SUMMARY
Shigella is an important bacterial cause of infectious diarrhoea globally. The Shigella human challenge model has been used since 1946 for a variety of objectives including understanding disease pathogenesis, human immune responses and allowing for an early assessment of vaccine efficacy. A systematic review of the literature regarding experimental shigellosis in human subjects was conducted. Summative estimates were calculated by strain and dose. While a total of 19 studies evaluating nine strains at doses ranging from 10 to 1 × 1010 colony-forming units were identified, most studies utilized the S. sonnei strain 53G and the S. flexneri strain 2457T. Inoculum solution and pre-inoculation buffering has varied over time although diarrhoea attack rates do not appear to increase above 75–80%, and dysentery rates remain fairly constant, highlighting the need for additional dose-ranging studies. Expansion of the model to include additional strains from different serotypes will elucidate serotype and strain-specific outcome variability.
Key words: Gastrointestinal infections, Shigella, travellers' infection
INTRODUCTION
Infection with Shigella causes bacillary dysentery and has been recognized as a major cause of inflammatory diarrhoeal disease in endemic regions. The low infective dose and faecal–oral route of transmission facilitates spread through contaminated food and water and personal contact. Shigella infections remain problematical for young children in endemic regions, travellers and deployed military personnel [1, 2]. Since 1946, the Shigella human challenge model has been used to investigate pathogenesis and host immune responses induced by Shigella infection and to evaluate the efficacy of investigational products. In the inaugural study, subjects were challenged with Shigella paradysenteriae (Flexner W) strain in a series of dose-finding and vaccine efficacy studies [3]. While early preclinical data on these vaccines were promising, they failed to protect against shigellosis following challenge. Despite the lack of protective efficacy, this inaugural study paved the way for future development and utilization of the Shigella human challenge model.
Shigella is categorized into four species, each further classified into one or more serotypes: S. dysenteriae (17 serotypes), responsible for epidemic outbreaks; S. flexneri (14 serotypes), more common in the developing world; S. sonnei (one serotype), primarily found in developed countries; S. boydii, relatively rare and found mostly in the Indian subcontinent [2]. Presently, there is a lack of pathogen specific clinical data from field studies.
The purpose of this study was to conduct a systematic review of the published literature on experimental human shigellosis enabling an analysis of study-specific factors and their associations with clinical outcomes. This type of review can be an important resource for researchers considering experimental human challenge studies. Specific to human challenge models, continual review of the published literature is important to minimize risk to future study participants and to ensure the safe application of the experimental infection for evaluating novel study hypotheses.
METHODS
A systematic review of the published literature was conducted to examine specific outcomes in human subjects participating in experimental Shigella infection studies. A Pubmed database search was performed to identify all articles for potential inclusion. Searches began with the term ‘Shigella’, and were followed by one of the following: ‘challenge’ or ‘human challenge’, ‘efficacy’ or ‘vaccine efficacy’, and ‘model’. Only experimental infection studies in which human subjects received live, wild-type Shigella strains were included. Subjects receiving investigational products prior to, or during experimental infection were excluded. The search was limited to studies conducted in Shigella non-endemic regions and published in the English language prior to June 2010. Studies were also identified through a detailed review of references and through discussion with experts in the field.
Bibliographical information, inoculum and strain information, subject demographics, specific clinical outcomes, and immunogenicity data were indentified in the included studies and entered into a pre-tested Microsoft Access (Microsoft Inc., USA) database. Heterogeneity was assessed using a χ2 heterogeneity statistic, and potential sources of heterogeneity were assessed graphically by Forest plots and using non-parametric methods (e.g. Kruskal–Wallis, Mann–Whitney U test) to compare differences in incidence between two or more groups of a given study characteristic. In the case of parameters where only a few studies were found, a median and range of estimates were reported. For summarization purposes, point estimates and standard 95% confidence intervals were combined using a random-effects model with methodology developed by DerSimonian & Laird [4]. As the principal purpose of this systematic review was to summarize studies reporting diarrhoea and dysentery incidence following experimental infection, publication bias was not assessed; as such, the concern for non-published findings due to negative studies or disappointing results was considered minimal. Individual study characteristics evaluated included strain and quantity of inoculum administered and inoculum administration procedures. These were assessed in relation to their effect on multiple outcomes such as diarrhoeal attack rates and disease severity. Statistical analyses were conducted using SAS version 8.2 (SAS Institute Inc., USA).
RESULTS
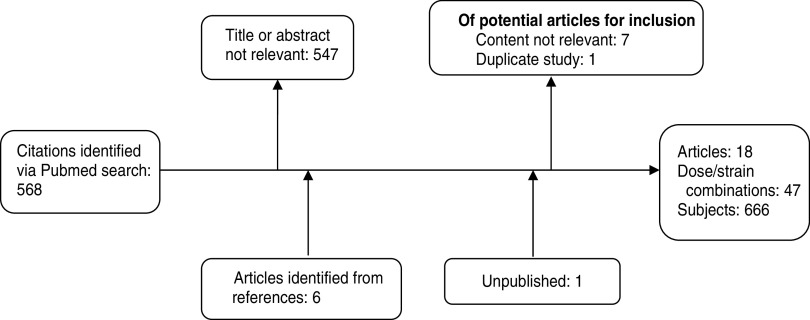
A total of 568 citations of published literature on experimental Shigella infection were identified through the Pubmed database search yielding a total of 18 individual studies and 47 dose/strain combinations for entry into a Microsoft Access database (Fig. 1). Because one reference included a review of a previous studies, but contained data of greater relevance than the original, only the review was included. A complete listing of all included studies is shown in Table 1.
Fig. 1.
Flow diagram for studies included in the systematic review.
Table 1.
List of experimental Shigella infection studies that met inclusion criteria for systematic review and meta-analysis
| Ref. | Pub. year | First- named author | Study type* | NaHCO3 buffer | Inoculum solution | Diarrhoea definition† | Strain | Dose (c.f.u.) | N | N (%) diarrhoea | N (%) dysentery | N (%) fever | Mean no. LLS (volume) | Mean incub. (h) | Mean duration | % colonized | Immune response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] | 2006 | Taylor | C | Yes | NaHCO3 | A | 2457T | 1.5 × 103 | 10 | 8 (80) | 1 (10) | 4 (40) | 2 (0.3 l) | 78.5 | 50 | ||
| [13] | 1999 | Coster | B | Yes | NaHCO3 | A | 2457T | 1 × 103 | 15 | 6 (40) | 4 (27) | 6 (40) | 11 | 40 | ASC (LPS) –IgG: 40% –IgA: 40% | ||
| [5] | 1996 | Van de Verg | D | NR | NR | NR | 2457T | 1 × 103 | 14 | NR | NR | Serum (LPS) –IgG: 50% –IgA: 65% | |||||
| [14] | 1995 | Kotloff | B | Yes | NaHCO3 | A | 2457T | 1 × 103 | 14 | 9 (64) | 9 (64) | 12 (86) | 93 | Serum (LPS) –IgG: 50% –IgA: 79% ASC (LPS) –IgA:100% –IgG:93% | |||
| [6] | 1995 | Kotloff | A | Yes | NaHCO3 | A | 2457T | 1.4 × 103 | 12 | 10 (83) | 10 (83) | 100 | |||||
| [24] | 1995 | Munoz | A | NR | Milk | A | 53G | 5 × 102 | 11 | 6 (55) | 7 (64) | 43.9 | 82 | ASC (LPS) –IgA: 72% | |||
| [25] | 1992 | Tacket | C | Yes | Milk | A | 2457T | 1.2 × 103 | 7 | 1 (14) | 3 (43) | 4 (57) | (3.4 l) | 3 days | Serum (LPS) –IgG: 43% –IgA: 29% ASC (LPS) –IgG: 57% –IgA: 71% | ||
| 1.6 × 103 | 11 | 4 (36) | 4 (36) | 4 (36) | (0.8 l) | 2.6 days | 91 | Serum (LPS) IgG: 45% IgA: 82% ASC (LPS) –IgG: 64% –IgA: 73% | |||||||||
| [26] | 1992 | Kotloff | B | NR | Milk | A | 2457T | 1 × 103 | 21 | 8 (38) | 7 (33) | 8 (38) | 48 | ||||
| [27] | 1992 | Mackowiak | B | NR | Milk | A | 53G | 5 × 102 | 85 | 40 (47) | NR | 33 (39) | 54 | ||||
| [28] | 1990 | Van de Verg | B | NR | Milk | NR | 53G | 5 × 102 | 12 | 5 (42) | NR | Serum (O Ag) –IgA: 50% ASC (O Ag) –IgA: 50% | |||||
| [10] | 1990 | Herrington | B | NR | Milk | A | 53G | 4 × 102 | 12 | 7 (58) | 8 (67) | 6 (50) | |||||
| [29] | 1989 | DuPont‡ | A | No | Milk | D | A-1 | 2 × 102 | 8 | 3 (38) | NR | 3 (38) | 144 | ||||
| No | D | A-1 | 1 × 104 | 6 | 2 (33) | NR | 2 (33) | 72 | |||||||||
| No | D | M-131 | 10 | 10 | 1 (10) | NR | 1 (10) | 36 | |||||||||
| No | D | M-131 | 2 × 102 | 4 | 2 (50) | NR | 2 (50) | 72 | |||||||||
| No | D | M-131 | 2 × 103 | 10 | 7 (70) | NR | 7 (70) | 146.4 | |||||||||
| No | D | M-131 | 1 × 104 | 6 | 5 (83) | NR | 5 (83) | 124.8 | |||||||||
| NR | C | 53G | 5 × 102 | 20 | 7 (35) | NR | 7 (35) | ||||||||||
| [7] | 1987 | Black | B | NR | Milk | A | 53G | 5 × 102 | 38 | 20 (53) | 19 (50) | 20 (53) | 9.2 (1.3 ml) | Serum (O Ag) –IgG: 45% –IgA: 68% | |||
| [30] | 1977 | Levine | B | NR | Milk | D | 2457T | 1 × 102 | 36 | 14 (39) | 8 (22) | 7 (19) | Serum (LPS) –HA: 22% | ||||
| 1 × 104 | 15 | 5 (33) | 5 (33) | 3 (20) | 60 | Serum (LPS) –HA: 60% | |||||||||||
| [9] | 1972 | DuPont | B | NR | Milk | E | 2457T | 1 × 104 | 88 | 52 (59) | NR | 52 (59) | 75 | Serum (LPS) –HA: 44% | |||
| 1.8 × 102 | 36 | 9 (22) | NR | 9 (22) | 17 | Serum (LPS) –HA: 27% | |||||||||||
| 1 × 103 | 24 | 14 (58) | NR | 14 (58) | 63 | Serum (LPS) –HA: 50% | |||||||||||
| 5 × 103 | 49 | 28 (57) | NR | 49 (28) | 67 | Serum (LPS) –HA: 49% | |||||||||||
| [31] | 1969 | DuPont | A | NR | Milk | C | 2457T | 1 × 104 to 1 × 108 | 43 | 27 (63) | 25 (58) | 13 (30) | 96 | 168 h | 67 | ||
| [3] | 1946 | Shaughnessy | A | Yes | Milk | NR | FW I | 1 × 108 | 4 | 0 (0) | NR | 100 | |||||
| Milk | FW I | 1 × 109 | 4 | 1 (25) | NR | 0 | 100 | ||||||||||
| Milk | FW I | 1 × 1010 | 4 | 2 (50) | NR | 0 | 100 | ||||||||||
| Water | FW I | 1 × 1010 | 4 | 2 (50) | NR | 0 | 100 | ||||||||||
| Water | FW II | 6.25 × 108 | 3 | 2 (67) | 2 (67) | 2 (67) | |||||||||||
| Water | FW III | 6.25 × 108 | 3 | 2 (67) | 2 (67) | 2 (67) | |||||||||||
| Water | FW IV | 6.25 × 108 | 3 | 2 (67) | 2 (67) | 2 (67) | |||||||||||
| Water | FW V | 6.25 × 108 | 3 | 2 (67) | 2 (67) | 2 (67) | |||||||||||
| B | Yes | Water | FW II | 6.25 × 108 | 20 | 14 (70) | 15 (75) | 15 (75) | |||||||||
| FW II | 2.5 × 108 | 5 | NR | 2 (40) | 2 (40) | ||||||||||||
| FW II | 1 × 109 | 5 | NR | 2 (40) | 2 (40) | ||||||||||||
| FW III | 6.25 × 108 | 20 | 14 (70) | 15 (75) | 15 (75) | ||||||||||||
| FW III | 2.5 × 108 | 5 | NR | 2 (40) | 2 (40) | ||||||||||||
| FW III | 1 × 109 | 5 | NR | 2 (40) | 2 (40) | ||||||||||||
| FW IV | 6.25 × 108 | 20 | 14 (70) | 15 (75) | 15 (75) | ||||||||||||
| FW IV | 2.5 × 108 | 5 | NR | 2 (40) | 2 (40) | ||||||||||||
| FW IV | 1 × 109 | 5 | NR | 2 (40) | 2 (40) | ||||||||||||
| FW V | 6.25 × 108 | 20 | 14 (70) | 15 (75) | 15 (75) | ||||||||||||
| FW V | 2.5 × 108 | 5 | NR | 2 (40) | 2 (40) | ||||||||||||
| FW V | 1 × 109 | 5 | NR | 2 (40) | 2 (40) |
NA, Not applicable; NR, not reported, abx, antibiotic; HA, humoral antibody determined by haemagglutination; incub., incubation; LPS, lipopolysaccharide.
Study types: A, pathogenesis; B, vaccine efficacy; C, treatment or prophylaxis; D, other.
Diarrhoea definitions: A, 1 loose or liquid stool (LLS) ⩾300 ml or ⩾2 LLS ⩾200 ml over 48 h; B, ⩾2 LLS in 24 h or 1 LLS ⩾300 ml; C, 2 or 3 LLS in 24 h; D, ⩾3 LLS in 24 h; E ⩾4 loose stools in 24 h.
Data on strains A-1 and M-131 in the DuPont 1989 study was previously reported in Levine et al. [32].
The majority (n = 11, 61%) of the studies were conducted between 1989 and 1999. Additionally, a majority (n = 10, 55%) of the studies were conducted to evaluate vaccine efficacy, although one of the studies was also an infective dose-finding study [3]. Most of the remaining studies were performed to describe strain/dose pathogenesis (n = 6, 33%), or evaluate the protection conferred by an oral antibiotic (n = 1, 6%) or prophylactic (n = 1, 6%). An additional study was conducted to assess the cross-reactivity of S. flexneri 2a antibodies generated subsequent to challenge [5].
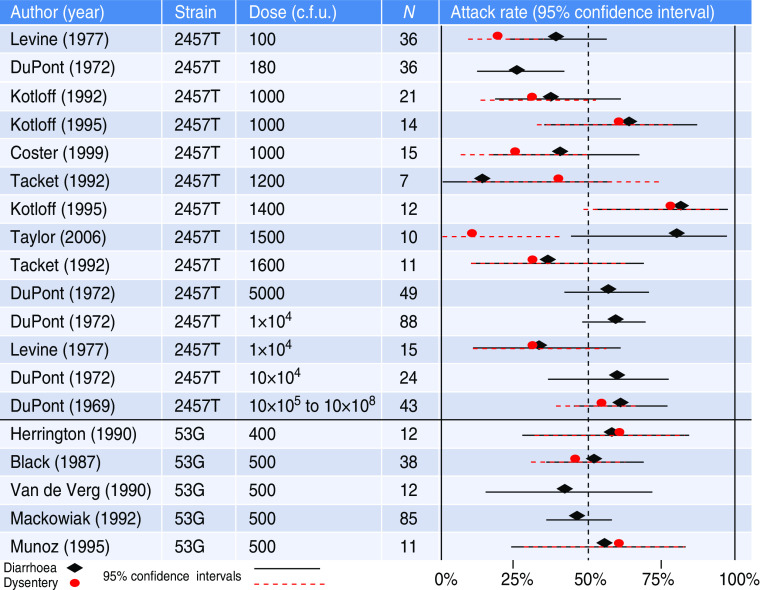
Strains from three Shigella species were used in these studies: S. flexneri, S. sonnei, and S. dysenteriae. The following strains were used: 2457T (S. flexneri 2a), 53G (S. sonnei), M-131 and A-1 (S. dysenteriae 1), and five Flexner W (previous designation for S. flexneri 2a) strains, FW I, FW II, FW III, FW IV, and FW V. Summary information about each strain is provided in Table 2. The two most commonly used strains, 2457T and 53G, were included in 94% of the published studies. The diarrhoea and dysentery rates following inoculation with various doses of those two strains are shown in Figure 2.
Table 2.
Strains of Shigella utilized in experimental human infection
| Species | Sero- type | Strain | Initial strain description | Country of origin | No. of subjects | Clinical information on index case | Dose (c.f.u.) |
|---|---|---|---|---|---|---|---|
| S. flexneri | 2a | 2457T | [31] | Japan | 407 | Isolated from a patient with clinical illness in Japan in early 1950s | 8 × 102 to 1.6 × 103 |
| S. sonnei | – | 53G | [29] | Japan | 178 | Isolated from 5 year-old boy who had experienced diarrhoea for 2 days in a Tokyo, Japan hospital in August 1954 | 4 × 102 to 5 × 102 |
| S. dysenteriae | 1 | M-131 | [32] | Guatemala | 30 | Isolated from patient with severe dysentery acquired during pandemic of 1970 in Guatemala | 2 × 102 to 1 × 104 |
| S. dysenteriae | 1 | A-1 | [32] | Guatemala | 14 | Isolated from patient with mild disease in Guatemala in late 1960s, early in pandemic | 10 to 1 × 104 |
| S. paradysenteriae (S. flexneri) | 2a | FW I, | [3] | – | 49 | – | 1 × 108 to 1 × 1010 |
| FW II, | |||||||
| FW III, | |||||||
| FW IV, | |||||||
| FW V |
Fig. 2.
[colour online]. Diarrhoea and dysentery rates (with 95% confidence intervals) for experimental infections with Shigella flexneri strain 2457T and Shigella sonnei strain 53G.
Several studies (n = 7, 37%) reported the use of a sodium bicarbonate (NaHCO3) pre-treatment prior to inoculation, although a considerable number (n = 11, 58%) did not report any pre-treatment. The studies by Shaughnessy et al. in 1946, used water as the inoculum vehicle [3]. However, the majority (n = 12, 63%) of studies, and nearly all before 1995, administered the challenge strain in milk. Subsequently, sodium bicarbonate has become the inoculum vehicle (n = 5, 26%) following the 1995 study which found that this buffer yielded a higher and more consistent attack rate than milk [6].
A consistent definition for the primary clinical outcome, i.e. diarrhoea, was not used until the study published by Black et al. in 1987 [7]. Thereafter, excluding two studies, the definition for diarrhoea was most commonly (n = 9, 50%) one loose or liquid stool (LLS) ⩾300 ml or ⩾2 LLS ⩾200 ml over 48 h. The 1946 study by Shaughnessy et al. did not report a definition for diarrhoea, as dysentery was the primary clinical outcome [3]. Additional studies by Taylor et al. and Van de Verg et al. similarly failed to include diarrhoea definitions [5, 8]. For the remaining studies, most definitions were for LLS within 24 h: ⩾3 LLS (11%), 2 or 3 LLS (11%), ⩾4 LLS (6%), and ⩾2 LLS or 1 LLS ⩾300 ml (6%).
We found a significantly (P < 0·05) higher diarrhoea attack rate in studies of strain 2457T in which the inoculum was administered in NaHCO3 [67%, 95% confidence interval (CI) 52–81] compared to studies utilizing milk (42%, 95% CI 32–53). Rates of dysentery were similar between inoculum vehicles. Fasting prior to inoculation may be associated with higher attack rates, although data are insufficient.
Excluding strain 2457T, a small range of doses has been evaluated for each strain. Despite no statistically significant dose response for diarrhoea or dysentery, a borderline positive association (P = 0·1) was noted between dysentery rates and dose at doses ⩽1400 colony-forming units (c.f.u.) for strain 2457T. However, the rate of dysentery following administration of 2457T (41%, 95% CI 30–52) was lower than the other tested strains (56%, 95% CI 48–65).
Homologous protection has been assessed in three separate studies of two strains (2457T and 53G) [6, 9, 10]. The inaugural study by DuPont et al. showed a 64% reduction in disease in subjects receiving a second inoculum of 104 organisms of S. flexneri strain 2457T administered in skim milk [9]. Similar results were observed by Kotloff et al. following a second inoculation of 103 organisms of 2457T administered in NaHCO3 [6]. A single study has assessed homologous protection using the S. sonnei strain 53G and shown 100% protection in subjects who received a prior dose (400 organisms) of the inoculum compared to 67% with disease in naive participants [10].
DISCUSSION
Since its inception, the Shigella human challenge model has been useful for researchers seeking to understand the disease pathogenesis, clinical manifestations, and immune responses associated with Shigella infection. Its utility has also been borne out in assessing the efficacy of vaccine candidates and antibiotics or other prophylactic products used to control bacterial infections. However, significant variability in study protocols including the methods utilized to administer the challenge inoculum, the outcome definitions and variable challenge strains confound result interpretation.
A major finding of this study is that diarrhoea attack rates appear somewhat dose-related but do not appear to increase above 75–80% with the two most commonly studied strains, 2457T and 53G. Higher doses generally did not result in higher diarrhoea or dysentery attack rates, nor are they representative of natural infection. Doses tested ranged from 10 to 1 × 1010 c.f.u., with doses as high as 1 × 104 to 1 × 108 tested for strain 2457T, even though as little as 10–100 organisms are needed to cause infection in the field [11, 12]. The dose range tested for the S. paradysenteriae (flexneri) strains from the inaugural 1946 study reached 1 × 109 to 1 × 1010 organisms, the highest doses tested for all studies; even this high dosage, however, failed to produce significant virulence in humans [3]. Unfortunately, detailed information on the origin or processing of these strains is unavailable. Recent studies have begun reporting rates of shigellosis which are further categorized by diarrhoea, vomiting and fever. No clear dose response for fever or dysentery is observable from the published results.
Only three studies to date have assessed homologous protection following an initial infection with an experimental challenge inoculum with clear evidence of a reduction in disease risk upon a second exposure [6, 9, 10]. There appears to be inherent variability in the induction of protective immune responses across the studied strains; however, more studies are needed to confirm this variability. Importantly, low attack rates in naive participants increase the sample size requirements to ensure such studies are adequately powered.
Despite a significant amount of effort to develop immune correlates of protection, data are lacking and limited to vaccination challenge studies where only a small number of immune parameters have been measured. A study in 1987 by Black et al. [7] identified a reduction in disease outcomes following challenge with S. sonnei strain 53G in subjects with prechallenge immune titres (⩾40) against the O antigen of S. sonnei. Most recently, studies by Kotloff et al. and Coster et al. indicate a similar reduction in disease risk in subjects with anti-lipopolysaccharide (LPS) antibody-secreting cells circulating in peripheral blood [13, 14]. Despite these data, evidence of immune correlates against naturally occurring shigellosis is more limited. Serotype-specific immunity is supported by epidemiological studies as well [15]. However, only Cohen et al. identified a specific immune parameter (anti-LPS serum antibodies) associated with significant protection from naturally occurring disease [16].
Increased dose-ranging for a variety of strains is needed in future studies. The greatest range of doses has been tested for strain 2457T, in which doses have ranged from 100 to 1 × 108. In comparison, the second most commonly studied strain (S. sonnei, 53G) has only reported inoculum doses ranging between 400 and 500 organisms, with relatively low diarrhoea and dysentery attack rates. Future studies should also focus on the development of models for other Shigella strains. Specifically, current vaccine development efforts have identified S. flexneri 2a, 3a, 6 and S. sonnei as likely targets that would provide broad-based coverage to travel and endemic populations [17]. Presently, there are no challenge models for either S. flexneri 3a or 6. Given the unpredictability of species-specific disease incidence in field settings, the human challenge is likely to play a significant role in vaccine development and highlights the need to expand efforts on Shigella challenge model development [17].
One underappreciated concern with the utilization of the human challenge model is the need for adequate sample sizes to ensure adequate confidence around estimates of disease outcomes. The confidence interval around a single estimate for disease decreases as sample sizes increase, but remains about 10% with as many as 60 subjects at a given inoculum dose. This is critical for adequately powering subsequent studies of vaccine efficacy. For example, assuming an 80% attack rate at a fixed inoculum dose in 60 people, the 95% CI is 70–90%. Presuming an efficacy study was adequately powered (80%) to detect 70% reduction in disease, total sample size requirements would range from 40 to 24 presuming a 70% or 90% placebo attack rate, respectively (assuming a 1:1 randomization).
Other challenges also complicate the development and utilization of these models. First, unlike challenge models with malaria, these studies are required to be conducted in an inpatient setting where appropriate oral and intravenous hydration fluids are readily available. Additionally, closed or treated water systems are often required to prevent release or spread of the experimental organism into the environment.
Other limitations include variability in challenge seed inoculum. Dose preparation is traditionally performed by preparing a bacterial suspension to a fixed optical density, a method with inherent imprecision, especially at low inoculum doses. Quantification of the number of organisms administered then relies on post-dose colony counts. One potential improvement in this area may be to include a more rapid technique for quantifying viable bacteria in the challenge inoculum. Implementation of a flow cytometry-based assay for viable bacteria may help ensure accurate target inocula are more frequently administered. Another approach could involve using lyophilized challenge inocula to more accurately estimate dose.
All clinical trials must weigh the ethical dilemmas involved with placing human subjects at risk against the potential benefit of a future drug, vaccine or other product designed to treat or prevent disease. This is no difference in experimental Shigella infections, which are used in drug and vaccine efficacy studies, but due to the unique aspects of experimental infection, challenge key aspects of this paradigm. The ethical framework developed by Miller & Grady offers a structure by which to evaluate experimental infection studies and can serve as a guide for future Shigella infection research [18]. These authors highlight seven ethical issues (rationale, risks, discomforts, vulnerable subjects, informed consent, financial compensation, and right to withdraw) that should be used to evaluate proposed studies and the application of several of these issues in relation to past and potentially future experimental Shigella challenge studies is warranted. One historical issue is that of the use of vulnerable subjects. Initial studies were conducted in inmates at local prisons, a vulnerable population that is unlikely to be utilized for future experimental infection studies. At present, however, factors associated with ensuring ongoing informed consent, financial compensation and the subjects' rights to withdraw seem no more intricate in human challenge studies than in other research in which subjects receive an experimental product.
An area that is an increasing topic of discussion related to the ethics of human challenge studies is that of risks and discomforts. While Shigella-associated diarrhoea in healthy adults is generally self-limiting and easily treatable in a controlled environment, there are known long-term health outcomes that can result from shigellosis. The most well-described of these sequelae is reactive arthritis (ReA), a sterile joint inflammation that occurs subsequent to a preceding infection [19]. The mechanisms by which a distal infection results in joint inflammation are largely unknown, although there appear to be genetic factors, specifically HLA-B27 predisposing some individuals to an increased risk. Efforts to exclude HLA-B27–positive subjects from experimental infection studies have been made and should persist in future studies.
In addition to ReA, recent literature has highlighted a potential association with other post-infectious sequelae. Three separate meta-analyses have shown a sevenfold increase in the risk of developing post-infectious irritable bowel syndrome (PI-IBS), following an episode of acute infectious gastroenteritis (IGE), of which Shigella is a cause [20–22]. Similar to ReA, the pathogen-specific attributable risk for these post-infectious sequelae is currently unknown; however, unlike ReA, PI-IBS occurs at a rate much higher than ReA and suggests a new paradigm in the understanding of the probability of long-term risks and discomforts possible following experimental Shigella infections. Given the rate of these outcomes, future studies should consider active surveillance for PI-IBS and other functional gastrointestinal disorders subsequent to experimental infections. It is important to note that the simple rate of these disorders in the general population will confound attribution to the experimental infection. Furthermore, the prompt treatment of infection in these models may obviate the sequelae risk. Moreover, there is growing evidence of immunological markers (IL-10, IL-12) which may predict risk of IBS following Shigella infection and could be used to screen for healthy volunteers (as is already done with HLA-B27).
Focusing on human challenge studies, Hope & McMillan [23] conclude that the simple act of experimentally infecting someone does not solely make a study morally wrong. They argue that the inherent concern arising from such studies is more associated with the general perception that experimental infection (and subsequent disease) leads to harm; however, in reality, that harm is no greater than what is widely acceptable in other human subjects' research. Future researchers should continue to strive for an adequate risk/benefit ratio and implement more stringent enrolment criteria to minimize the risk of long-term adverse health outcomes as well as increase more prolonged surveillance to identify incident outcomes.
The Shigella human challenge model has served as an invaluable tool in understanding the mechanisms of disease, clinical presentation, assessing vaccine efficacy and identifying immunological outcomes. While there has been variability in the model over time it is clear that several prototypic human challenge strains have emerged. Although more work is needed to refine methods for inoculum preparation and utilizing consistent, well-specified outcome definitions, it is clear that these models will serve an important role in furthering our understanding of this intestinal pathogen and as an early screen for potentially viable vaccine candidates. Future studies designed to further refine the dose–response relationship and to expand the repertoire of available challenge strains should be considered a priority.
ACKNOWLEDGEMENTS
This study was conducted under support of Military Infectious Disease Research Program funding. The opinions and assertions herein should not be construed as official or representing the views of the Department of the Navy, Department of the Army, the Department of Defense, or the U.S. Government. This is a U.S. Government work. There are no restrictions on its use.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Riddle MS, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. American Journal of Tropical Medicine and Hygiene 2006; 74: 891–900. [PubMed] [Google Scholar]
- 2.Kotloff KL, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization 1999; 77: 6516–66. [PMC free article] [PubMed] [Google Scholar]
- 3.Shaughnessy HJ, et al. Experimental human bacillary dysentery; polyvalent dysentery vaccine in its prevention. Journal of the American Medical Association 1946; 132: 362–368. [DOI] [PubMed] [Google Scholar]
- 4.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 5.Van de Verg LL, et al. Cross-reactivity of Shigella flexneri serotype 2a O antigen antibodies following immunization or infection. Vaccine 1996; 14: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 6.Kotloff KL, et al. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 1995; 13: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 7.Black RE, et al. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. Journal of Infectious Disease 1987; 155: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 8.Taylor DN, et al. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clinical Infectious Diseases 2006; 42: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 9.DuPont HL, et al. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. Journal of Infectious Disease 1972; 125: 12–16. [DOI] [PubMed] [Google Scholar]
- 10.Herrington DA, et al. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 1990; 8: 353–357. [DOI] [PubMed] [Google Scholar]
- 11.DuPont HL, Hornick RB. Clinical approach to infectious diarrheas. Medicine (Baltimore) 1973; 52: 265–270. [PubMed] [Google Scholar]
- 12.Guerrant R. Gastrointestinal infectious and food poisoning. In: Mandell G, Douglas R, Bennet J, eds. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone, 1990, pp. 837–847. [Google Scholar]
- 13.Coster TS, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infection and Immunity 1999; 67: 3437–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotloff KL, et al. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine 1995; 13: 495–502. [DOI] [PubMed] [Google Scholar]
- 15.Ferreccio C, et al. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. American Journal of Epidemiology 1991; 134: 614–627. [DOI] [PubMed] [Google Scholar]
- 16.Cohen D, Slepon R, Green MS. Sociodemographic factors associated with serum anti-Shigella lipopolysaccharide antibodies and shigellosis. International Journal of Epidemiology 1991; 20: 546–550. [DOI] [PubMed] [Google Scholar]
- 17.Levine MM, et al. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nature Reviews Microbiology 2007; 5: 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller FG, Grady C. The ethical challenge of infection-inducing challenge experiments. Clinical Infectious Diseases 2001; 33: 1028–1033. [DOI] [PubMed] [Google Scholar]
- 19.Rihl M, et al. Infection and musculoskeletal conditions: reactive arthritis. Best Practice & Research: Clinical Rheumatology 2006; 20: 1119–1137. [DOI] [PubMed] [Google Scholar]
- 20.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome – a meta-analysis. American Journal of Gastroenterology 2006; 101: 1894–1899; quiz 942. [DOI] [PubMed] [Google Scholar]
- 21.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Alimentary Pharmacology and Therapeutics 2007; 26: 535–544. [DOI] [PubMed] [Google Scholar]
- 22.Schwille-Kiuntke J, et al. Post-infectious irritable bowel syndrome – a review of the literature. Zeitschrift für Gastroenterologie 2011; 49: 997–1003. [DOI] [PubMed] [Google Scholar]
- 23.Hope T, McMillan J. Challenge studies of human volunteers: ethical issues. Journal of Medical Ethics 2004; 30: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz C, et al. Characteristics of Shigella sonnei infection of volunteers: signs, symptoms, immune responses, changes in selected cytokines and acute-phase substances. American Journal of Tropical Medicine and Hygiene 1995; 53: 47–54. [PubMed] [Google Scholar]
- 25.Tacket CO, et al. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. American Journal of Tropical Medicine and Hygiene 1992; 47: 276–283. [DOI] [PubMed] [Google Scholar]
- 26.Kotloff KL, et al. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infection and Immunity 1992; 60: 2218–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackowiak PA, Wasserman SS, Levine MM. An analysis of the quantitative relationship between oral temperature and severity of illness in experimental shigellosis. Journal of Infectious Diseases 1992; 166: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 28.Van de Verg L, et al. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infection and Immunity 1990; 58: 2002–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuPont HL, et al. Inoculum size in shigellosis and implications for expected mode of transmission. Journal of Infectious Diseases 1989; 159: 1126–1128. [DOI] [PubMed] [Google Scholar]
- 30.Levine MM, et al. Studies with a new generation of oral attenuated shigella vaccine: Escherichia coli bearing surface antigens of Shigella flexneri. Journal of infectious Diseases 1977; 136: 577–582. [DOI] [PubMed] [Google Scholar]
- 31.DuPont HL, et al. The response of man to virulent Shigella flexneri 2a. Journal of Infectious Diseases 1969; 119: 296–299. [DOI] [PubMed] [Google Scholar]
- 32.Levine MM, et al. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. Journal of Infectious Diseases 1973; 127: 261–270. [DOI] [PubMed] [Google Scholar]