SUMMARY
A temporal relationship of Japanese encephalitis virus (JEV) transmission in pigs, mosquitoes and humans revealed that sentinel pig seroconversions were significantly associated with human cases 4 weeks before (P = 0·04) their occurrence, highly correlated during the same time and 2 weeks before case occurrence (P < 0·001), and remained significantly correlated up to 2 weeks after human case occurrence (P < 0·01). JEV was detected in the same month in pigs and mosquitoes, and peaks of pig seroconversion were preceded by 1–2 months of peaks of infection in vectors. Kaplan–Meier analysis indicated that detection of JEV-positive mosquitoes was significantly associated with the median time to occurrence of seroconversion in pigs (P < 0·05). This study will not only help in predicting JEV activity but also accelerate timely vector control measures and vaccination programmes for pigs and humans to reduce the Japanese encephalitis risk in endemic areas.
Key words: Arboviruses, epidemiology, Japanese encephalitis
INTRODUCTION
Japanese encephalitis virus (JEV) is a member of the family Flaviviridae, genus Flavivirus. JEV is maintained in nature by a cycle that involves pigs and ardeid birds, which act as amplifying hosts [1]. Mosquitoes belonging to the Culex vishnui subgroup (Culex vishnui Theobald sensu stricto, Culex pseudovishnui Colless, Culex tritaeniorhynchus Giles) are the most important vector species in India [2]. An outbreak of JEV infection was first observed in Vellore district in Tamil Nadu, India in 1955 [3]. Since then the virus has been active in almost all parts of India [4]. The incidence of Japanese encephalitis in recent times has shown an increasing trend in India and has become a major public health problem [5]. JEV has been endemic in Assam since 1976 [6] and in recent years clinical cases of JE have increased markedly [7]. Furthermore, the primary mosquito vectors and both the effective amplifying hosts for JEV, i.e. pigs and ardeid birds, can be found in this region [8–10]. The temporal pattern of JEV transmission, especially within its host and vectors, and the possible source of viral amplification within its host system remains indistinguishable in this region. It is generally accepted that the JE seroconversion rates in sentinel pigs reflect the prevalence of JEV in the area [11]. Sentinel pigs have the advantages of monitoring enzootic transmission events at a specific location [12] and being more cost-effective than other arboviral surveillance indicators, especially ardeid birds.
In Assam, pigs are likely to be the major amplifying host as there is a high prevalence of JEV antibody in swine sera [9]. The high occurrence of JE cases in Assam may be related to the increasing number of pigs in recent years. Pigs are currently not vaccinated against JEV in India, including Assam. Reliable prediction of epidemiological concordance of JEV infection in swine, mosquito and human populations during any specified period in a specific endemic locality is important for practical reasons that include initiation of mosquito control and vaccination programmes in pigs and humans and to analyse the mechanism of seasonal spread of infection from one host to another. So far, no systematic study of this nature has been conducted in India, the outcome of which is very important in order to develop a strategic plan for JE prevention and control and to assess the success of these programmes in endemic areas. Therefore, the present study aims at identifying the temporal transmission pattern of JEV infection by tracking the sentinel pigs, mosquito vectors and incidence of human JE cases in Assam. This information will provide baseline data to design and implement JE prevention programmes not only in the state of Assam but also in other JE endemic areas of the country.
METHODS
Study area
The study was conducted during 2009 and 2010 in two areas, Barbaruah and Lahoal in Dibrugarh district, Assam, India (Fig. 1). These two areas were selected due to JEV endemicity, abundance of breeding places for JE vector mosquitoes and extensive pig rearing by the villagers [9]. Table 1 provides details of the increasing pig population in the study areas. Rice is the major crop cultivated between May and August and rice fields are generally located within a 2 km radius of human habitation.
Fig. 1.
Map showing the location of Assam in India and Barbaruah and Lahoal in Dibrugarh district of Assam.
Table 1.
Increasing trend of pig population in Lahoal and Barbaruah primary health centre, 2006–2009
| Study area | Years | |||
|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | |
| Lahoal | 4936 | 5765 | 9897 | 13 796 |
| Barbaruah | 5674 | 7973 | 12 463 | 18 775 |
Source: Directorate of Animal Husbandry and Veterinary Department, Government of Assam.
Sentinel pig seroconversion
Twenty locally procured, post-weaned pigs (aged 3–6 months) of both sexes and JEV antibody-negative by haemagglutination inhibition (HI) test were distributed to locally identified households for rearing in each area in January 2009. A similar process was followed in the next year after allowing the beneficiaries to dispose of the first set of pigs in December 2009. Blood samples were collected every 2 weeks from the sentinel pigs. JEV antibody seroconversion in pigs was determined by HI test [13]. Sera were serially diluted twofold from 1:10 to 1:20480. Sera with an HI titre of ⩾1:20 were considered as positive [14].
JEV antigen preparation
Formalin inactivated antigen was prepared from cell culture propagated virus using the BHK-21 cell line [15] at the Regional Medical Research Centre, ICMR, Dibrugarh, Assam, India. The maximum haemagglutination titre of the prepared antigen usually reached 1:10240. This antigen was used to test pig sera by HI [13].
Mosquitoes
Adult mosquitoes were collected bi-weekly around sentinel pig sheds and in human habitats during dusk hours by using suction tubes (aspirators). Species identification was done by using the mosquito identification keys [16–18]. Mosquitoes were stored, in species order, in Eppendorf vials (2 ml) in pools of up to 50 mosquitoes in each vial. Species-wise per man hour density (PMHD) of mosquitoes was calculated according to the formula:
where, N = number of mosquitoes collected; T = time spent (in minutes); P = number of persons involved in collection.
Each pool was triturated mechanically in a sterile mortar and pestle in 3–5 ml of 2% fetal calf serum in minimum essential medium containing 50 μg gentamicin, 50 IU penicillin, 50 μg streptomycin and 50 μg amphotericin B per ml and centrifuged at 1100 g for 15 min at 4 °C. The supernatant was filtered through a 0·2 μm filter, aliquoted and tested for the presence of JEV antigen using an antigen-capture ELISA [19]. The supernatant was subjected to RNA extraction by using a QIAmp viral RNA mini kit (Qiagen, Germany). Purified RNA was used as template for cDNA synthesis using First Strand cDNA Synthesis kit (Fermentas, USA). Reverse transcriptase–polymerase chain reaction (RT–PCR) was performed to amplify the 348 bp C-prM gene region [20] of JEV by using cDNA (2 μl) as template.
Minimum infection rate (MIR)
The virus infection rate in mosquitoes was expressed as MIR/1000 mosquitoes of a particular species tested [21].
Human JE cases
Collection of samples from the study area (Barbaruah and Lahoal) was limited to those that matched the World Health Organization clinical case definition of Acute Encephalitis Syndrome [22] and which were laboratory-confirmed, typically by detecting IgM antibodies in sera or cerebrospinal fluid (MAC ELISA kit; NIV, India) as well as through a fourfold rise of antibody titre in paired sera. A second sample was collected from patients with JEV infection 14-21 days after the first sample had been collected. The fourfold rise in neutralizing antibody titre in paired sera was determined by a microseroneutralization assay [23]. Written informed consent was obtained from the patient or guardian before collection of the sample. Ethical clearance for the present study was obtained from the institutional ethics committee of the Regional Medical Research Centre, ICMR, NE Region, Dibrugarh, Assam, India.
Data analysis
JEV infection in mosquitoes, i.e. MIR/1000 mosquitoes, was calculated for each month. Cross-correlation analysis was applied to detect the lag period between pig seroconversion and occurrence of human JE cases. A survival analysis was performed on the pig serology results stratified by the presence or absence of JEV-positive mosquito pools and a Kaplan–Meier survival curve was plotted. Data were electronically analysed using statistical software (SPSS version 13, USA).
RESULTS
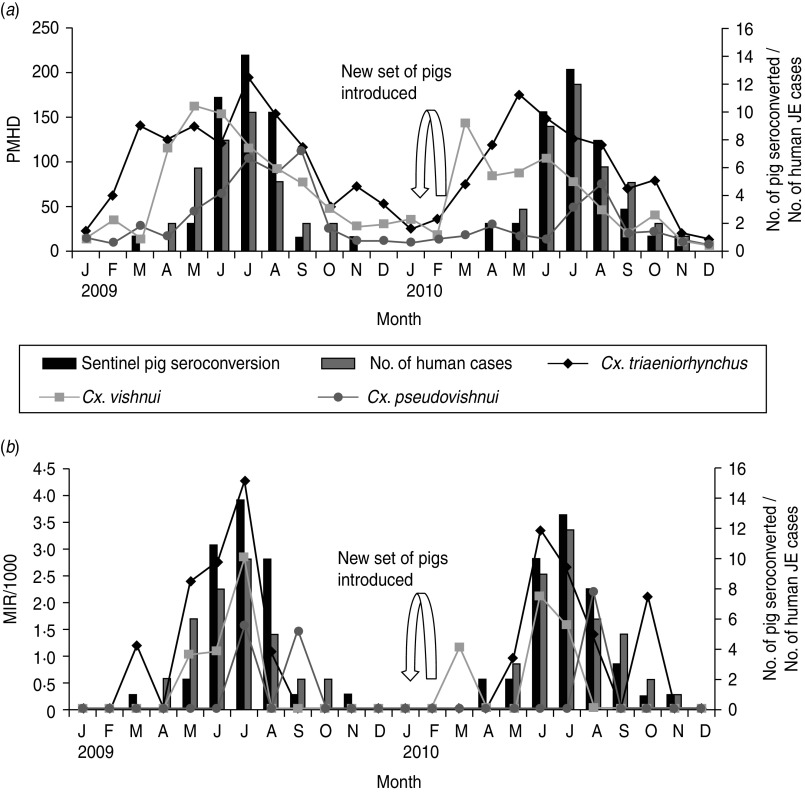
JEV seroconversion was seen in all 40 sentinel pigs during 2009 and 2010, starting from March and April of the first and second year, respectively. Most of the sentinel pigs became positive within 3 months, i.e. June–August, with a peak in July in both years (Fig. 2).
Fig. 2.
Relationship between pig seroconversion, (a) abundance and (b) JEV infection in Cx. tritaeniorhynchus, Cx. vishnui and Cx. pseudovishnui vector mosquitoes. PMHD, Per man hour density; MIR, minimum infection rate.
A total of 28503 mosquitoes, i.e. Cx. tritaeniorhynchus, Cx. vishnui and Cx. pseudovishnui collected and stored in 586 pools were screened for JEV over 2 years. Nineteen of 281 Cx. tritaeniorhynchus pools, 7/198 Cx. vishnui pools and 3/107 Cx. pseudovishnui pools collected during March to September were found positive by antigen-capture ELISA and RT–PCR. The results of this study clearly indicated the seasonal variation in occurrence of JEV infection in Cx. tritaeniorhynchus, Cx. vishnui and Cx. pseudovishnui, the principal JE vectors of India, in both PMHD and MIR peaks (Fig. 2). The MIR of Cx. tritaeniorhynchus was found to be highest with an average of 0·9 (range 0·0–4·3) during the 24-month study period followed by Cx. vishnui (average 0·4, range 0–2·9) and Cx. pseudovishnui (average 0·2, range 0–2·1)
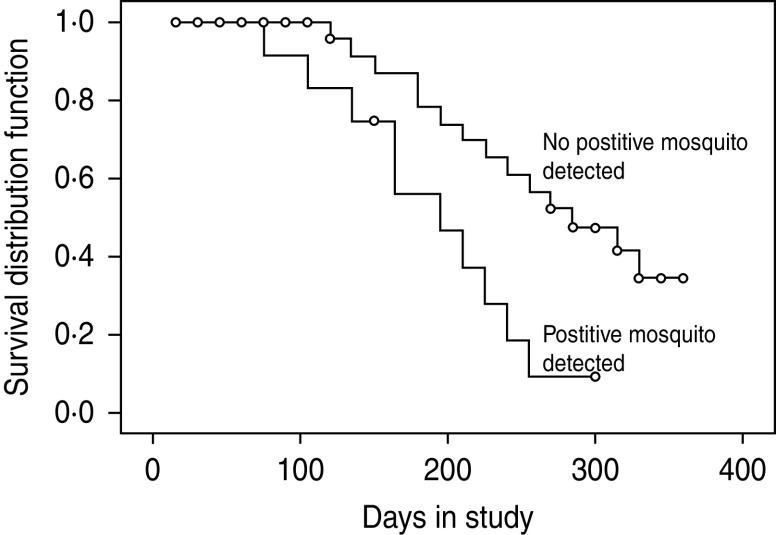
The monthly number of pig seroconversions during 2009 and 2010 compared to the monthly abundance of the Cx. vishnui subgroup of mosquitoes (PMHD) and JEV infection (MIR/1000) is shown in Figure 2. In 2009, the peak in pig seroconversions coincided with the increase in abundance of all three Cx. vishnui subgroup mosquitoes. In 2010, the pig seroconversion peak occurred after the increase in abundance of Cx. tritaeniorhynchus and Cx. vishnui (Fig. 2). In 2009, the first pig seroconversion and JEV-positive mosquito pools occurred in the same month, March, but in 2010, the first sentinel pig seroconversion in April preceded the detection of positive mosquitoes in March. The highest MIR peaks of Cx. tritaeniorhynchus and Cx. vishnui and the pig seroconversion peak occurred in July 2009, whereas in 2010, the highest MIR peak in June was followed by the peak seroconversion in July. The last seroconversion peak of both years occurred in November which was preceded by a peak in MIR in September and October of the first and second year, respectively. Thus, from this observation, JEV infection was detected in the same month in pigs as well as mosquitoes and pig seroconversions were also preceded by 1–2 months of peaks of occurrence of JEV infection in the vectors. Kaplan–Meier analysis revealed that the detection of positive mosquitoes is significantly associated with the median time to occurrence of seroconversion of pigs (P < 0·01) (Fig. 3).
Fig. 3.
Kaplan–Meier analysis to pig seroconversion stratified by mosquito positivity (P < 0·01).
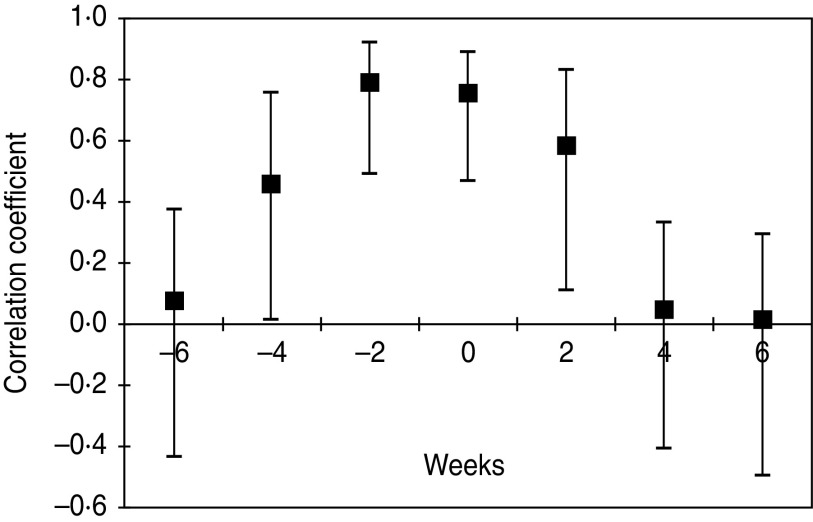
A graph based on plotting the onset dates of human JE cases and the seroconversion dates in sentinel pigs revealed a temporal concordance (Fig. 4). The same was evaluated by cross-correlation analysis by holding the number of human case counts constant by date of onset and shifting the number of pig seroconversions progressively earlier or later in time. Because of the seasonal trend present in both datasets, the time period was restricted from May to September. Cross-correlation coefficients with 95% confidence intervals revealed that sentinel pig seroconversions were significantly associated with human cases 4 weeks prior (r = 0·48, P = 0·04) to their occurrence and highly correlated during the same time period (r = 0·76, P< 0·001) as well as 2 weeks prior to case occurrence (r = 0·79, P< 0·001) and remained significantly correlated up to 2 weeks after cessation of human cases (r = 0·60, P < 0·01). These findings in the present study have revealed that initiation of porcine seroconversion predicted the incidence of JE cases in humans.
Fig. 4.
Relationship between human case counts and lagged sentinel pig seroconversions (lag is represented on the x-axis) depicted by correlation coefficients and 95% confidence intervals.
DISCUSSION
The present study demonstrated that JE seronegative sentinel pigs were sensitive indicators for detecting JEV transmission in a JE endemic area. Although various cross-sectional studies reported that sentinel pigs used for surveillance in various countries did not reflect JEV transmission to humans [24–27], in the Indian subcontinent porcine infections provided a means of detecting JEV activity in humans [19, 28, 29]. The present study further clearly illustrates how pig infections can be sensitive indicators for tracking tangential transmission of JEV in vector mosquitoes and subsequently to humans in an endemic area over a definite time period. The pattern of occurrence of JEV infection in mosquitoes, pigs and humans observed in this study area was striking in its synchrony.
In the present study, sentinel pig seroconversion which had been used to track JEV transmission subsequently in humans in endemic areas was significantly associated with human cases 4 weeks before their occurrence and up to 2 weeks after human case detection. This was also found to be highly correlated during the same time period and 2 weeks before human case occurrence. However, the concordance of sentinel pig seroconversion and human cases could be better assessed by shortening the time interval of blood sampling from 2 weeks to 1 week [30].
Our study revealed that both MHD and MIR in the Cx. vishnui subgroup of mosquitoes, the principal JE vector in India [31] showed seasonal variation [8, 32, 33]. There were three distinct peaks of MIR in June, July and August in both years. It is noteworthy that JEV infection was detected in the same month in pigs and mosquitoes, and peaks of pig seroconversion preceded by 1–2 months the peaks of JEV infection in vectors. The present study revealed that the median time to seroconversion in pigs is significantly associated with the MIR of vector mosquitoes. More particularly, the MIR of vector mosquitoes can be used as a tool to predict the appearance of JEV infection in pigs [24, 34].
Our study gives a clear understanding of the temporal transmission patterns of JE in mosquitoes, pigs and humans. This information will be extremely helpful in developing a strategic plan for JE prevention and control and to assess the success of the programme. There is no drug treatment for JE. Vaccination of pigs represents an alternative strategy to control JE [35, 36]. An alternative approach of moving domestic pigs away from human habitation to encompass the flight range of the local Culex vectors has been suggested as a potential method of reducing JEV transmission to humans [37, 38].
The marked increase of unorganized pig farming and trading may also play a major role in the increase of JE incidence [35]. Intensive and extensive IEC (information, education, communication) activities among the people in JE endemic areas should be undertaken to increase understanding of the necessity for implementation of JE control programmes and proper maintenance of pig populations in these areas [9]. Assessment of the success of such a programme can be measured by strict surveillance of JE incidence in humans, animals and its vector.
In conclusion, our study has established the temporal relationship of transmission of JE in an Indian setting which may depict identical scenarios in other pig-rearing areas of South East Asia with a similar ecological niche and potential mosquito vectors. This temporal relationship will not only help in predicting JEV activity in a particular JE endemic area but also accelerate the initiation of timely vector control measures and animal vaccination programmes to reduce the risk of JEV infection in both pigs and humans. This will also alert the local health authority and the administrator for management of any future outbreak of JE in areas under their jurisdiction.
ACKNOWLEDGEMENTS
J. Borah acknowledges the Indian Council of Medical Research, Government of India, for providing a senior research fellowship (SRF) to carry out the study. We thank the Directorate of Animal Husbandry and Veterinary Department, Government of Assam, India for providing pig population data. Technical assistance received from N. K. Baruah, P. K. Doloi, R. Doloi, L. J. Borah, P. Chowdhury and R. Topno in laboratory and field work is gratefully acknowledged.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, eds. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins, 2007, pp. 1101–1190. [Google Scholar]
- 2.Sunish P, Reuben R. Factors influencing the abundance of Japanese encephalitis vectors in ricefields in India. Medical and Veterinary Entomology 2001; 15: 381–392. [DOI] [PubMed] [Google Scholar]
- 3.Grascenkov NI. Japanese encephalitis in the USSR. Bulletin of World Health Organization 1964; 30: 161. [PMC free article] [PubMed] [Google Scholar]
- 4.Saxena V, Dhole TN. Preventive strategies for frequent outbreaks of Japanese encephalitis in Nothern India. Journal of Bioscience 2008; 33: 505–514. [DOI] [PubMed] [Google Scholar]
- 5.Kabilan L, et al. Japanese encephalitis in India: an overview. Indian Journal of Pediatric 2004; 71: 609–615. [DOI] [PubMed] [Google Scholar]
- 6.Khan SA, et al. West Nile virus infection, Assam, India. Emerging Infectious Diseases 2011; 17: 947–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borah J, et al. A comparison of clinical features of Japanese encephalitis virus infection in the adult and pediatric age group with Acute Encephalitis Syndrome. Journal of Clinical Virology 2011; 52: 45–49. [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, et al. Role of some environmental factors in modulating seasonal abundance of potential Japanese encephalitis vectors in Assam, India. Southeast Asian Journal of Tropical Medicine and Public Health 1996; 27: 382–391. [PubMed] [Google Scholar]
- 9.Dutta P, et al. Effect of insecticide–treated mosquito nets (ITMNs) on Japanese encephalitis virus seroconversion in pigs and humans. American Journal of Tropical Medicine and Hygiene 2011; 84: 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Environmental Information System, India (http://www.wetlandsofindia.org/wetlandwiki/index.php/Assam). Accessed 4 March 2011.
- 11.Arai S, et al. vaccine preventable disease surveillance programme of Japan. Japanese Journal of Infectious Diseases 2008; 61: 333–338. [PubMed] [Google Scholar]
- 12.Nidaira M, et al. Survey of Japanese encephalitis virus in pigs on Miyako, Ishigaki, Kume, and Yonaguni Islands in Okinawa, Japan. Japanese Journal of Infectious Diseases 2009; 62: 220–224. [PubMed] [Google Scholar]
- 13.Clark DH, Casals J. Techniques for hemagglutination and haemagglutination inhibition with arthropod borne viruses. American Journal of Tropical Medicine and Hygiene 1958; 7: 561–573. [DOI] [PubMed] [Google Scholar]
- 14.Yang DK, et al. Serosurveillance for Japanese encephalitis, Akabane, and Aino viruses for Thoroughbred horses in Korea. Journal of Veterinary Science 2008; 9: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appaiahgari MB, Sudhanshu V. Immunogenicity and protective efficacy in mice of a formaldehyde-inactivated Indian strain of Japanese encephalitis virus grown in Vero cells. Vaccine 2004; 22: 27–28. [DOI] [PubMed] [Google Scholar]
- 16.Christophers SR. The Fauna of British India, including Ceylon and Burma. Diptera. Vol. IV. Family Culicidae. Tribe Anophilinae. London: Taylor and Francis, 1933, pp. 371. [Google Scholar]
- 17.Barraud PJ. The Fauna of British India, including Ceylon and Burma. Diptera. Vol. V, FamiIy Culicidae. Tribes Megarhinini and Culicinii. London: Taylor & Francis, 1934, pp. 463. [Google Scholar]
- 18.Reinert JF. Revised list of abbreviations for genera and subgenera of Culicidae (Diptera) and notes on generic and subgeneric changes. Journal of American Mosquito Control Association 2001; 17: 51–55. [PubMed] [Google Scholar]
- 19.Gajanana A, et al. Comparative evaluation of bioassay and ELISA for detection of Japanese encephalitis virus in field collected mosquitoes. Southeast Asian Journal of Tropical Medicine and Public Health 1995; 26: 91–97. [PubMed] [Google Scholar]
- 20.Sapkal GN, et al. Detection and isolation of Japanese encephalitis virus from blood clots collected during the acute phase of infection. American Journal of Tropical Medicine and Hygiene 2007; 77: 1139–1145. [PubMed] [Google Scholar]
- 21.Chiang CL, Reeves WC. Statistical estimation of virus infection rates in mosquito vector populations. American Journal of Tropical Medicine and Hygiene 1962; 75: 377. [DOI] [PubMed] [Google Scholar]
- 22.Solomon T, et al. A cohort study to assess the new WHO Japanese Encephalitis surveillance standards. Bulletin of World Health Organization 2008; 86: 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui J, et al. Detection of Japanese encephalitis virus antibodies in bats in Southern China. American Journal of Tropical Medicine and Hygiene 2008; 78: 1007–1011. [PubMed] [Google Scholar]
- 24.Rajendran R, et al. Longitudinal studies in South Indian villages on Japanese encephalitis virus infection in mosquitoes and seroconversion in goats. Tropical Medicine and International Health 2003; 8: 174–181. [DOI] [PubMed] [Google Scholar]
- 25.Simpson DI, et al. Japanese encephalitis in Sarawak: virus isolation and serology in a land dyak village. Transaction of Royal Society of Tropical Medicine and Hygiene 1970; 64: 503–510. [DOI] [PubMed] [Google Scholar]
- 26.Wuryadi S, Suroso T. Japanese encephalitis in Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health 1989; 20: 575–580. [PubMed] [Google Scholar]
- 27.Peiris JS, et al. Japanese encephalitis in Sri Lanka: comparison of vector and virus ecology in different agro-climatic areas. Transaction of Royal Society of Tropical Medicine and Hygiene 1993; 87: 541–548. [DOI] [PubMed] [Google Scholar]
- 28.Geevarghese G, et al. Domestic pigs as sentinels to monitor the activity of Japanese encephalitis and West Nile viruses in Kolar district, Karnataka. Indian Journal of Medical Research 1987; 86: 413–418. [PubMed] [Google Scholar]
- 29.Thenmozhi V, et al. Temporal relationship of swine infection of Japanese encephalitis virus to human cases in a Japanese encephalitis endemic area of Tamil Nadu. Virus Information Exchange Newsletter 1991; 8: 64. [Google Scholar]
- 30.Kwan JL, et al. Sentinel chicken seroconversions track tangential transmission of West Nile virus to humans in the greater Los Angeles area of California. American Journal of Tropical Medicine and Hygiene 2010; 83: 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanojia PC, et al. A long–term study on vector abundance & seasonal prevalence in relation to the occurrence of Japanese encephalitis in Gorakhpur district, Uttar Pradesh. Indian Journal of Medical Research 2003; 117: 104–110. [PubMed] [Google Scholar]
- 32.Murty US, Rao MS, Arunachalam N. The effects of climatic factors on the distribution and abundance of Japanese encephalitis vectors in Kurnool district of Andhra Pradesh, India. Journal of Vector Born Diseases 2010; 47: 26–32. [PubMed] [Google Scholar]
- 33.Reuben R, et al. Illustrated keys to species of Culex (Culex) associated with Japanese encephalitis in Southeast Asia (Diptera: Culicidae). Mosquito Systematics 1994; 26: 75–96. [Google Scholar]
- 34.Gingrich JB, et al. A longitudinal study of Japanese encephalitis in suburban Bangkok, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1987; 18: 558–566. [PubMed] [Google Scholar]
- 35.Erlanger TE, et al. Past, present, and future of Japanese encephalitis. Emerging Infectious Diseases 2009; 15: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umenai T, et al. Japanese encephalitis: current worldwide status. Bulletin of World Health Organization 1985; 63: 625. [PMC free article] [PubMed] [Google Scholar]
- 37.Mackenzie JS, Williams DT, Smith W. Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. In: Tabor E, ed. Emerging Viruses in Human Populations. Amsterdam: Elsevier, 2007, pp. 201–268. [Google Scholar]
- 38.Williams DT, et al. Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. American Journal of Tropical Medicine and Hygiene 2001; 65: 379–387. [DOI] [PubMed] [Google Scholar]