.177.21.6027-6032.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angsuthanasombat C, Crickmore N, Ellar D J. Comparison of Bacillus thuringiensis subsp. israelensis CryIVA and CryIVB cloned toxins reveals synergism in vivo. FEMS Microbiol Lett. 1992;94:63–68. doi: 10.1016/0378-1097(92)90584-b. [DOI] [PubMed] [Google Scholar]
- 3.Angsuthanasombat S, Crickmore N, Ellar D J. Effects on toxicity of eliminating a cleavage site in a predicted interhelical loop in Bacillus thuringiensis CryIVB δ-endotoxin. FEMS Microbiol Lett. 1993;111:255–262. doi: 10.1111/j.1574-6968.1993.tb06395.x. [DOI] [PubMed] [Google Scholar]
- 4.Aronson A I, Beckman W, Dunn P. Bacillus thuringiensis and related insect pathogens. Microbiol Rev. 1986;50:1–24. doi: 10.1128/mr.50.1.1-24.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll J, Convents D, Van Damme J, Boets A, Van Rie J, Ellar D J. Intramolecular proteolytic cleavage of Bacillus thuringiensis Cry3A δ-endotoxin may facilitate its Coleopteran toxicity. J Invertebr Pathol. 1997;70:41–49. doi: 10.1006/jipa.1997.4656. [DOI] [PubMed] [Google Scholar]
- 6.Carroll J, Li J, Ellar D J. Proteolytic processing of a coleopteran-specific δ-endotoxin produced by Bacillus thuringiensis var. tenebrionis. Biochem J. 1989;261:99–105. doi: 10.1042/bj2610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chungjatupornchai W, Höfte H, Seurinck J, Angsuthanasombat C, Vaeck M. Common features of Bacillus thuringiensis toxins specific for Diptera and Lepidoptera. Eur J Biochem. 1988;173:9–16. doi: 10.1111/j.1432-1033.1988.tb13960.x. [DOI] [PubMed] [Google Scholar]
- 8.Dean D H, Rajamohan F, Lee M K, Wu S-J, Chen X J, Alcantara E, Hussain S R. Probing the mechanism of action of Bacillus thuringiensis insecticidal proteins by site-directed mutagenesis—a minireview. Gene. 1996;179:111–117. doi: 10.1016/s0378-1119(96)00442-8. [DOI] [PubMed] [Google Scholar]
- 9.Gazit E, La Rocca P, Sansom M S, Shai Y. The structure and organization within the membrane of the helices composing the pore-forming domain of Bacillus thuringiensis delta-endotoxin are consistent with an “umbrella-like” structure of the pore. Proc Natl Acad Sci USA. 1998;95:12289–12294. doi: 10.1073/pnas.95.21.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazit E, Shai Y. The assembly and organization of the α5 and α7 helices from the pore-forming domain of Bacillus thuringiensis δ-endotoxin. J Biol Chem. 1995;270:2571–2578. doi: 10.1074/jbc.270.6.2571. [DOI] [PubMed] [Google Scholar]
- 11.Gill S S, Cowles E A, Pietrantonio P V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 12.Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz J, Brousseau R, Cygler M. Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation. J Mol Biol. 1995;254:447–464. doi: 10.1006/jmbi.1995.0630. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann C, Luthy P, Hutter R, Pliska V. Binding of the delta endotoxin from Bacillus thuringiensis to brush-border membrane vesicles of the cabbage butterfly (Pieris brassicae) Eur J Biochem. 1988;173:85–91. doi: 10.1111/j.1432-1033.1988.tb13970.x. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann C, Vanderbruggen H, Höfte H, Van Rie J, Jansens S, Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins is correlated with the presence of high-affinity binding sites in the brush border membrane of target insect midguts. Proc Natl Acad Sci USA. 1988;85:7844–7848. doi: 10.1073/pnas.85.21.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Höfte H, Whiteley H R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Insell J P, Fitz-James P C. Composition and toxicity of the inclusion of Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 1985;50:56–62. doi: 10.1128/aem.50.1.56-62.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knowles B H, Ellar D J. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different insect specificity. Biochim Biophys Acta. 1987;924:509–518. [Google Scholar]
- 18.Komano T, Yamagiwa M, Nishimoto T, Yoshisue H, Tanabe K, Sen K, Sakai H. Activation process of the insecticidal proteins CryIVA and CryIVB produced by Bacillus thuringiensis subsp. israelensis. Isr J Entomol. 1998;32:185–198. [Google Scholar]
- 19.Lambert B, Buysse L, Decock C, Jansens S, Piens C, Saey B, Seurinck J, Van Audenhove K, Van Rie J, Van Vliet A, Peferoen M. A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family Noctuidae. Appl Environ Microbiol. 1996;62:80–86. doi: 10.1128/aem.62.1.80-86.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Carroll J, Ellar D J. Crystal structure of insecticidal δ-endotoxin from Bacillus thuringiensis at 2.5Å resolution. Nature. 1991;353:815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- 21.Lilley M, Ruffell R N, Somerville H J. Purification of the insecticidal toxin in crystals of Bacillus thuringiensis. J Gen Microbiol. 1980;118:1–11. doi: 10.1099/00221287-118-1-1. [DOI] [PubMed] [Google Scholar]
- 22.Nicholls C N, Ahmad W, Ellar D J. Evidence for two different types of insecticidal P2 toxins with dual specificity in Bacillus thuringiensis subspecies. J Bacteriol. 1989;171:5141–5147. doi: 10.1128/jb.171.9.5141-5147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimoto T, Yoshisue H, Ihara K, Sakai H, Komano T. Functional analysis of block 5, one of the highly conserved amino acid sequences in the 130-kDa CryIVA protein produced by Bacillus thuringiensis subsp. israelensis. FEBS Lett. 1994;348:249–254. doi: 10.1016/0014-5793(94)00604-0. [DOI] [PubMed] [Google Scholar]
- 24.Rajomohan F, Lee M K, Dean D H. Bacillus thuringiensis insecticidal proteins: molecular mode of action. Prog Nucleic Acid Res Mol Biol. 1998;60:1–27. doi: 10.1016/s0079-6603(08)60887-9. [DOI] [PubMed] [Google Scholar]
- 25.Schnell D J, Pfannenstiel M A, Nickerson K W. Bioassay of solubilized Bacillus thuringiensis var. israelensis crystals by attachment to latex beads. Science. 1984;223:1191–1193. doi: 10.1126/science.6701520. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz J L, Juteau M, Grochulski P, Cygler M, Prefontaine G, Brousseau R, Masson L. Restriction of intramolecular movements within the Cry1Aa toxin molecule of Bacillus thuringiensis through disulfide bond engineering. FEBS Lett. 1997;410:397–402. doi: 10.1016/s0014-5793(97)00626-1. [DOI] [PubMed] [Google Scholar]
- 27.Slatin S L, Abrams C K, English L. Delta-endotoxins form cation-selective channels in planar lipid bilayers. Biochem Biophys Res Commun. 1990;169:765–772. doi: 10.1016/0006-291x(90)90397-6. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K, Matsushima Y, Sen K, Sakai H, Komano T. Insecticidal activity of a peptide containing the 30th to 695th amino acid residues of the 130-kDa protein of Bacillus thuringiensis var. israelensis. Agric Biol Chem. 1989;53:2121–2127. [Google Scholar]